Abstract
Specific glycosphingolipids (GSL), found on the surface of target immune cells, are recognized as alternate cell surface receptors by the human immunodeficiency virus type 1 (HIV-1) external envelope glycoprotein. In this study, the globotriose and 3’-sialyllactose carbohydrate head groups found on two GSL were covalently attached to a dendrimer core to produce two types of unique multivalent carbohydrates (MVC). These MVC inhibited HIV-1 infection of T cell lines and primary peripheral blood mononuclear cells (PBMC) by T cell line-adapted viruses or primary isolates, with IC50s ranging from 0.1 – 7.4 µg/ml. Inhibition of Env-mediated membrane fusion by MVC was also observed using a dye-transfer assay. These carbohydrate compounds warrant further investigation as a potential new class of HIV-1 entry inhibitors. The data presented also shed light on the role of carbohydrate moieties in HIV-1 virus-host cell interactions.
Keywords: human immunodeficiency virus-1, multivalent carbohydrates, 3’ sialyllactose, globotriose, peripheral blood mononuclear cells, T cell lines
Introduction
Inhibition of HIV-1 entry into its target cells in vitro is a valuable starting point for both drug and vaccine discovery. The identification of the HIV-1 entry receptor and co-receptors, and derivation of the crystal structures of various viral envelope (Env) glycoproteins, have paved the way for the rational development of viral entry inhibitors, and for design of improved candidate vaccines (Berger et al., 1998; Feng et al., 1996; Koff, 2010; Kwong et al., 1998; Sattentau et al., 1993). Productive HIV-1 infection proceeds primarily by CD4 engagement by the Env glycoprotein gp120, followed by engagement of co-receptor (principally CCR5 or CXCR4), and Env gp41-induced membrane fusion. The fusion of the viral and target cell membranes is followed by entry of the viral core into the cytoplasm of the infected cell (Borkow and Lapidot, 2005; Hartley et al., 2005; Haynes and Montefiori, 2006). However, this process is highly complex and viral entry kinetics may be dependent upon several parameters inherent in the in vitro assay chosen.
The Env proteins interact not only with receptor and co-receptor, but also with other cell surface molecules, including complex lipids that may be utilized for efficient viral attachment and/or entry (Haynes and Montefiori, 2006). For example, galactosylceramide (GalCer) a differentiation marker for oligodendrocytes (Gard and Pfeiffer, 1989), is also found in the vaginal and rectal epithelia, major sites of initial HIV-1 entry in vivo (Bomsel, 1997; Bomsel and Alfsen, 2003; Bomsel et al., 1998; Fantini et al., 2000). Antibodies directed against GalCer are able to inhibit viral infection of CD4− cells (Harouse et al., 1991; Magerus-Chatinet et al., 2007). Peptides from the V3 loop of gp120 inhibit HIV-1 infection of CD4− cells by adhering to GalCer, and inhibit infection of CD4+ cells by adhering to the more complex GSL globotriosyl ceramide (Gb3), or the monosialoganglioside, hematoside (GM3) (Fantini et al., 1993; Hammache et al., 1998a; Hammache et al., 1999; Hammache et al., 1998b; Harrison et al., 2010; Nehete et al., 2002). Gb3 and GM3 are major glycosphingolipid constituents of B- (Mangeney et al, 1991) and T-cell membranes (Degroote, et al., 2004; Delezay et al., 1996; Sorice et al., 2004) respectively. Of direct relevance to HIV-1 infection models, mitogen activation of primary PBMC results in increased expression of GSL, similar to the increased levels of GSL found on PBMC from HIV-1 positive (but not HIV-1 negative) individuals (Fantini et al., 1998b; Lund et al., 2006). Interestingly, levels of Gb3 expression on the surface of PBMC have been shown to correlate inversely with susceptibility to infection by HIV-1 (Lund et al., 2009). Taken together, these studies indicate that the interaction of HIV-1 with complex GSL involves certain surface determinants that are host cell-specific.
The observation that aggregated carbohydrate moieties of GSL are clustered in cell surface lipid rafts or microdomains (Simons and Ikonen, 1997) and that gp120 binds to glycosphingolipid carbohydrate head groups (Kensinger et al., 2004a) suggests that multivalent carbohydrates (MVC) synthesized using the carbohydrate portions of Gb3 and GM3 might inhibit the interaction between HIV and the cell surface and impede viral entry. MVC have been previously used as molecular mimics for inhibition of a number of host-pathogen and host-toxin interactions (Schengrund, 2003), but relatively few studies have been performed using HIV-1 (Kensinger et al., 2004a; Lund et al., 2006).
Reported here is the synthesis of novel MVC derivatized with the carbohydrate head groups of either Gb3 or GM3 covalenty attached to a cationic dendrimer core, and evaluation of their effects on HIV-1 infection of transformed T cells, primary PBMC, and the epithelial HeLa cell line-derived TZM-bl reporter cells. Potent inhibition of HIV-1 infection of PBMC was observed using MVC derivatized with either globotriose or 3’-sialyllactose. The MVC also inhibited the membrane fusion between CD4+ T cells and cells expressing the HIV-1 envelope protein. The inhibition of HIV-1 primary isolates by novel MVC suggests that these compounds should be further evaluated as a potential new class of HIV-1 entry inhibitors.
