Abstract
The small GTPase Rap1A has a critical role in regulating cell-matrix and cell-cell adhesion. In T lymphocytes, Rap1A mediates LFA-1 activation and LFA-1-mediated adhesion. LFA-1 reduces the threshold of TCR signals for low affinity ligands. Previously, we determined that mice expressing constitutively active Rap1A on T cells have increased frequency of CD103+ T regulatory cells (Treg). We hypothesized that Rap1A-GTP might affect the differentiation of Treg by regulating LFA-1 activation. Using Foxp3-GFP-KI, LFA-1-KO and Rap1A-GTP-Tg mice we determined that Rap1A has an active role in the development of thymic Treg but LFA-1 is not mandatory for this function. Rap1A is also involved in the generation of peripheral Treg and this effect is mediated via LFA-1-dependent and LFA-1-independent mechanisms. Identification of the signaling pathways via which Rap1-GTP contributes to the differentiation of Treg will provide new insights to the function of Rap1A and to designing targeted approaches for generation of Treg for therapeutic applications.
Introduction
Rap1A, a member of the Ras family of GTPases has a critical role in regulating cell-matrix and cell-cell adhesion. Activation of Rap1A (thereafter referred to as Rap1), initiates inside-out signaling to integrins, resulting in conformational changes that convert integrin to high-affinity states [1]. Inside-out signaling enhances the function of a wide range of integrins including aLb2 (LFA-1), VLA-4 and VLA-5 on T cells [2; 3; 4], aIIbb3 on megakaryocytes and platelets [5; 6], and CD11b on monocytes and macrophages [7]. This action of Rap1 regulates a variety of processes in hematopoietic cells, including aggregation, migration, extravagation and homing to target tissues. “Outside-in” signaling refers to intracellular signals that are generated following the binding of integrins with their extracellular ligands [8]. Rap1 itself is a target of outside-in signaling [9] suggesting a positive feedback loop further enhancing integrin function.
The role of integrin activation on the functional outcome of TCR mediated signals is well-established. LFA-1 (aLb2 or CD11a/CD18) is the only b2 integrin expressed in mature T cells and reduces the threshold of TCR signals especially for low affinity ligands [10; 11; 12; 13; 14; 15]. The physiologic importance of LFA-1 is documented by the fact that individuals lacking the b2 subunit (CD18) display a constellation of pathological findings known as leukocyte adhesion deficiency (LAD). These individuals display inability to clear pathogens, develop recurrent infections and often die at an early age [16]. Interestingly, there are reports on patients with LAD, who develop inflammatory bowel disease (IBD) [17].
To investigate the role of LFA-1 in vivo, two different types of experimental mice have been generated one in which the b2 chain (CD18) of the integrin has been targeted [18] and a second one, in which the aL (CD11a) chain has been deleted [19]. The CD11a-/- and the CD18-/- mice display reduced T cell numbers in peripheral lymph nodes and extralymphoid organs including lung and liver and impaired T cell effector function. In spite of the impaired T cell responses these animals also display inflammatory manifestations. Because these observations were consistent with a mechanism of impaired T regulatory cell (Treg) function, the role of LFA-1 in Treg development and function was examined. Studies using CD18-/- mice have shown that CD18 is required for the development of Treg [20]. These mice have reduced numbers of Treg in the thymus and in the peripheral lymphoid organs and impaired Treg suppressive function. In contrast, mice deficient for CD11a displayed normal Treg frequencies in the thymus but reduced Treg frequencies in peripheral lymphoid organs and impaired Treg function [21].
Because Rap1 is indispensable for activation of LFA-1, we sought to determine whether Rap1 might regulate the development of Treg. Previously, we determined that mice expressing the constitutively active Rap1 mutant, Rap1E63, in their T cells displayed increased frequencies of CD103+ Treg cells in the spleen and lymph nodes [22]. Because CD103+ Treg represent post-thymic, peripherally generated Treg [23; 24] those studies did not determine the role of Rap1 in the development of natural Treg in the thymus. In the present study, we examined the role of Rap1 in the generation of thymic and peripheral Treg and we investigated whether Rap1-mediated activation of LFA-1 might be involved in this function. Using Foxp3-GFP-KI, LFA-1-KO and Rap1-GTP-Tg mice we determined that Rap1 has an active role in the development of thymic Treg but LFA-1 is not required for this function. Rap1 is also involved in the generation of peripheral Treg from naïve CD4+CD25- T cells in vitro and in vivo and this effect is mediated via LFA-1-dependent and LFA-1-independent mechanisms. However, although Rap1-GTP can regulate generation Treg via LFA-1-independent pathways, LFA-1 expression is mandatory for Treg suppressive function.
Materials and Methods
Animals
Mouse strains used were: eight to 12-week-old C57BL/6 mice (Charles River Laboratory); LFA-1 null mice (CD11a-/-, C57BL/b background, Jackson laboratory); Rag2-/- mice were obtained from Taconic; Foxp3-IRES-GFP knock-in (Foxp3GFP-KI) reporter mice were obtained from Dr. Mohamed Oukka [25]; Rap1E63 transgenic (Rap1E63-Tg) mice were previously described [22] and maintained in our laboratory; LFA-1 null mice (LFA-1-KO) were crossed on Rap1E63 transgenic mice resulting in Rap1E63-Tg T cells lacking CD11a (LFA-1-KO/Rap1E63-Tg mice). Rap1E63-Tg mice, Foxp3GFP-KI and Foxp3GFP-KI/LFA-1-KO/Rap1E63-Tg mice were generated in our laboratory by crossing Rap1E63-Tg mice or LFA-1-KO/Rap1E63-Tg mice with Foxp3GFP-KI mice. All the experimental animals were maintained in Beth Israel Deaconess Medical Center animal housing facility (Boston MA).
