Abstract
A microwave-assisted one-pot, three-step Sonogashira cross coupling-desilylation-cycloaddition sequence was developed for the convenient preparation of 1,4-disubstituted 1,2,3-triazoles starting from a range of halides, acyl chlorides, ethynyltrimethylsilane and azides.
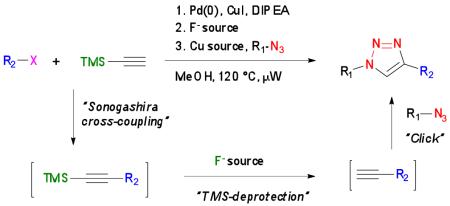
The exquisite regioselectivity, high tolerance to the presence of functional groups and exceptional high yields of Cu(I)-catalyzed 1,3-dipolar cycloadditions of azides with terminal alkynes (CuAAC) to give 1,4-disubstituted triazoles1 have made it a powerful approach for bioconjugation,2 construction and modification of materials3 and the parallel combinatorial synthesis of libraries of compounds for drug discovery.4 Recent efforts to further develop CuAAC have focused on one-pot multi-component reactions in which azides are prepared in-situ prior to the cycloaddition. The attraction of such an approach is that it minimizes time-consuming work-up and purification protocols and avoids handling of potentially explosive azides. Most methods for in-situ preparation of azides take advantage of the nucleophilicity of sodium azide towards electrophiles such as aromatic fluorides, iodides, benzylic bromides as well as epoxides and boronic acids.5 Organic azides have also been generated in-situ by Cu(II)-catalyzed diazo-transfer of triflyl azide or azidotrimethylsilane to amines.6 Surprisingly, one-pot multi-step reactions involving in-situ generation of terminal alkynes for click chemistry have not been reported. The attraction of such an approach is that a wide variety of alkynes can be generated from simple and readily available starting materials, such as aromatic halides.
Herein, we describe a microwave-assisted one-pot three-step procedure entailing Sonogashira cross-coupling of aromatic halides with ethynyltrimethylsilane (TMS-acetylene) to give TMS-protected alkynes, which were desilylated and then employed in 1,3-dipolar cycloadditions with various azides to give a range of 1,2,3-triazoles (Scheme 1). In addition, it was found that the use of aromatic acyl chlorides in the Sonogashira cross-coupling gave TMS-protected ynones, which after desilylation and Cu(I)-catalyzed cycloadditions with azides led to the regioselective formation of 1-substituted 4-phenylacyl-1H-1,2,3-triazoles.
Scheme 1.
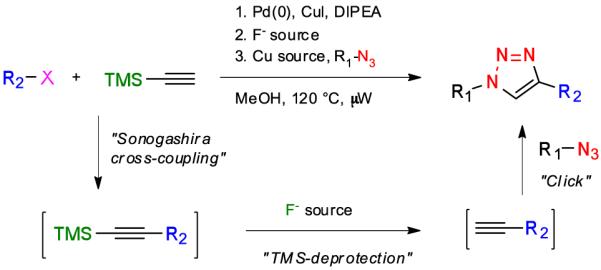
One-pot three-step synthesis of 1,2,3-triazoles by in-situ formation of a terminal alkyne by Sonagashira cross-coupling and desilylation followed by a cycloaddition with an azide.
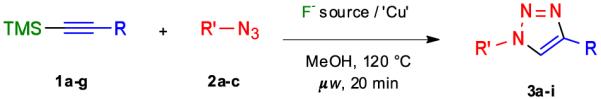
First, optimal reaction conditions were established for a fast and high yielding one-pot desilylation-cycloaddition reaction sequence (Table 1). It has been reported that TMS-modified acetylenes can readily be deprotected using reagents such as tetrabutylammonium fluoride (TBAF)7 or silver salts8 to give terminal alkynes, which can then undergo cycloadditions with azides. It was expected that these two steps can be performed as a one-pot process and accelerated by microwave irradiation.9 A temperature survey revealed that reaction of trimethyl(phenylethynyl)silane (1a) with benzyl azide (2a) in the presence of TBAF (1 equiv), CuI (10 mol %) and N,N-diisopropylethylamine (20 mol %) in methanol under microwave irradiation at 120 °C was complete within 20 min to afford the 1,4-disubstituted triazole 3a in an almost quantitative yield as a single regioisomer (Table 1, entry 1, method A). A similar reaction without TBAF resulted in no triazole formation, clearly establishing that the TMS-alkyne must be deprotected to undergo a 1,3 dipolar-cycloaddition. Furthermore, when the transformation was performed in the absence of CuI, only a trace amount of triazole was isolated as a mixture of regioisomers.10 The desilylation-cycloaddition reaction could also be performed at ambient temperature, however, in this case a prolonged reaction time of 12 h was required to achieve complete conversion of the starting materials.