Materials and Methods
Preparation and Characterization of the Multivalent Carbohydrates (MVC)
Carbohydrates used, globotriose [Gal(α1–4)Gal(β1–4)Glc] and 3’-sialyllactose [NeuNAc(α2–3)Gal(β1–4)Glc], were obtained by material transfer agreements from Kyowa Hakko Kogyo Co., Ltd. (Tokyo, Japan) and NEOSE Technologies, Inc. (Horsham, PA), respectively. Globotriose was linked to the amino termini of different sizes (generations) of polypropylenimine dendrimer cores (Sigma-Aldrich, St. Louis, MO) via a thiopropionic acid spacer arm linked to the C(1) position of the reducing sugar (Kensinger et al., 2004b). Briefly, globotriose was peracetylated with anhydrous pyridine and acetic anhydride as described previously (Wolfrom, 1963). The thiopropionic acid globotriose derivative was prepared from peracetylated globotriose by incubation with a five-fold molar excess of 3-mercaptopropionic acid (Sigma-Aldrich, St. Louis, MO) and borontrifluoride diethyletherate (Sigma-Aldrich) in anyhdrous dichloromethane (Elofsson, et al., 1991; Magnusson, 1981). High performance thin layer chromatography (HPTLC) (VWR Scientific Products, Westchester, PA) was used to monitor completeness of the reaction and purity of the isolated products (solvent - butanol: methanol: water, 2:1:1, v:v:v). The product was characterized by NMR after deacetylation using methanolic sodium methoxide (Zemplen, 1926).
Peracetylated 3-(β-D-globotrihexosylthio)propionic acid was coupled to a polypropylenimine dendrimer core using O-(7-Azabenzotriazol-1-yl)-N,N,N’,N’,-tetramethyluronium hexafluorophosphate (HATU) and diisopropylethylamine to activate the carboxyl group (Poirot et al., 2001). In brief, an amount of derivatized dendrimer equal to 1.5 equivalents per terminal amine on the dendrimer core was resuspended in acetonitrile to which one equivalent each of HATU (Sigma-Aldrich) and diisopropylethylamine (Sigma-Aldrich) were added. Dendrimer core, dried from dichloromethane under nitrogen, was added dropwise while stirring. After adjusting the solvent to a 1:1 (v:v) ratio of dichloromethane:acetonitrile, the reaction was stirred overnight at room temperature. Product formation was monitored by the presence of derivatized dendrimer at the origin after HPTLC (solvent - butanol:methanol:water, 2:1:1, v:v:v) (data not shown). Carbohydrate containing compounds were visualized using 5% sulfuric acid in ethanol. When the reaction was complete, the sample was dried and deacetylated (Zemplen, 1926). Purified derivatized dendrimer was obtained by chromatography on BioGel P2 (Biorad, Hercules, CA) using 1 M pyridine acetate (pH 5.5) as eluant.
The presence of the carboxyl group on the sialosyl residue on 3’-sialyllactose precluded use of the same method to link it to the primary amines on the dendrimer. Therefore, reductive amination was used as the coupling method (Gray, 1978). Two mole equivalents of 3’-sialyllactose per amino terminal on the dendrimer core were resuspended in 0.2 M borate buffer, pH 10.5, prior to the addition of one mole equivalent of dendrimer amines. Sodium cyanoborohydride was then added with stirring and the reaction incubated at 37°C for up to 120 hrs. Product was purified by chromatography on a BioGel P2 column as described above. Fractions containing carbohydrate-derivatized dendrimer were pooled, taken up in a small amount of water and then centrifuged through a 3 kDa molecular weight cut-off filter to remove residual low molecular weight materials (including unreacted 3’-sialyllactose). Matrix Assisted Laser Desorption Ionization Time-of-Flight Mass Spectrometry (MALDI-TOF MS) was used to characterize the carbohydrate-derivatized dendrimers. Analyses were done using a Perspective Biosystems Voyager DE-PRO spectrometer (Perspective Biosystems, Foster City, CA). 100–200 sample spectra were generated in a linear positive ion mode using a trans-3-indoacrylic acid (IAA) (Acros Organic, Pittsburgh, PA) matrix (20mg/ml in dimethylformamide) that was diluted 10:1 by the addition of 1–10mM aqueous solution of sample. This gave a working ratio of IAA to analyte of about 1000:1 (Kensinger et al., 2004b; Woller and Cloninger, 2001). Average molecular weights and polydispersities of the dendrimers were calculated using DataExplorer software, version 4.0 (Applied Biosystems, Foster City, CA). The average number of carbohydrate units conjugated to a dendrimer core was calculated by subtracting the theoretical MW of the native, underivatized dendrimer core from the MW of the corresponding derivatized dendrimer and dividing the difference by the weight of the attached sugar moiety minus 18 Da for the water molecule displaced during formation of multivalent globotriose and 16 Da during addition of sialyllactose using reductive amination. NMR spectra for the thiopropionic acid derivative of globotriose [3-(β-D-globotrihexosylthio)propionic acid] were obtained using a Bruker DRX-400 NMR operating in the quadrature mode at 25°C using a triple-axis-gradient broadband inverse probe as previously described (Kensinger et al., 2004b).