Cell purification
Total lymphocytes were isolated from the spleen and lymph nodes (axillary, brachial, and inguinal) of various mouse strains of C57BL/6 Foxp3-GFP reporter mice. CD4+ T cells were obtained using CD4 isolation kit and negatively selected through LS columns (Miltenyi Biotec). Subsequently, CD4+Foxp3- cells were sorted on MoFlo based on expression of GFP on Foxp3+ cells. The resultant CD4+Foxp3- cells were used in TGF-b induced Treg assay or were incubated with CD62L microbeads and naïve CD4+CD62LhiGFP- cells were positively selected through LS columns. The resultant cells were used for in vivo adoptive transfer experiments.
In vitro iTreg development
Naïve, CD4+CD62LhiGFP- cells were cultured with antigen presenting cells were stimulated with 1ug/ml soluble anti-CD3 antibody in the absence or presence of 0.02, 0.2, 2, or 20ng/ml of TGF-b for 3 days.
In vivo adoptive transfer
Purified naïve CD4+CD62L+Foxp3-GFP- cells were i.v. injected into the tail veins of Rag-/- mice (1 × 106 cell/mouse). Mice were monitored for 5 weeks euthanized for cellular analysis.
Cell surface and intracellular staining
Surface antigen expression was determined with Abs specific for CD8a. CD4. For Foxp3 staining, cells were stained with anti-CD4 antibody, fixed and permeabilized with the fixation and permeabilization solution (eBioscience). Subsequently, the cells were intracellular stained with Foxp3. Data were collected on LSRII and analyzed by FlowJO software.
Results and Discussion
Active Rap1 promotes development of thymic Treg via LFA-1 independent mechanisms
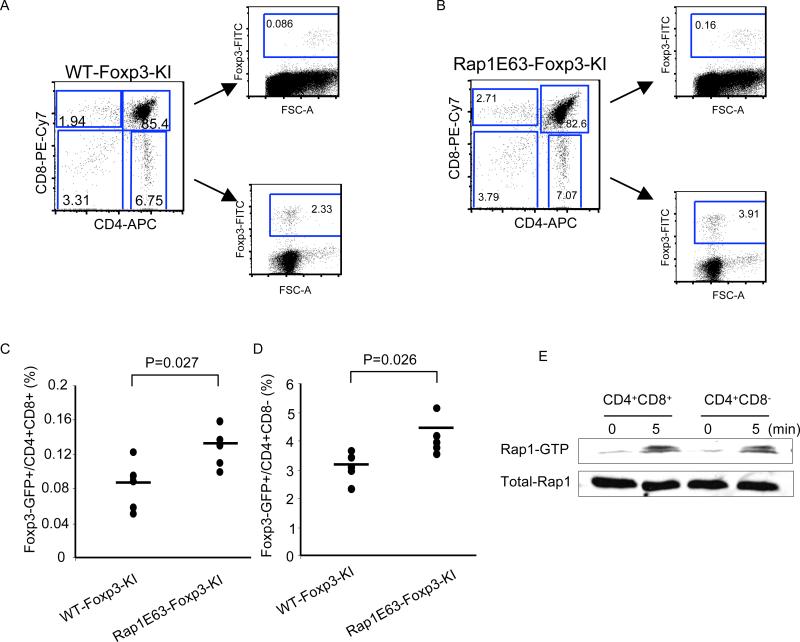
Our previous studies revealed that mice expressing Rap1E63 in T cells (Rap1E63-Tg) displayed a four-fold increase in the frequency of Treg in spleens and lymph nodes (p=0.007 RapE63-Tg vs. NLC) [22]. Peripheral Treg originate from natural Treg (nTreg) generated in the thymus but may also develop from post thymic CD4+CD25- naïve T cells, which undergo conversion into inducible Treg (iTreg) in the peripheral tissues [26]. To dissect the potential role of Rap1 in the development of thymic and peripheral Treg we used the Foxp3GFP knock-In (KI) strain, in which GFP is expressed under the control of Foxp3 promoter [25] and we generated Foxp3GFP-KI/Rap1E63-Tg mice. Treg differentiation in the thymus occurs at the double positive (DP) stage [27; 28]. Consistent with this pattern, in control Foxp3GFP-KI mice, Foxp3+ cells were detected among immature CD4+CD8+ DP thymocytes but the majority of Foxp3+ cells was detected within the CD4+CD8- single positive (SP) thymic cell population (Fig. 1A). The frequencies of Foxp3+ cells within the CD4+CD8+ DP and within the CD4+ SP thymocyte populations were significantly increased in Foxp3GFP-KI/Rap1E63-Tg mice compared to control Foxp3GFP-KI mice (Fig. 1B-D). Because our previous studies showed that Rap1E63-Tg and control wild type (WT) mice had comparable absolute thymocyte numbers and differentiation profiles [22], our present data suggest that Rap1 plays a positive role in the development of Foxp3+ Treg in the thymus. Notably, DP thymocytes from WT mice could activate endogenous Rap1 in response to CD3 mediated signals (Fig. 1E), suggesting that Rap1 activation might be a physiologic event that occurs at the DP stage of thymocyte maturation.