Table 1.
Microwave-assisted one-pot TMS-deprotection of compounds 1a-g and cycloaddition with azides 2a-c to give triazoles 3a-i
 | ||||
|---|---|---|---|---|
| entry | R | R’ | method Aa yield (%) |
method Bb yield (%) |
| 1 | C6H5 | Bn | 98 | 98c |
| 2 | 4-MeO-C6H4 | Bn | 97 | 96 |
| 3 | 4-CF3-C6H4 | Bn | 98 | 97 |
| 4 | 4-Cl-C6H4 | Bn | 89 | 95 |
| 5 | 4-Br-C6H4 | Bn | 81 | 91 |
| 6 | 2-Br-C6H4 | Bn | 89 | 90 |
| 7 | CH2OH | Bn | 82 | 98 |
| 8 | C6H5 | 4-MeO-Bn | 87 | 98 |
| 9 | C6H5 | 4-NO2-Bn | 98 | 99 |
A mixture of TBAF (1.0 M, 2.0 equiv) and CuI (10 mol %) and DIPEA (20 mol %) was used
CuF2 (2.0 equiv) was used
3a was isolated in yields of 22% and 57% when the reaction was performed at 60 °C or 90 °C, respectively.
We have found that CuF2 is an efficient reagent for deprotecting TMS-modified alkynes and promoting 1,3-dipolar cycloadditions with azides to give 1,4-disubstituted triazoles.11 As expected, the use of CuF2 (2 equiv) also gave the 1,2,3-triazole 3a in an excellent yield (Table 1, entry 1, method B). It was found that the scope of the one-pot process is excellent and 1,4-disubstituted triazoles containing electron donating (entry 2), withdrawing (entry 3) and bulky functionalities (entry 6) could be obtained in excellent yield. Furthermore, the use of CuF2 was found to be most convenient and gave in general slightly higher yields of product compared to the use of TBAF/CuI. It is also possible to employ a catalytic amount of CuF2 (10 mol %) however in this case the addition of TBAF (1 equiv) is required to facilitate desilylation.
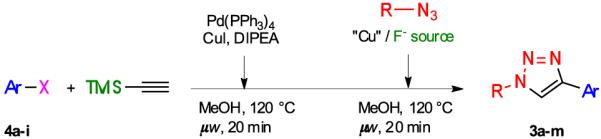
TMS-substituted alkynes are readily accessible by Sonogashira cross-coupling between aromatic halides and ethynyltrimethylsilane.12 Furthermore, it has been found that microwave irradiation can significantly reduce the reaction time of metal-catalyzed cross-coupling reactions.13 Thus, we explored whether Sonogashira cross-coupling, desilylation and cycloaddition can be performed under microwave conditions as a one-pot procedure. Various aromatic iodides (Table 2, entries 1-6 and 10-11) were reacted with ethynyltrimethylsilane using a catalytic amount of Pd(PPh3)4 (5 mol %) and CuI (10 mol %) in the presence of N,N-diisopropylethylamine (2 equiv) in methanol under microwave irradiation at 120 °C for 20 min, followed by the introduction of an azide and TBAF/CuI or CuF2 and further microwave irradiation at 120 °C for 20 min. Gratifyingly, the one-pot three-step process produced the expected 1,4-disubstituted triazoles (3) in most cases in high yield. As expected, aromatic bromides (entries 7-9), which are known to couple poorly under classical Sonogashira cross-coupling conditions, required optimization of the palladium source, choice of amine and reaction time. It was found that triazoles were obtained in acceptable yields when a mixture of an aromatic bromide, ethynyltrimethylsilane, Pd(PPh3)2Cl2 (5 mol %) and CuI (10 mol %) in Et3N was heated under reflux prior to the desilylation-cycloaddition protocol (entries 8 sand 9).