Titration of primary HIV-1 isolates
Donor PBMC were obtained by leukophoresis of blood from HIV-1-negative donors (BRT Laboratories, Inc, Baltimore, MD); PBMC were isolated by Ficoll-Hypaque density gradient centrifugation and cryopreserved. All incubations were in a 95% humidified-5% CO2 environment. Viral titrations were set up by serially diluting samples for all cell line assays with cRPMI [RPMI 1640 (Quality Biologics, Gaithersburg, MD) containing 100 U/ml penicillin (Quality Biologics), 100 µg/ml streptomycin (Quality Biologics), 2mM L-glutamine (Quality Biologics), 15% fetal calf serum (FCS) (Gemini-Bio, Woodland, CA)] or for PBMC with cRPMI/IL-2 medium [cRPMI supplemented with 20 U/ml of recombinant IL-2]. Titrations were performed in quadruplicate wells in 96-well microtiter plates, using an equal amount of medium or normal human serum (NHS) (Gemini-Bio, Woodland, CA) without inhibitors. Virus was incubated for 30 min in medium at 37°C and then 50 µl aliquots containing 1.5 × 105 PHA (DIFCO, Detroit, MI/Roche, Indianapolis, IN)-activated PBMC or 1 × 105 T cells were added to each well for infection overnight at 37°C. Plates were washed three times with media to remove excess virus and cells were transferred to a round-bottom, 96-well plate. On days 4, 6, and 8 post-infection, 100 µl of medium were harvested and an equal volume of new medium was added back. On days 4, 6, and 8, p24 was quantified using an antigen capture assay (Beckman Coulter, Miami, FL); sample wells with a p24 concentration > 60 pg/ml were scored positive. An endpoint virus titer, or 50% tissue culture infectious dose (TCID50), was calculated for day 8 by the Spearman-Karber method (DAIDS, 1997). The nearest dilution of virus that yielded >10 ng/ml of p24 in control wells by day 4 or day 6 was used in inhibition assays.
HIV-1 Inhibition Assay Using T Cell Lines or PBMC
These assays were performed in triplicate for each virus/inhibitor combination, including 8 control wells of virus and cells only, in 96-well deep-bottom (0.5 ml) plates. A dilution of virus stock yielding >10 ng/ml of p24 at day 4–6 in previous titrations (on the relevant cell type) was used. After 30 min pre-incubation of virus (25 µl) and test reagent (25 µl) at 37°C, a 50 µl aliquot containing either 1.5 × 105 PHA/IL-2 PBMC or 1 × 105 H9 or 1 × 105 A3/R5 [T cell line engineered to over express CCR5 (R McLinden, unpublished results)] was added to each well. Cells were infected for 18–20 hours and then washed twice. After a third wash, cells were resuspended in 250 µl of appropriate growth medium and transferred to 96-well U-bottom plates. On days 4, 6 and 8, 100 µl of supernatant was harvested for p24 analysis, and replaced with 100 µl of growth medium. When p24 levels in the virus controls were above 10 ng/ml, pooled triplicate supernatants were tested for p24. The percent inhibition was calculated by the percent reduction of p24 production at day 4 or 6, depending on viral growth kinetics. Infection of H9 cells was tested using three H9-adapted viruses: 92UG_029/H9 (clade A, X4), 93RW_024/H9 (clade A, dual tropic), and MN (clade B). Infection of A3/R5 cells was assayed with A3R5-adapted A08483M1/A3R5 (clade D, R5) and BaL/A3R5 (clade B, R5) viruses (Table 2), and PBMC were tested using a panel of primary isolates (Table 3). Dextran sulfate was used as a control for viral fusion and a neutralizing HIV+ pooled serum collected from HIV+ subtype B patients under informed consent through an IRB approved protocol was used as an assay control for viral inhibition. Percent inhibition was plotted vs. concentration of MVC, and IC50s were calculated using a quadratic projection based program (Mascola, 1999).
Table 2.
MVC effects on HIV-1 infection using cell line models.
| Target Cell | Viral Strain | Clade | Coreceptor | IC50a GBT | IC50 3SL |
|---|---|---|---|---|---|
| H9 | 92UG_029 | A | X4 | 0.24 | 0.23 |
| H9 | 93RW_024 | A | R5/X4 | 0.19 | 0.28 |
| H9 | 84US_MN | B | X4 | 3.6 | 0.92 |
| A3/R5 | 85US_BaL | B | R5 | 0.1 | 15.6 |
| A3/R5 | 99UG_A08483M1 | D | R5 | 10.4 | 3.1 |
| TZM-bl | 92UG_029.ec1 | A | X4 | >100 | >100 |
| TZM-bl | 93RW_024.ec5 | A | R5/X4 | >100 | >100 |
| TZM-bl | 99KE_KNH1135.ec3 | A | R5 | >100 | >100 |
| TZM-bl | 84US_MN.ec1 | B | X4 | >100 | >100 |
| TZM-bl | 92FR_BX08.ec5 | B | R5 | >100 | >100 |
| TZM-bl | 91US_US-1.ec6 | B | R5 | >100 | >100 |
| TZM-bl | 01TZ_911.vrca | C | R5 | >100 | >100 |
| TZM-bl | 02ET_288.vrc38a | C | R5 | >100 | >100 |
| TZM-bl | 98UG_57128.vrc15 | D | R5 | >100 | >100 |
| TZM-bl | 93UG_065.ec3 | D | X4 | >100 | >100 |
50% inhibitory concentrations (IC50s) presented in µg/ml are the means of at least two independent assays performed in triplicate (H9 or A3/R5 T cells) or in duplicate (TZM-bl cells).
Table 3.
MVC inhibit infection of PBMC by HIV-1 primary isolates from multiple clades.
| Viral Strain | Clade | Coreceptor | IC50a GBT | IC50 3SL |
|---|---|---|---|---|
| 92UG_029 | A | X4 | 3.8 | 1.3 |
| 00KE_KER2008 | A | R5/X4 | 5.6 | 2.0 |
| 85US_BaL | B | R5 | 2.4 | 2.9 |
| 92FR_BX08 | B | R5 | 1.7 | 1.5 |
| 91US_US-1. | B | R5 | 7.4 | 3.6 |
| 98US_MSC5016 | C | R5 | 2.8 | 1.6 |
| 02ET_14 | C | R5 | 2.8 | 2.1 |
| 99UG_A08483M1 | D | R5 | 0.2 | 0.5 |
| 00UG_D26830M4 | D | R5 | 3.1 | 1.9 |
| 90TH_CM244 | CRF01_AE | R5 | 2.9 | 1.6 |
| 96TH_NI1046 | CRF01_AE | R5 | 5.3 | 2.3 |
IC50s (means of two independent experiments performed in triplicate) are presented (in µg/ml) for a panel of eleven primary isolates representing five of the major clades of the HIV pandemic.