Figure 1.
Rap1 promotes the generation of thymus-derived Treg. A-B, Thymi were harvested from WT (A) and Foxp3GFP-KI/Rap1E63-Tg mice (B). Differentiation of thymocytes and Foxp3 expression within CD4+CD8+ and CD4+CD8- thymocytes were assessed by flow cytometry. C-D, The percentages of Foxp3+ cells in CD4+CD8+ thymocytes (C) and CD4+CD8- thymocytes (D) obtained from five Foxp3GFP-KI/Rap1E63-Tg mice were compared with those obtained from five Foxp3GFP-KI age-matched littermate control mice. Data are representative of two independent experiments. E, WT CD4+CD8+ and CD4+CD8- thymocytes were stimulated with anti-CD3 and anti-CD28 antibodies for 5 min and expression of GTP-bound Rap1 was examined by pull-down assay. Immunoblot with Rap1-specific antibody was done to confirm equal Rap1 expression.
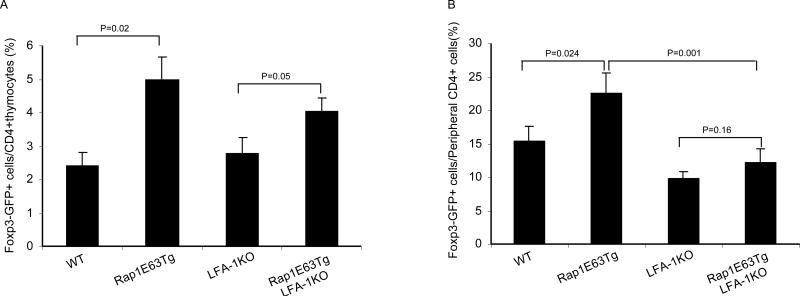
Rap1-GTP induces activation of LFA-1 [2; 4; 29], which enhances low affinity TCR signals [10; 13]. Treg selection is facilitated by TCR with affinities for self peptide-ligands falling within a range between the low affinity of CD4+ T cells that undergo positive selection and the high-affinity of self-reactive T cells that undergo negative selection [30]. We hypothesized that Rap1 might affect the development of Treg because it alters the strength of TCR signals. To test this hypothesis, we generated Foxp3GFP-KI/Rap1E63-Tg mice deficient for CD11a-/- (the aL chain of LFA-1; thereafter termed LFA-1-KO). In contrast to Foxp3GFP-KI/Rap1E63-Tg thymocytes, which displayed increased adhesion to ICAM-1, the ability of Foxp3GFP-KI/LFA1-KO and Foxp3GFP-KI/LFA1-KO/Rap1E63-Tg T cells for adhesion to ICAM-1 was abrogated (Fig. S1). These results confirmed that in spite of the expression of Rap1-GTP, Foxp3GFP-KI/LFA-1-KO/Rap1E63-Tg thymocytes lack LFA-1-mediated adhesion. Assessment of GFP+ cells revealed that Foxp3GFP-KI/LFA1-KO mice did not have significant difference in the frequency of Foxp3+ thymic Treg cells compared to control Foxp3GFP-KI animals (Fig. 2A). This finding is consistent with previous results indicating that CD11a deficiency did not reduce thymic Treg [21]. However, Foxp3GFP-KI/LFA1-KO/Rap1E63-Tg mice displayed a significant increase in the frequency of Foxp3+ thymic Treg compared to Foxp3GFP-KI and Foxp3GFP-KI/LFA-1-KO mice (Fig. 2A). These results suggest that Rap1-GTP regulated the development of thymic Treg in an LFA-1-independent manner.
Figure 2.
Rap1-GTP leads to increase of natural Treg in the thymus via a mechanism independent of LFA-1 and to increase of peripheral Treg via a mechanism predominantly dependent on LFA-1. A-B, Foxp3 expression was analyzed on freshly isolated thymocytes and splenocytes from WT, Rap1E63Tg, LFA-1-/-, Rap1E63Tg-LFA-1-/- mice by flow cytometry. The percentages of Foxp3+ cells in CD4+ thymocytes (A) and CD4+ splenocytes (B) are presented. Data in are mean ± SD of ≥ 3 mice/group.
When we analyzed frequencies of peripheral Foxp3+ cells in the same mice, we observed a reproducible, significant increase of Foxp3+ T cells in the peripheral CD4+ T cells of Foxp3GFP-KI/Rap1E63-Tg compared to Foxp3GFP-KI mice (p=0.024). In contrast, expression of Foxp3+ T cells was reduced in Foxp3GFP-KI/LFA-1-KO and in Foxp3GFP-KI/LFA-1-KO/Rap1E63-Tg mice compared to Foxp3GFP-KI wild type and to Foxp3GFP-KI/LFA-1-KO/Rap1E63-Tg animals (Fig. 2B). These results indicate that the effect of Rap1-GTP on the quantitative expression of peripheral Treg is mediated predominantly via LFA-1 dependent mechanisms.