Table 2.
One-pot, three-step synthesis of 1,4-disubstituted triazoles 3
 | ||||
|---|---|---|---|---|
| entry | Ar-X | R | method Aa yield (%) |
method Bb yield (%) |
| 1 | Ph-I | Bn | 96 | 91 |
| 2 | 4-MeO-C6H4-I | Bn | 41 | 54 |
| 3 | 4-CF3-C6H4-I | Bn | 97 | 98 |
| 4 | 4-F-C6H4-I | Bn | 98 | 98 |
| 5 | 4-Me-C6H4-I | Bn | 93 | 95 |
| 6 | 2-Me-C6H4-I | Bn | 97 | 98 |
| 7 | Ph-Br | Bn | 42 | 65 |
| 8 | 4-MeO-C6H4-Br | Bn | 13 | 15(57c) |
| 9 | 4-NO2-C6H4-Br | Bn | 12 | 11(53c) |
| 10 | Ph-I | 4-MeO-Bn\ | 84 | 91 |
| 11 | Ph-I | 4-NO2-Bn | 97 | 98 |
A mixture of TBAF (1.0 M, 2.0 equiv) and CuI (10 mol %) was used
CuF2 (2.0 equiv) was used
The Sonogashira cross coupling was performed using Pd(PPh3)2Cl2 (5 mol %), CuI (10 mol %) in refluxing Et3N for 10 h.
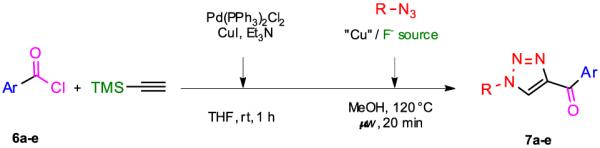
The scope of the one-pot three-step protocol was further extended to the synthesis of 1-substituted 4-acyl-1H-1,2,3-triazoles by using acyl chlorides as the starting material in the Sonogashira cross-coupling reaction (7, Table 3).12b,14 Acyl chlorides did not tolerate the use of microwave irradiation,15 however, the desired TMS-protected ynones could be obtained by simply reacting an acyl chloride (6) with ethynyltrimethylsilane in the presence of Pd(PPh3)2Cl2 and triethylamine in THF at room temperature for 1 h. The resulting compounds were not isolated but immediately submitted to the deprotection-cycloaddition protocol and reaction with benzyl azide in the presence of CuF2 or the TBAF/CuI afforded 1-benzyl,4-acyl-1H-1,2,3-triazoles (7). The Sonogashira cross-coupling reaction was found to be sensitive to the nature of the employed acyl chloride and the use of electron-rich acyl chlorides (entries 2 and 3 vs entry 4) gave the best conversions of the starting materials. Full conversions of the 1-substituted 4-acyl-1H-1,2,3-triazoles were achieved when TMS-modified ynones were purified prior to reaction with azides, establishing the Sonogashira coupling of the acyl chlorides with ethynyltrimethylsilane as the yield-limiting step of the one-pot, three-step protocol.
Table 3.
One-pot, three-step synthesis of 1-substituted 4-acyl-1H-1,2,3-triazoles 7a-e
 | ||||
|---|---|---|---|---|
| entry | Ar | R | method Aa yield (%) |
method Bb yield (%) |
| 1 | Ph | Bn | 40 | 42 |
| 2 | 4-MeO-C6H4 | Bn | 80 | 83 |
| 3 | 4-F-C6H4 | Bn | 82 | 88 |
| 4 | 4-NO2-C6H4 | Bn | 32 | 36 |
| 5 | 2-Me-C6H4 | Bn | 35 | 34 |
A mixture of TBAF (1.0 M, 2.0 equiv) and CuI (10 mol %) was used
CuF2 (2.0 equiv) was used.