Cell Viability Assay
Effect of MVC on cell viability was assessed by monitoring activity of mitochondrial deyhydrogenases as described in the ATCC cell viability protocol (http://www.atcc.org/common/documents/pdf/30-1010k.pdf). In brief, cells, seeded in deep-well or standard 96-well plates, respectively, were grown in medium alone (control wells), or in the presence of serially diluted MVC, and incubated at 37°C. After incubation for 24 hrs, medium was removed and the cells washed with PBS, (and in the case of PBMC, transferred to a clear bottom 96-well standard plate) and then co-incubated with MTT reagent in the dark for 30 min; the A490 was read on an ELISA plate reader. The percent viable cells was calculated by:
Pseudovirus Preparation and Titration
Pseudoviruses were prepared by transfecting 5 × 106 exponentially dividing 293T cells in 20 ml growth medium (DMEM) in a T-75 culture flask with 8 µg of env expression plasmid and 24 µg of an env-deficient HIV-1 backbone vector (pSG3ΔEnv) using FuGene as the transfection reagent (Roche, Indianapolis, IN). Pseudovirus-containing culture supernatants were harvested 3 days after transfection, centrifuged and stored at −80°C in 1 ml aliquots. TCID50 measurements were done in triplicate using 8 serial four-fold dilutions of each pseudovirus. For each dilution, 100 µl were placed in a 96-well culture plate and trypsinized TZM-bl cells (10,000 cells in 100 µl of growth medium) were added to each well. Plates were incubated at 37°C in a 5% CO2 incubator with 95% humidity for 48 hrs. To measure luciferase activity, the culture medium was removed, the monolayers were washed once with PBS, and a 20µl aliqout of lysis buffer (Promega Corp, Madison, WI) was added to the cells. Plates were subjected to two freeze/thaw cycles to lyse cells, and 100 µl of reconstituted Luciferase Assay Substrate (Promega Corp) was then added to each well and luminescence was measured using a Victor 2 luminometer (Perkin-Elmer Life Sciences, Shelton, CT). Wells producing relative light units (RLU) >2.5× background wells (containing cells only) were scored positive for infection. An endpoint virus titer, or TCID50 was calculated by the Spearman-Karber method (DAIDS, 1997). Ten pseudoviruses presented in this study were prepared with env clones from a panel of primary viruses (Brown et al., 2005). The pseudoviruses represent four of the major clades of the HIV pandemic and are coded by year of isolation, country of origin and strain identification as follows: clade A: 99KE_KNH1135.ec3, 92UG_029.ec1, 93RW_024.ec5; clade B: 84US_MNp.ec1, 92FR_BXO8.ec5, 91US_1.ec6; clade C: 02ET_288.vrc38a, 01TZ_911.vrca; clade D: 98UG_57128.vrc15, 93UG_065.ec3.
Reporter Cell Line Infectivity Assay
Each pseudovirus was incubated with various dilutions of 0.22 µm filter sterilized MVC in duplicate for 1 h at 37°C in a total volume of 50 µl DMEM in 96-well flat-bottom culture plates (Corning-Costar, Corning, NY). Pseudovirus infection assays were conducted in the absence of DEAE-dextran to not avoid any interference with virus-dendrimer interactions. Trypsinized TZM-bl cells (104) were then added in 50 µl of growth medium. Six wells contained cells and virus (virus control), and another six wells contained cells only (background control). After 48 hrs, luciferase activity was quantified, as described above. The IC50 was calculated as the concentration of sample that reduced RLU by 50% compared to that obtained in the virus-only control, after subtraction of background RLU.
HIV Env-mediated Fusion Assay
Fusion was measured by quantification of syncytium formation between PHA-activated, CD8 T cell-depleted PBMC and TF228 B cells, as described previously (Puri et al., 1999). The target cells, PHA-stimulated PBMC(CD8-), were labeled with CMFDA, a green fluorescent marker (Ex/Em 492/517, Molecular Probes, Junction City, OR). The effector cells expressing HIV env TF228, were labeled with CMRA, an orange fluorescent marker (Ex/Em 548/576, Molecular Probes). Both target and effector cells were labeled in serum-free media containing probe at a concentration of 1 µg/ml; cells were incubated at a density of 106 cells/ml for 1hr at 37°C. Labeling media was then removed and cells washed twice with PBS, suspended in IL-2 containing RPMI-10 media, and further incubated at 37°C for 30 minutes. Cells were then centrifuged and resuspended in cRPMI/IL-2 medium; PBMC were at 6×106 cell/ml and TF228 cells were at 2×106 cells/ml. To score fusion, these cells were co-cultured (50 µl of each cell type) in a flat bottom 96-well plate at a 3:1 ratio (PBMC to TF228 cells). Cells were allowed to undergo fusion for 4 to 6 hours in the presence of MVC, medium only (negative control), or dextran sulfate (positive control). Five replicate images were captured for each well on a Nikon fluorescent microscope using a 20× objective lens. Fusion events were scored as syncytia that were positive for both CMFDA and CMRA markers. Percent fusion was calculated by dividing the number of double-positive cells by the total number of target cells (positive for green dye only). Results are presented as the mean of all five images ± standard deviation. Fusion was normalized to 100% of control, media only wells.