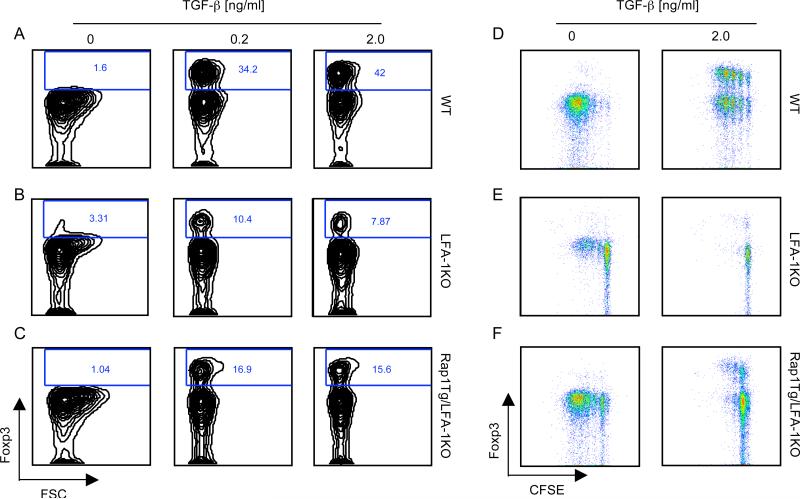
LFA-1 deficiency leads to impaired generation of iTreg by TGF-b in vitro
Peripheral Treg originate from nTreg generated in the thymus but may also develop from post thymic naïve T cells, which undergo conversion into iTreg in the peripheral tissues [26]. Although the role of TGF-b in the development of Treg in vivo remains controversial [31; 32; 33], culture with TGF-b represents the best-studied approach to generate Foxp3+ iTreg from naïve T cells in vitro [34; 35]. To investigate whether LFA-1 plays a role in the conversion of peripheral naïve T cells to iTreg in vitro, naïve Foxp3-CD4+ T cells (CD4+CD62LhiGFP-) from Foxp3GFP-KI and Foxp3GFP-KI/LFA1-KO mice were cultured with anti-CD3 antibody and antigen presenting cells (APC) in the presence of TGF-b. Naïve T cells from Foxp3GFP-KI mice were converted into Foxp3+ cells by a range of TGF-beta concentrations (Fig. 3A). In sharp contrast, naïve T cells from Foxp3GFP-KI/LFA1-KO mice displayed a dramatically impaired ability to convert into Foxp3+ cells under the identical conditions (Fig. 3B). Compared to Foxp3GFP-KI/LFA1-KO cells, a two-fold increase in the induction of Foxp3+ cells was observed in naïve Foxp3GFP-KI/LFA1-KO/Rap1E63-Tg T cells expressing constitutively active Rap1 (Fig. 3C), but the generation of Foxp3+ cells remained significantly impaired compared to wild type Foxp3GFP-KI T cells (Fig. 3A). These results indicate that Rap1 can promote in vitro conversion of naïve T cells in response to TGF-b and this effect is mediated in an LFA-1 independent manner.
Figure 3.
Rap1 is involved in generation of Treg by TGF-b via LFA-1-dependent and independent mechanisms. A, Naive (CD4+CD62Lhigh) Foxp3GFP-KI T cells from the indicated mouse strains were cultured with anti-CD3-plus-APC in the presence of the indicated concentrations of TGF-beta. Generation of Foxp3+ cells was assessed by flow cytometric analysis after 3 days of culture. Data from one representative of two similar experiments are shown. B, Naïve Foxp3GFP-KI T cells were labeled with CFSE and subsequently were cultured with anti-CD3-plus-APC in the presence of indicated concentrations of TGF-b. Foxp3 expression and cell proliferation were simultaneously analyzed by flow cytometry after 3 days of culture. Data are representative of two independent experiments.
Conversion of naïve T cells into Treg by TGF-b requires TCR engagement and T cell activation [34; 35]. In contrast to WT Foxp3GFP-KI, Foxp3GFP-KI/LFA-1-KO T cells proliferated poorly in response to anti-CD3-plus-APC as determined by CFSE dilution assay (Fig. 3D and E, left panels). Addition of TGF-b abrogated proliferation of Foxp3GFP-KI/LFA-1-KO T cells (Fig. 3E, right panel) whereas the inhibitory effect was less prominent in WT T cells (Fig. 3D, right panel). The poor proliferation of Foxp3GFP-KI/LFA-1-KO T cells in response to anti-CD3-plus-APC that was abrogated by TGF-b might be responsible for their impaired ability to convert into Foxp3+ cells (Fig. 3B). Expression of Rap1-GTP in LFA-1-KO/Rap1E63-Tg T cells significantly enhanced proliferation in response to anti-CD3-plus-APC compared to Foxp3GFP-KI/LFA-1-KO T cells (Fig. 3F, left panel). This response might be due to an effect of Rap1E63 on activation of other integrins distinct from LFA-1 that are involved in T cell activation and proliferation. Strikingly, TGF-b mediated a potent inhibitory effect in proliferating Foxp3GFP-KI/LFA-1-KO/Rap1E63-Tg cells (Fig. 3F, right panel) that was more prominent than the inhibitory effect of TGF-b in wild type Foxp3GFP-KI cells (Fig. 3D, right panel), indicating that Foxp3GFP-KI/LFA-1-KO/Rap1E63-Tg cells were more susceptible to the effects of TGF-b. Indeed, TGF-b-mediated anti-proliferative function and conversion of Foxp3GFP-KI/LFA-1-KO/Rap1-Tg T cells into Foxp3+ Treg was induced by very low concentrations of TGF-b (0.02ng/ml) that had a minimal effect on wild type Foxp3GFP-KI cells (Fig. S2A and B). Taken together, our results indicate that LFA-1 is required for optimal Treg generation by TGF-b in vitro but LFA-1-independent Rap1-mediated mechanisms are also operative in this process.