It is important to note that 1-substituted 4-acyl-1H-1,2,3-triazoles have rarely been prepared by CuAAC due to side product formation.16 Previous syntheses of this class of triazoles involved classical thermal Huisgen 1,3-dipolar cycloadditions with the inconvenience of separating the two triazole regioisomers17a and condensation of amines with α-diazo-1,3-dicarbonyls.16b The microwave conditions reported here provide easy and regioselective access to this class of biomedically important compounds.
In conclusion, an expedient one-pot, three-step protocol has been developed for the preparation of 1,4-disubstituted triazoles involving Sonogashira cross-coupling to generate in-situ a variety of TMS-protected alkynes, which could immediately be desilylated and reacted with azides in the presence of a mixture of TBAF/CuI or CuF2. The reaction sequence could be completed within 1 h by employing microwave-assisted heating. Furthermore, we have shown that 2-yn-1-ones, which were obtained by Sonogashira cross-coupling of aromatic acyl chlorides with ethynyltrimethylsilane, undergo Cu-catalyzed cycloadditions with azides when subjected to microwave heating to give regioselective formation of 1-substituted 4-phenylacyl-1H-1,2,3-triazoles. The new one-pot three-step process will make it possible to rapidly prepare compound libraries for drug discovery programs as it avoids time consuming and costly purification protocols of synthetic intermediates. Furthermore, it is to be expected that the one-pot procedure can be combined with in-situ formation of azides5 thereby further increasing the convenience of CuAAC.
Supplementary Material
Acknowledgment
This research was supported by the National Cancer Institute of the US National Institutes of Health (Grant No. R01 CA88986, G.-J.B.).
Footnotes
Supporting Information Available: Experimental procedures, characterization data, and copies of 1H NMR and 13C NMR spectra of all products are available. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.For the original publications, see: Tornøe CW, Christensen C, Meldal M. J. Org. Chem. 2002;67:3057. doi: 10.1021/jo011148j. Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. Angew. Chem. Int. Ed. 2002;41:2596. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4.
- 2.a) Pieters RJ, Rijkers DTS, Liskamp RM. J. QSAR Comb. Sci. 2007;26:1181. [Google Scholar]; b) Nájera C, Sansano JM. Org. Biomol. Chem. 2009;7:4567. doi: 10.1039/b913066g. [DOI] [PubMed] [Google Scholar]; c) van Dijk M, Rijkers DTS, Liskamp RMJ, van Nostrum CF, Hennink WE. Bioconjugate Chem. 2009;20:2001. doi: 10.1021/bc900087a. [DOI] [PubMed] [Google Scholar]
- 3.a) Lutz J-F. Angew. Chem. Int. Ed. 2007;46:1018. doi: 10.1002/anie.200604050. [DOI] [PubMed] [Google Scholar]; b) Golas PL, Matyjaszewski K. Chem. Soc. Rev. 2010;39:1338. doi: 10.1039/b901978m. [DOI] [PubMed] [Google Scholar]
- 4.a) Moorhouse AD, Moses JE. Chem. Med. Chem. 2008;3:715. doi: 10.1002/cmdc.200700334. [DOI] [PubMed] [Google Scholar]; b) Tron GC, Pirali T, Billington RA, Canonico PL, Sorba G, Genazzani AA. Med. Res. Rev. 2008;28:278. doi: 10.1002/med.20107. [DOI] [PubMed] [Google Scholar]; c) Mamidyala SK, Finn MG. Chem. Soc. Rev. 2010;39:1252. doi: 10.1039/b901969n. [DOI] [PubMed] [Google Scholar]