Results
Synthesis, Structure and Characterization of MVC
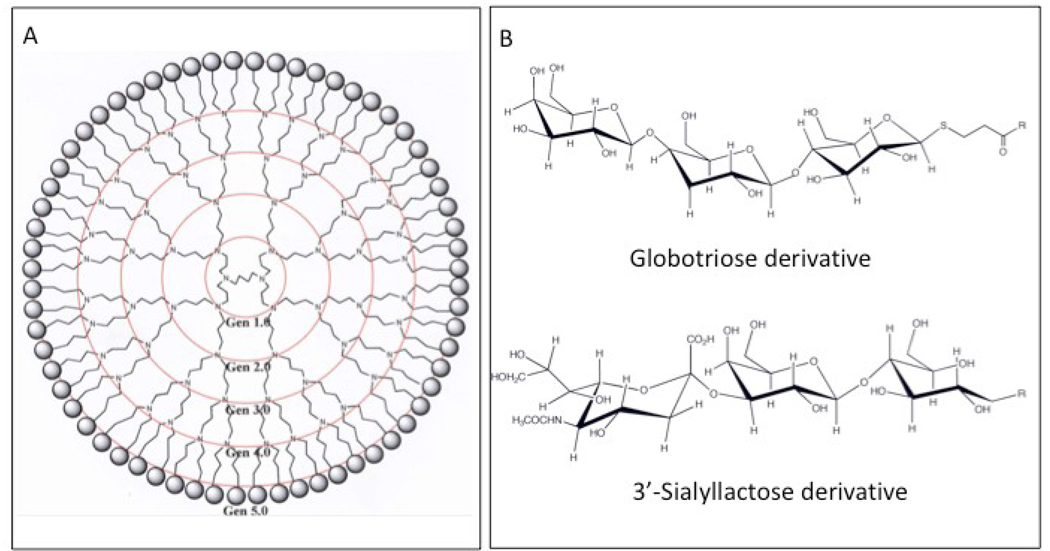
The MVC were synthesized by linking the headgroups of either Gb3 (globotriose) or GM3 (3’-sialyllactose) to a highly cationic Gen 5.0 polypropylenimine dendrimer core. A two-dimensional schematic of the dendrimer is shown in Figure 1A. The structures of the terminal globotriose and 3'-sialyllactose moieties linked to the dendrimer core are shown in Figure 1B. Table 1 provides details of the characterization of these compounds; identity of the globotriose oligosaccharide was verified by NMR (data not shown). Reaction products were monitored for purity and completeness by high performance thin layer chromatography (HPTLC) (data not shown). The mass and polydispersities of the purified compounds, and the calculated average number of carbohydrates per molecule, were obtained using Matrix Assisted Laser Desorption Ionization Time-of-flight Mass Spectrometry (MALDI-TOF MS) as described previously (Kensinger et al., 2004b). The MVC used in this study are the two compounds with the highest number of linked sugars, globotriose 64mer (MVC GBT) and 3’-sialyllactose 64mer (MVC 3SL) (Table 1) with average masses of 35,507 (average of 46 sugars/64mer) and 25,200 (average of 28 sugars/64mer), respectively. Selection of the 64mers for this study was based upon previous observations that smaller derivatized dendrimers were less effective at inhibiting HIV laboratory strains (Kensinger et al., 2004b).
Figure 1. Schematic representation of multivalent carbohydrates (MVC) studied.
A) A two-dimensional structural diagram of a fully conjugated MVC (from Kensinger et. al., 2004b). Concentric circles denote the various dendrimer sizes (the largest being ‘Gen 5.0’with 64 branches), terminal spheres represent linked carbohydrates, whose structures are shown in panel B. B) Representative structures of 3-(β-D-globotrihexosylthio)propionic acid and 3’-sialyllactose moieties.
Table 1.
MALDI TOF MS characterization of multivalent carbohydrates.
| Compound | Theoretical Mw (Da)a |
Average Mw (Da) |
Polydispersity | Average # of linked sugars |
|---|---|---|---|---|
| Globotriose 16mer | 9363 | 8084 | 1.01 | 11 |
| Globotriose 64mer | 43940 | 35507 | 1.01 | 46 |
| 3’-Sialyllac 16mer | 11808 | 4967 | 1.01 | 5 |
| 3’-Sialyllac 64merb | 47654 | 15977 | 1.02 | 14 |
| 3’-Sialyllac 64merc | 47654 | 25200 | 1.02 | 28 |
Theoretical Mw (Da) refers to the molecular weight, in daltons, the compound would have if each arm of the dendrimer were derivatized with the sugar indicated; Average Mw (Da) is the actual molecular weight, in daltons, determined by MALDI-TOF MS; Polydispersity gives an indication of the distribution of molecular mass; and the Average # of linked sugars indicates the average number of dendrimer arms derivatized with the sugar.
XXmer indicates the number of available arms on the polypropylenimine dendrimer
denotes an 18hr reaction time
denotes a 120hr reaction time
MVC inhibit T cell line infection by T cell line-adapted (TCLA) HIV-1, but not pseudovirus infection of TZM-bl cells
The ability of MVC to block HIV-1 infection of lymphocytes was first assessed using the CXCR4+ H9 T cell line, a model system that is typically sensitive for inhibition of HIV-1 CXCR4-utilizing cell line adapted isolates. Both MVC inhibited infection of H9 cells by three H9-adapted viruses, with IC50s ranging from 0.2–3.6 µg/ml (Table 2). To further assess inhibition of lymphocyte infection, the A3/R5 T cell line that was derived from CCR5-transfected, CXCR4+ A3.01 cells (McLinden et al., in preparation) was employed. Two CCR5-utilizing, A3/R5 cell line adapted HIV-1 isolates from clades B and D were also strongly inhibited by the MVC (Table 2). These compounds did not reduce cell line or PBMC viability or proliferation at concentrations up to 1 mg/ml (data not shown).