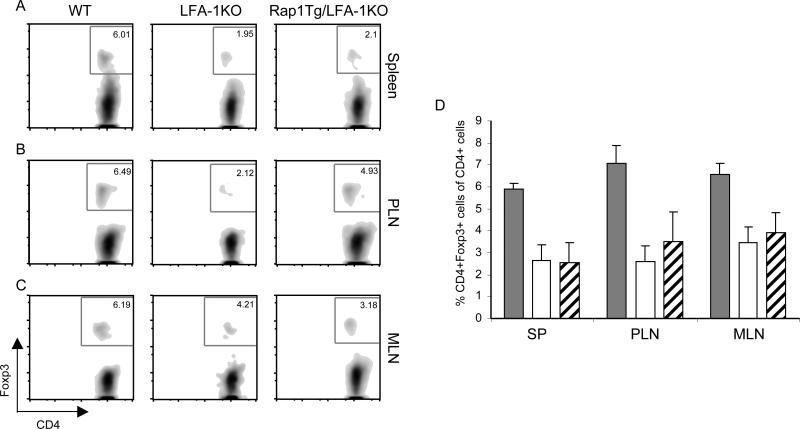
LFA-1 deficiency leads to impaired iTreg cell development in vivo
Naïve CD4+ T cells can convert in vivo into Foxp3+ iTreg as a result of lymphopenia-driven homeostatic proliferation [36; 37]. . To investigate the role of LFA-1 and Rap1 in the conversion of naïve T cells to iTreg in vivo, we adoptively transferred naïve (CD4+CD62LhiGFP-) T cells from WT Foxp3GFP-KI or Foxp3GFP-KI/LFA-1-KO mice into Rag2-/- recipients, in which donor T cells could undergo homeostatic expansion, and we analyzed Foxp3 expression on donor CD4+ cells 5 weeks after transfer. In vivo conversion of WT naïve CD4+ donor T cells into Foxp3+ Treg was readily detected (Fig. 4A-D). In contrast, conversion of naïve donor CD4+ T cells from Foxp3GFP-KI/LFA-1-KO mice into Foxp3+ Treg was significantly reduced (Fig. 4A-D) indicating that these cells were deficient in their ability to convert into Foxp3+ Treg cells in vivo (Fig. 4A-B). Expression of Rap1-GTP in naïve CD4+ T cells from Foxp3GFP-KI/LFA-1-KO/Rap1E63-Tg mice slightly increased the percentage of Foxp3+ cells in the peripheral lymphoid organs (Fig. 4A-D). These results demonstrate the critical role of LFA-1 in regulating de novo generation and/or maintenance of Foxp3+ iTreg in vivo in response to lymphopenia-driven homeostatic proliferation and indicate that Rap1-GTP has only a minimal LFA-1-independent role in this process.
Figure 4.
LFA-1 deficiency results in impaired conversion of naïve T cells into Treg in vivo during lymphopenia-induced homeostatic proliferation. A-B, naïve (CD4+CD62LhighGFP-) Foxp3GFP-KI T cells (1×106cells/mouse) from WT, LFA-1-KO, LFA-1-KO/Rap1E63-Tg mice were adoptively transferred i.v. into Rag2-/- recipients. Spleens, peripheral lymph nodes (PLN), and mesenteric lymph nodes (MLN) were analyzed for GFP(Foxp3) expression five weeks after in vivo transfer (A). B, The percentage of GFP(Foxp3) expressing cells in the spleen, PLN, and MLN are shown as the mean ± SD of three independent mice.
TCR mediated signals are mandatory for Foxp3 induction and generation of Treg [38]. Because Rap1 induces LFA-1 activation, we hypothesized that Rap1 might affect the development of Treg because it alters the strength of TCR signals. Surprisingly, our data showed that Rap1-GTP significantly increased the frequency of Foxp3+ Treg cells in the thymus in WT but also in LFA-1 deficient mice, indicating that Rap1 mediated this effect in a manner independent of LFA-1. In our studies, LFA-1 deficiency resulted in reduced Treg frequency in the peripheral lymphoid organs but not in the thymus consistent with previous results using CD11a-/- mice [21]. Expression of Rap1-GTP significantly increased the frequency of Foxp3+ Treg in the thymus of LFA-1-KO mice but had an insignificant impact on the peripheral Foxp3+ T cell population.
Because LFA-1 is involved not only in cell adhesion but also in homing and migration [15], the inability of Rap1-GTP to increase the frequency of peripheral Foxp3+ T cells in LFA-1-KO mice might reflect reduced thymic egress of cells lacking LFA-1 in spite of the expression of Rap1-GTP. To dissect whether this disproportional effect of Rap1-GTP on thymic versus peripheral Treg might be related to thymic output or to the development of peripheral Treg in vivo, we used a model of lymphopenia-induced conversion of naïve T cells into Treg after in vivo transfer in Rag-/- background [36; 37]. This approach allowed us to study development of peripheral Treg in vivo in a manner not affected by thymus-dependent regulation. We determined that development of Treg was impaired in recipients of Foxp3GFP-KI/LFA-1-KO naïve T cells and was only slightly increased when Foxp3GFP-KI/LFA-1-KO/Rap1E63-Tg naïve T cells were used for the adoptive transfer. Taken together these observations indicate that in the thymus Rap1-GTP increases the frequency of Treg via LFA-1 dependent and LFA-1-independent mechanisms, whereas Rap1-GTP increases the frequency of peripheral Treg predominantly in an LFA-1-dependent manner.