- 5.a) Dururgkar KA, Gonnade RG, Ramana CV. Tetrahedron. 2009;65:3974. [Google Scholar]; b) Feldman AK, Colasson B, Fokin VV. Org. Lett. 2004;6:3897. doi: 10.1021/ol048859z. [DOI] [PubMed] [Google Scholar]; c) Ackermann L, Potukuchi HK, Landsberg D, Vicente R. Org. Lett. 2008;10:3081. doi: 10.1021/ol801078r. [DOI] [PubMed] [Google Scholar]; d) Appukkuttan P, Dehaen W, Fokin VV, Van der Eycken E. Org. Lett. 2004;6:4223. doi: 10.1021/ol048341v. [DOI] [PubMed] [Google Scholar]; e) Yadav JS, Reddy BVS, Reddy M, Chary DN. Tetrahedron Lett. 2007;48:8773. [Google Scholar]; f) Campbell-Verduyn LS, Szymanski W, Postema CP, Dierckx RA, Elsinga PH, Janssen DB, Feringa BL. Chem. Commun. 2010;46:898. doi: 10.1039/b919434g. [DOI] [PubMed] [Google Scholar]; g) Tao C-Z, Cui X, Li J, Liu A-X, Liu L, Guo Q-X. Tetrahedron Lett. 2007;48:3525. [Google Scholar]
- 6.a) Beckmann HSG, Wittmann V. Org. Lett. 2007;9:1. doi: 10.1021/ol0621506. [DOI] [PubMed] [Google Scholar]; b) Barral K, Moorhouse AD, Moses JE. Org. Lett. 2007;9:1809. doi: 10.1021/ol070527h. [DOI] [PubMed] [Google Scholar]; c) Lee C-T, Huang S, Lipshutz BH. Adv. Synth. Catal. 2009;351:3139. doi: 10.1002/adsc.200900323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valverde IE, Delmas AF, Aucagne V. Tetrahedron. 2009;65:7597. [Google Scholar]
- 8.Aucagne V, Leigh DA. Org. Lett. 2006;8:4505. doi: 10.1021/ol061657d. [DOI] [PubMed] [Google Scholar]
- 9.a) Kappe CO, Van der Eycken E. Chem. Soc. Rev. 2010;39:1280. doi: 10.1039/b901973c. [DOI] [PubMed] [Google Scholar]; b) Appukuttan P, Mehta V, Van der Eycken E. Chem. Soc. Rev. 2010;39:1467. doi: 10.1039/b815717k. [DOI] [PubMed] [Google Scholar]
- 10.Heating under microwave irradiation trimethyl(phenylethynyl)silane (1a) with benzyl azide (2a) in the presence of TBAF (1 equiv) at 120 °C for 20 min resulted in less than 5% yield of the corresponding triazole in a 1:1 mixture of regioisomers.
- 11.Mechanistic studies and DFT calculations support the notion that CuF2 catalyzes the cycloaddtion. Friscourt F, Ledin PA, Boons G-J. Abstracts of Papers, 239th ACS National Meeting; San Francisco, CA, USA. March 21-25; 2010. manuscript in preparation.
- 12.a) Sonogashira K, Tohda Y, Hagihara N. Tetrahedron Lett. 1975;16:4467. [Google Scholar]; b) Tohda Y, Sonogashira K, Hagihara N. Synthesis. 1977:777. [Google Scholar]
- 13.a) Kappe CO. Angew. Chem. Int. Ed. 2004;43:6250. doi: 10.1002/anie.200400655. [DOI] [PubMed] [Google Scholar]; b) Nilsson P, Olofsson K, Larhed M. Top Curr. Chem. 2006;266:103. [Google Scholar]
- 14.Karpov AS, Muller TJJ. Org. Lett. 2003;5:3451. doi: 10.1021/ol035212q. [DOI] [PubMed] [Google Scholar]
- 15.Sashida H. Synthesis. 1998:745. [Google Scholar]
- 16.Synthesis of 1-substituted 4-acyl-1H-1,2,3-triazoles using CuAAC exhibits moderate yield (< 60%), see: Chassaing S, Kumarraja M, Sido ASS, Pale P, Sommer J. Org. Lett. 2007;9:883. doi: 10.1021/ol0631152. Xie J, Seto CT. Bioorg. Med. Chem. 2007;15:458. doi: 10.1016/j.bmc.2006.09.036. Pardin C, Roy I, Lubell WD, Keillor JW. Chem. Biol. Drug Des. 2008;72:189. doi: 10.1111/j.1747-0285.2008.00696.x.
- 17.a) Calderone V, Giorgi I, Livi O, Martinotti E, Mantuano E, Martelli A, Nardi A. Eur. J. Med. Chem. 2005;40:521. doi: 10.1016/j.ejmech.2005.01.010. [DOI] [PubMed] [Google Scholar]; b) Dabak K, Sezer O, Akar A, Anaç O. Eur. J. Med. Chem. 2003;38:215. doi: 10.1016/s0223-5234(02)01445-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.