To assess the breadth of MVC activity against multiple strains of HIV-1 in a model commonly used for both drug development and vaccine testing, we assessed pseudovirus infection of the TZM-bl luciferase reporter cell line (Mascola et al., 2005; Montefiori, 2004; Wei et al., 2002). Of note, DEAE-dextran (DEAE-dex) is typically used with pseudoviruses in the TZM-bl assay to enhance virus infection. As shown in Table 2, neither of the MVC compounds inhibited pseudovirus infection of TZM-bl cells, up to concentrations of 100ug/ml. In fact, the compounds displayed differential effects in this cell line, depending upon the virus stocks utilized in the assay (data not shown, RosaBorges et al., manuscript in preparation).
MVC inhibit HIV-1 primary isolate infection of PBMC
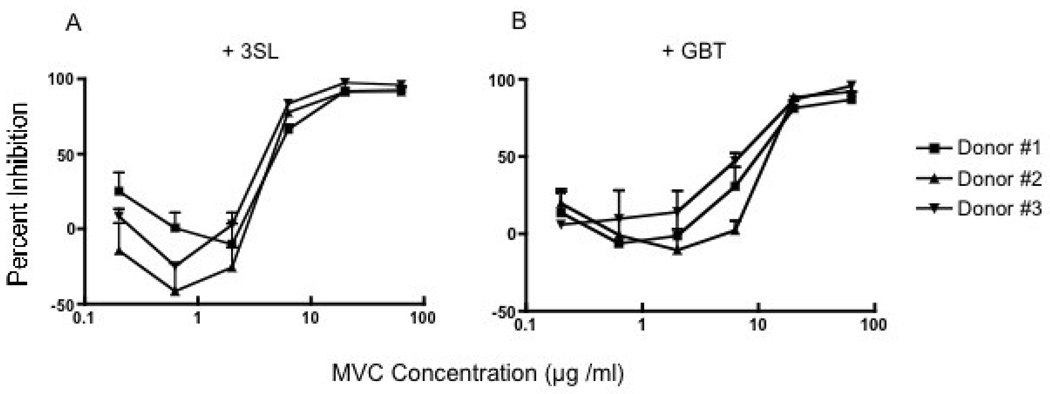
Given the discrepant results observed in the T cell and epithelial cell line models, and to test the compounds in a more physiologic model with CCR5-utilizing viruses, the effects of the MVC on primary isolate infection of activated PBMC were studied. The HIV-1 subtype B 91US_US-1 primary isolate, which displays a moderate level of sensitivity to antibody-mediated neutralization (Brown et al, 2005), was initially assessed. As can be seen in Figure 2, both the 3SL (A) and the GBT (B) MVC inhibited HIV-1 infection in a dose dependent manner. The possibility of PBMC donor-related target cell variability or specificity was addressed by performing replicate assays on PBMC from three different donors. The downward inflection in the curve (negative inhibition [about −45%] or potential enhancement in viral growth) seen for 3SL using donors 2 and 3 is within the range of variation observed in the PBMC inhibition assay. The inhibition curves and IC50 values observed were relatively comparable for the three HIV-negative PBMC donors tested (Figure 2A and 2B). The breadth and magnitude of MVC inhibition of PBMC infection was subsequently assessed using 11 HIV-1 primary isolates (with different co-receptor usages) from subtypes A–D and CRF01_AE. The MVC IC50s for these isolates in a PBMC assay ranged from 0.2 to 7.4 µg/ml, as shown in Table 3.
Figure 2. MVC inhibit infection of PBMC by a primary isolate of HIV-1.
Panels A and B show the inhibition of HIV-1 infection by MVC 3SL and MVC GBT, respectively, using the clade B, R5 isolate 91US_US-1. PBMC were isolated from three different donors, as indicated by the diamond, square, and triangular-shaped symbols. Error bars indicate the standard deviations for three independent experiments.
In certain PBMC assay formats, the presence of endotoxin in samples has been shown to cause an inhibition of HIV-1 infection as an artifact, with no apparent effects of endotoxin observed in the TZM-bl assay (Geonnotti et al., 2010). The endotoxin has been reported to stimulate production of chemokines such as MIP-1-alpha or -beta, and inhibition of HIV infection may be observed. We therefore assayed the levels of chemokine production in our PBMC assay in the presence of MVC to assess any role that chemokines may play in the inhibition observed. The levels of MIP-1-alpha and -beta in the supernatants of PBMC cultured for 4 days (the length of the assay) in the presence of either of the two MVC or LPS was nearly undetectable (data not shown). The inhibition of HIV-1 in this PBMC assay thus appears to be attributable to MVC activity and not to endotoxin-induced chemokines. In support of this inference, the MVC also inhibited an X4-utilizing virus, as shown in Table 3.