Several signaling pathways have been shown to have selective implications on the development of nTreg in the thymus and iTreg in peripheral lymphoid organs. Among them, CD28 costimulation has an essential role in the differentiation of nTreg in the thymus, which is stressed by the marked decrease in frequencies of nTreg cells in CD28-deficient and CD80-CD86 deficient mice [39]. In contrast, CD28 costimulation may suppresses the generation of iTreg [40; 41]. Interestingly, CD28 costimulation enhances Rap1 activity in thymocytes but not in mature, peripheral T cells [42]. Thus, Rap1 might positively regulate Foxp3 expression in thymocytes in a CD28-dependent manner. Other studies have shown that Notch1 has a significant role in Foxp3 expression. Inhibition of Notch1 blocks TGF-b-induced Foxp3 expression, upregulation of Foxp3 target genes and the suppressive function of Treg [43]. A functional crosstalk between active Rap1 and Notch in development and expansion of T cell acute lymphoblastic leukemia (T-ALL) has been reported [44]. In that system, expression of active Rap1 correlated with increased expression of Notch target genes suggesting that Rap1 signaling might induce permissive conditions for Notch signaling [44]. Whether Notch has an active role in the differentiation of thymic Foxp3+ cells downstream of Rap1 remains to be determined. It has been reported that PI3K/Akt/mTOR has a negative effect on Foxp3 expression whereas inhibition of PI3K/Akt/mTOR during T cell stimulation resulted in robust Foxp3 expression [45]. Consistently, sustained Akt activation inhibits TGF-b mediated Foxp3 induction in peripheral CD4+ T cells [46]. Activation of Rap1 in B lymphocytes diminishes Akt activity induced by antigen receptor signaling [47]. If such pathway is also operative in T cells it may be responsible for the increased susceptibility of naïve Rap1-Tg T cells to TGF-b-mediated generation of Treg that we observed in our studies (Fig. S2).
Regardless of the role of LFA-1 and Rap1 in the differentiation of Foxp3+ Treg that was investigated in our study, LFA-1 was mandatory for the Treg suppressive function. We observed that LFA-1 deficient Treg had a dramatically reduced suppression capacity that remained unaffected by the expression of Rap1-GTP (data not shown). These data are consistent with previous reports indicating that LFA-1 is indispensable for Treg-mediated suppression [20; 21]. In conclusion, our studies indicated that Rap1-GTP increases the frequency of natural Treg in the thymus via LFA-1-independent mechanisms but affects the frequency of peripheral Treg predominantly in an LFA-1-dependent manner. Identification of the signaling pathways via which Rap1-GTP contributes to the differentiation of Treg will provide new insights on the function of Rap1 and on the design of targeted approaches for generation of Treg cells for therapeutic applications.
Supplementary Material
Acknowledgments
We thank Dr. Mohamed Oukka for providing the Foxp3GFP-KI reporter mice. This work was supported by NIH grants HL087870 (L.L.) and CA123855, AI43552, CA104596 (V.A.B.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Carman CV, Springer TA. Integrin avidity regulation: are changes in affinity and conformation underemphasized? Curr Opin Cell Biol. 2003;15:547–56. doi: 10.1016/j.ceb.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Reedquist KA, Ross E, Koop EA, Wolthuis RM, Zwartkruis FJ, van Kooyk Y, Salmon M, Buckley CD, Bos JL. The small GTPase, Rap1, mediates CD31-induced integrin adhesion. J Cell Biol. 2000;148:1151–8. doi: 10.1083/jcb.148.6.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Bruyn KM, Rangarajan S, Reedquist KA, Figdor CG, Bos JL. The small GTPase Rap1 is required for Mn(2+)- and antibody-induced LFA- 1- and VLA-4-mediated cell adhesion. J Biol Chem. 2002;277:29468–76. doi: 10.1074/jbc.M204990200. [DOI] [PubMed] [Google Scholar]
- 4.Katagiri K, Hattori M, Minato N, Irie S-K, Takatsu K, Kinashi T. Rap1 is a potent activation signal for leukocyte function-associated antigen 1 distinct from protein kinase C and phosphatidylinositol-3-OH kinase. Mol. Cel. Biol. 2000;20:1956–1969. doi: 10.1128/mcb.20.6.1956-1969.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertoni A, Tadokoro S, Eto K, Pampori N, Parise LV, White GC, Shattil SJ. Relationships between Rap1b, affinity modulation of integrin alpha IIbbeta 3, and the actin cytoskeleton. J Biol Chem. 2002;277:25715–21. doi: 10.1074/jbc.M202791200. [DOI] [PubMed] [Google Scholar]
- 6.de Bruyn KM, Zwartkruis FJ, de Rooij J, Akkerman JW, Bos JL. The small GTPase Rap1 is activated by turbulence and is involved in integrin [alpha]IIb[beta]3-mediated cell adhesion in human megakaryocytes. J Biol Chem. 2003;278:22412–7. doi: 10.1074/jbc.M212036200. [DOI] [PubMed] [Google Scholar]
- 7.Caron E, Self AJ, Hall A. The GTPase Rap1 controls functional activation of macrophage integrin alphaMbeta2 by LPS and other inflammatory mediators. Curr. Biol. 2000;10:974–978. doi: 10.1016/s0960-9822(00)00641-2. [DOI] [PubMed] [Google Scholar]
- 8.Shattil SJ, Kashiwagi H, Pampori N. Integrin signaling: the platelet paradigm. Blood. 1998;91:2645–57. [PubMed] [Google Scholar]
- 9.Franke B, van Triest M, de Bruijn KM, van Willigen G, Nieuwenhuis HK, Negrier C, Akkerman JW, Bos JL. Sequential regulation of the small GTPase Rap1 in human platelets. Mol Cell Biol. 2000;20:779–85. doi: 10.1128/mcb.20.3.779-785.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bachmann MF, McKall-Faienza K, Schmits R, Bouchard D, Beach J, Speiser DE, Mak TW, Ohashi PS. Distinct roles for LFA-1 and CD28 during activation of naive T cells: adhesion versus costimulation. Immunity. 1997;7:549–57. doi: 10.1016/s1074-7613(00)80376-3. [DOI] [PubMed] [Google Scholar]
- 11.Abraham C, Miller J. Molecular mechanisms of IL-2 gene regulation following costimulation through LFA-1. J Immunol. 2001;167:5193–201. doi: 10.4049/jimmunol.167.9.5193. [DOI] [PubMed] [Google Scholar]
- 12.Kandula S, Abraham C. LFA-1 on CD4+ T cells is required for optimal antigen-dependent activation in vivo. J Immunol. 2004;173:4443–51. doi: 10.4049/jimmunol.173.7.4443. [DOI] [PubMed] [Google Scholar]
- 13.Abraham C, Griffith J, Miller J. The dependence for leukocyte function-associated antigen-1/ICAM-1 interactions in T cell activation cannot be overcome by expression of high density TCR ligand. J Immunol. 1999;162:4399–405. [PubMed] [Google Scholar]
- 14.Berlin-Rufenach C, Otto F, Mathies M, Westermann J, Owen MJ, Hamann A, Hogg N. Lymphocyte migration in lymphocyte function-associated antigen (LFA)-1- deficient mice. J Exp Med. 1999;189:1467–78. doi: 10.1084/jem.189.9.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lub M, van Kooyk Y, Figdor CG. Ins and outs of LFA-1. Immunol Today. 1995;16:479–83. doi: 10.1016/0167-5699(95)80031-x. [DOI] [PubMed] [Google Scholar]
- 16.Bunting M, Harris ES, McIntyre TM, Prescott SM, Zimmerman GA. Leukocyte adhesion deficiency syndromes: adhesion and tethering defects involving beta 2 integrins and selectin ligands. Curr Opin Hematol. 2002;9:30–5. doi: 10.1097/00062752-200201000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Uzel G, Kleiner DE, Kuhns DB, Holland SM. Dysfunctional LAD-1 neutrophils and colitis. Gastroenterology. 2001;121:958–64. doi: 10.1053/gast.2001.28022. [DOI] [PubMed] [Google Scholar]
- 18.Scharffetter-Kochanek K, Lu H, Norman K, van Nood N, Munoz F, Grabbe S, McArthur M, Lorenzo I, Kaplan S, Ley K, Smith CW, Montgomery CA, Rich S, Beaudet AL. Spontaneous skin ulceration and defective T cell function in CD18 null mice. J Exp Med. 1998;188:119–31. doi: 10.1084/jem.188.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ding ZM, Babensee JE, Simon SI, Lu H, Perrard JL, Bullard DC, Dai XY, Bromley SK, Dustin ML, Entman ML, Smith CW, Ballantyne CM. Relative contribution of LFA-1 and Mac-1 to neutrophil adhesion and migration. J Immunol. 1999;163:5029–38. [PubMed] [Google Scholar]
- 20.Marski M, Kandula S, Turner JR, Abraham C. CD18 is required for optimal development and function of CD4+CD25+ T regulatory cells. J Immunol. 2005;175:7889–97. doi: 10.4049/jimmunol.175.12.7889. [DOI] [PubMed] [Google Scholar]
- 21.Wohler J, Bullard D, Schoeb T, Barnum S. LFA-1 is critical for regulatory T cell homeostasis and function. Mol Immunol. 2009;46:2424–8. doi: 10.1016/j.molimm.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li L, Greenwald RJ, Lafuente EM, Tzachanis D, Berezovskaya A, Freeman GJ, Sharpe AH, Boussiotis VA. Rap1-GTP is a negative regulator of Th cell function and promotes the generation of CD4+CD103+ regulatory T cells in vivo. J Immunol. 2005;175:3133–9. doi: 10.4049/jimmunol.175.5.3133. [DOI] [PubMed] [Google Scholar]
- 23.Shimizu J, Moriizumi E. Aging-dependent generation of suppressive CD4+CD25-R123loCD103+ T cells in mice. Eur J Immunol. 2003;33:2449–58. doi: 10.1002/eji.200324040. [DOI] [PubMed] [Google Scholar]
- 24.Banz A, Peixoto A, Pontoux C, Cordier C, Rocha B, Papiernik M. A unique subpopulation of CD4+ regulatory T cells controls wasting disease, IL-10 secretion and T cell homeostasis. Eur J Immunol. 2003;33:2419–28. doi: 10.1002/eji.200324205. [DOI] [PubMed] [Google Scholar]