MVC Inhibition of HIV-1 Envelope-mediated Cell Fusion
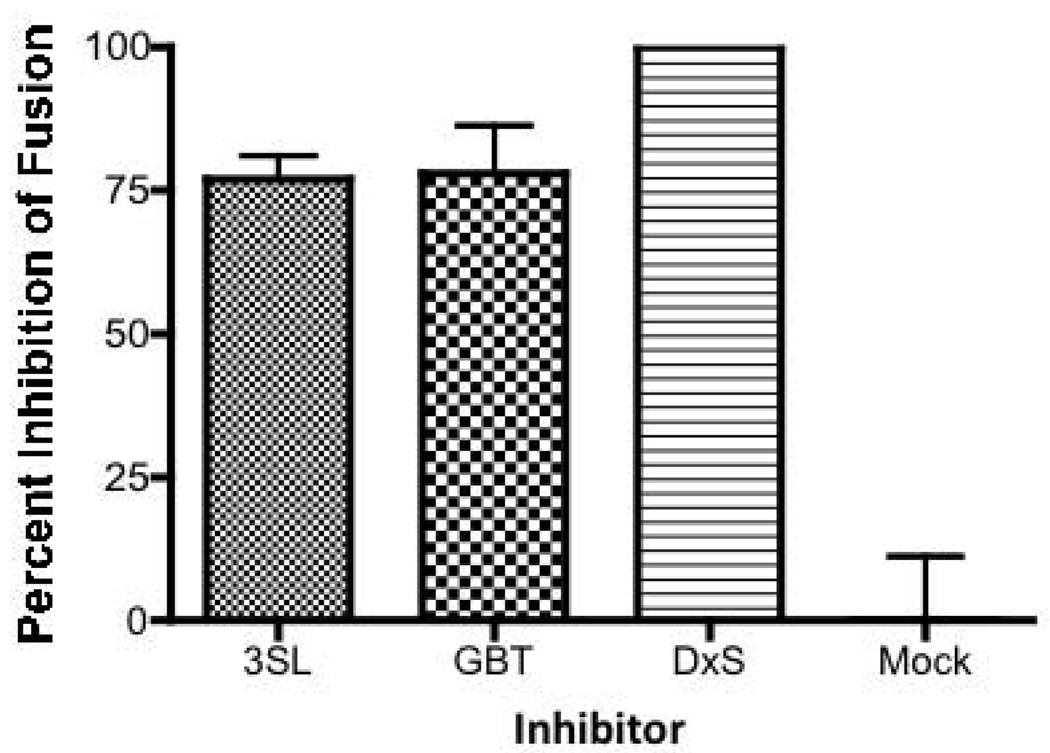
Because GM3 and Gb3 have been implicated in Env-mediated fusion (Hug et al., 2000; Puri et al., 1998; Puri et al., 1999), we assessed the effects of the MVC on Env-mediated fusion to begin to dissect the mechanisms involved in the inhibition of HIV-1 infection by MVC. HIV-1 fusion was measured between CD8 T cell-depleted PBMC and the TF228 cells, a B lymphocyte cell line which constitutively expresses HIV-1 IIIB envelope protein (Jonak et al., 1993). Both MVC showed >75% inhibition of fusion at 10 µg/ml, a concentration where potent inhibition was also observed in the PBMC infection assay (Figure 3). In the presence of dextran sulfate, the positive control for inhibition, fusion was blocked completely, while the mock (medium alone) control showed no inhibition (Figure 3). These results lead us to conclude that the mechanism(s) of MVC inhibition of HIV-1 infection involves the interference of events that lead to gp120-mediated fusion.
Figure 3. MVC inhibit HIV-1 Env-mediated fusion.
Fusion of PBMC with gp120 expressing cells was assessed by dye transfer in the presence of MVC 3SL (grey/white checked bar), MVC GBT (black/white checkered bar), or dextran sulfate (horizontally-hatched bars). Untreated cells are indicated as the mock (white bar). The percent inhibition of fusion is indicated and error bars indicate standard deviations for five independent experiments.
Discussion
In this report we have explored the potential use of novel multivalent carbohydrate compounds for the inhibition of HIV-1. Our results indicate that mimetics of the clustered carbohydrates of cell surface GSL involved in HIV-1 entry can inhibit infection of T cell lines and PBMC by a broad array of viruses, independent of clade or tropism. While complete inhibition of fusion was achieved using dextran sulfate, 100% inhibition was not observed using the two MVC. This may be indicative of failure of all cells to express similar numbers of cell surface GSL, or the mechanism of inhibition by the compounds may not be exclusively due to inhibition of fusion; other factors may play a role in MVC inhibition. It is also unknown whether or not these compounds may elicit drug-resistant variants in vivo, as happens with other anti-retrovirals (McKinnell and Saag, 2009). Future studies in vitro where resistant viruses are generated and then sequenced might reveal the site(s) in the envelope that might be responsible for development of resistance.
Glycosphingolipids have previously been invoked as key elements for efficient HIV-1 infection. GM3 and Gb3 are two of the major glycosphingolipid constituents on major HIV-1-susceptible immune cells (Degroote, et al., 2004; Delezay et al., 1996; Mangeney et al., 1991; Sorice et al., 2001). Several lines of evidence suggested that MVC made from the carbohydrates of these GSL could impact Env-mediated fusion in vitro. Initial work with neural cell lines deficient in CD4 highlighted GSL as ‘alternate receptors’ for the HIV-1 (Harouse et al., 1991). In the case of both T cell lines and primary PBMC, GM3 was closely associated with CD4; the two molecules could be co-precipitated from cell lysates by antibodies to either (Sorice et al., 2001; Sorice et al., 2000; Yohe et al., 2001). Characterization of gp120 and various glycosphingolipid monolayers or reconstituted membranes indicated that this viral glycoprotein interacts specifically with the carbohydrate head groups of both Gb3 and GM3 (Hammache et al., 2000; Hammache et al., 1998a; Hammache et al., 1999). While inhibition of synthesis and cell surface expression of GSL inhibited virus-mediated membrane fusion, the fusion process was reconstituted upon addition of either purified Gb3 or GM3 (Hug et al., 2000; Puri et al., 1998; Puri et al., 1999). Furthermore, in contrast to PBMC from HIV-1 negative donors, lipid membrane profiles of PBMC from HIV-1-infected patients or PBMC stimulated in vitro with PHA, had increased surface expression of complex GSL, specifically Gb3 and GM3 (Fantini et al., 1998a; Lund et al., 2006). Their role in the perturbation of the host immune system during HIV-1-associated pathogenesis is indicated by the increased antibody production against GSL in HIV-1-infected patients (Griggi et al., 1994; Misasi et al., 1993; Reimer et al., 1988). While induction of antibodies to GSLs represents an altered immune response in the setting of HIV infection, this may not affect the efficacy of the body to respond to other immunogens.