- 25.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 26.Josefowicz SZ, Rudensky A. Control of regulatory T cell lineage commitment and maintenance. Immunity. 2009;30:616–25. doi: 10.1016/j.immuni.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liston A, Nutsch KM, Farr AG, Lund JM, Rasmussen JP, Koni PA, Rudensky AY. Differentiation of regulatory Foxp3+ T cells in the thymic cortex. Proc Natl Acad Sci U S A. 2008;105:11903–8. doi: 10.1073/pnas.0801506105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–41. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 29.Suga K, Katagiri K, Kinashi T, Harazaki M, Iizuka T, Hattori M, Minato N. CD98 induces LFA-1-mediated cell adhesion in lymphoid cells via activation of Rap1. FEBS let. 2001;489:249–253. doi: 10.1016/s0014-5793(00)02222-5. [DOI] [PubMed] [Google Scholar]
- 30.Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, Naji A, Caton AJ. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2:301–6. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 31.Shevach EM, Davidson TS, Huter EN, Dipaolo RA, Andersson J. Role of TGF-Beta in the induction of Foxp3 expression and T regulatory cell function. J Clin Immunol. 2008;28:640–6. doi: 10.1007/s10875-008-9240-1. [DOI] [PubMed] [Google Scholar]
- 32.Marie JC, Letterio JJ, Gavin M, Rudensky AY. TGF-beta1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J Exp Med. 2005;201:1061–7. doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y, Zhang P, Li J, Kulkarni AB, Perruche S, Chen W. A critical function for TGF-beta signaling in the development of natural CD4+CD25+Foxp3+ regulatory T cells. Nat Immunol. 2008;9:632–40. doi: 10.1038/ni.1607. [DOI] [PubMed] [Google Scholar]
- 34.Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF. Cutting edge: TGF-beta induces a regulatory phenotype in CD4+CD25- T cells through Foxp3 induction and down-regulation of Smad7. J Immunol. 2004;172:5149–53. doi: 10.4049/jimmunol.172.9.5149. [DOI] [PubMed] [Google Scholar]
- 35.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–86. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haribhai D, Lin W, Edwards B, Ziegelbauer J, Salzman NH, Carlson MR, Li SH, Simpson PM, Chatila TA, Williams CB. A central role for induced regulatory T cells in tolerance induction in experimental colitis. J Immunol. 2009;182:3461–8. doi: 10.4049/jimmunol.0802535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Curotto de Lafaille MA, Lino AC, Kutchukhidze N, Lafaille JJ. CD25- T cells generate CD25+Foxp3+ regulatory T cells by peripheral expansion. J Immunol. 2004;173:7259–68. doi: 10.4049/jimmunol.173.12.7259. [DOI] [PubMed] [Google Scholar]
- 38.Singer A, Adoro S, Park JH. Lineage fate and intense debate: myths, models and mechanisms of CD4- versus CD8-lineage choice. Nat Rev Immunol. 2008;8:788–801. doi: 10.1038/nri2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–40. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 40.Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp Med. 2007;204:1765–74. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim JM, Rudensky A. The role of the transcription factor Foxp3 in the development of regulatory T cells. Immunol Rev. 2006;212:86–98. doi: 10.1111/j.0105-2896.2006.00426.x. [DOI] [PubMed] [Google Scholar]
- 42.Amsen D, Kruisbeek A, Bos JL, Reedquist K. Activation of the Ras-related GTPase Rap1 by thymocyte TCR engagement and during selection. Eur. J. Immunol. 2000;30:2832–2841. doi: 10.1002/1521-4141(200010)30:10<2832::AID-IMMU2832>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 43.Samon JB, Champhekar A, Minter LM, Telfer JC, Miele L, Fauq A, Das P, Golde TE, Osborne BA. Notch1 and TGFbeta1 cooperatively regulate Foxp3 expression and the maintenance of peripheral regulatory T cells. Blood. 2008;112:1813–21. doi: 10.1182/blood-2008-03-144980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang SF, Aoki M, Nakashima Y, Shinozuka Y, Tanaka H, Taniwaki M, Hattori M, Minato N. Development of Notch-dependent T-cell leukemia by deregulated Rap1 signaling. Blood. 2008;111:2878–86. doi: 10.1182/blood-2007-07-103119. [DOI] [PubMed] [Google Scholar]
- 45.Sauer S, Bruno L, Hertweck A, Finlay D, Leleu M, Spivakov M, Knight ZA, Cobb BS, Cantrell D, O'Connor E, Shokat KM, Fisher AG, Merkenschlager M. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc Natl Acad Sci U S A. 2008;105:7797–802. doi: 10.1073/pnas.0800928105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haxhinasto S, Mathis D, Benoist C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J Exp Med. 2008;205:565–74. doi: 10.1084/jem.20071477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Christian SL, Lee RL, McLeod SJ, Burgess AE, Li AH, Dang-Lawson M, Lin KB, Gold MR. Activation of the Rap GTPases in B lymphocytes modulates B cell antigen receptor-induced activation of Akt but has no effect on MAPK activation. J Biol Chem. 2003;278:41756–67. doi: 10.1074/jbc.M303180200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.