The principal of utilizing these GSL and ligand mulitivalency to increase avidity of a carbohydrate-protein interaction has been well demonstrated using GSL and bacterial toxin interactions (Schengrund, 2003). For example, the cell surface receptor for Shiga (Shigella dysenteriae) and Shiga-like toxins is Gb3. Globotriose headgroups of this lipid are bound by the toxin, and inhibition in vitro by multivalent globotriose inhibitors is over a million times more potent than that observed using monovalent globotriose (Kitov et al., 2000). This multivalent carbohydrate approach was previously shown to inhibit HIV-1 infection using MVC derivatized with sulfated-galactosyl moieties (Kensinger et al., 2004a). While the sulfated-galctose MVC inhibited HIV-1 infection, monovalent sugars did not. A recent study from Lund et al. (Lund et al., 2006) showed effective use of Gb3 micelles as inhibitors of infection by both CXCR4 and CCR5 viruses, but only clade B laboratory strains of HIV-1 were tested. Our observation that the globotriose-derivatized dendrimers inhibited CCR5 strains agrees with those of Lund et al. (2006). Hug et al (2000) found that addition of Gb3 to GSL-depleted cells markedly enhanced infection by both a CCR5 (BAL) and CXCR4 strain (IIIB) of HIV while addition of GM3 did so to a lesser extent. The X4 envelope-mediated fusion studied here was inhibited by both MVC, as expected. It will be important to test an R5 envelope in a fusion assay to see if the GBT compound is also effective in this assay. These future studies may also aid in further dissecting the mechanism of action of these compounds. Possibile mechanisms to consider include: 1) gp120 recognizes a structure on the MVC as a ligand, 2) they disrupt the lipid raft association of viral binding components needed for formation of a fusion pore complex (Nguyen e al, 2005), or 3) they block the interaction of glycosphingolipids present on the virus from functioning as needed for productive infection (Hatch et al, 2009).
A potential problem with using GSL per se as an inhibitor is the possibility that they can interact with cell membranes and become functional receptors. This was first shown for the monosialoganglioside, GM1, which serves as a receptor for cholera toxin (Moss et al., 1976), and a facilitator of HIV-1 infection (Puri et al., 1999). The multivalent presentation of the Gb3 and GM3 carbohydrate head groups on the MVC used in these studies reduced their abilities to interact with the cell surface and provide more HIV-1 binding sites. Our results using these dendrimeric scaffolds mirror those of others (Harrison et al., 2010; Lund et al., 2006) with the exception that our MVC GBT IC50 against HIV-1 Bal using PBMC was 70 nM, in comparison to >100 µM for their Gb3-lipid like inhibitors. The increased efficacy (more than 20-fold as calculated based on the number of sugar molecules present) of our inhibitor may be due to a greater valency of carbohydrates per molecule, a difference in size, or possible self-aggregation that could affect potency. Inhibition of PBMC infection was dependent on pre-incubation of MVC with the virus (data not shown), suggesting that a direct interaction with the HIV envelope is part of the inhibition process.
To mimic the interaction between HIV-1 clinical isolates and primary cells, cell line models are often used by drug or vaccine developers. HeLa-based (epithelial) reporter cells are commonly used to measure CCR5 and/or CXCR4 dependent viral entry. Several studies have now shown divergent neutralizing antibody results between assays using different cell types (Bagnarelli et al., 2003; Brown et al., 2007; Brown et al., 2008; Fenyo et al., 2009; Mann et al., 2009; Moody et al., 2010; Polonis et al., 2008; Polonis et al., 2009; Rusert et al., 2009). A potential explanation for the difference in the results in the two cell models may lie in differences in cell surface coreceptor expression for CCR5. It has been reported that certain genetically engineered HeLa cell lines have a much higher expression of cell surface CCR5 than do PBMC from HIV seronegative donors (Choudhry et al., 2006). Furthermore, the CCR5 surface density was shown to have an impact on HIV neutralizing antibody efficacy in that more potent neutralization was observed at lower CCR5 densities. Data from our laboratory indicate that TZM-bl cells express 1–2 logs more CCR5 than do either stimulated or unstimulated PBMC from six independent donors tested (Rosa Borges et al., manuscript in preparation). Thus our data using the MVC agree with the observations of Choudhry et al. (2006), as we have observed a lack of HIV inhibition in the setting of high CCR5 expression. In addition, in a separate study involving the effects of the CCR5-ligand (and CC-chemokine) RANTES on HIV-1 infection, it was reported that, while RANTES potently inhibited infection in most T cell models, varying effects on HIV infection were seen in non-lymphoid models, including macrophages and epithelial cells. In fact, RANTES enhanced HIV infection of CCR5-expressing HeLa cells, and the mechanisms for this enhancement were proposed to occur via cellular activation by RANTES, as well as by an increase in viral attachment to cells (Gordon et al., 1999).
Additionally, the host cell that HIV is produced in has an impact on the levels of HIV inhibition observed in in vitro models. Interestingly, Louder et al. (2005) have shown that infectious molecular clones (IMC) produced via 293T cell transfection are more sensitive to neutralizing antibodies than the matched IMC stocks after one passage through PBMC donor cells. Thus, the HIV envelope topography is clearly distinct and different when derived from 293T- versus PBMC hosts (Louder et al., 2005). Our data with the MVC further highlight the differences observed between viruses from different sources, as well as differences observed using different target cells (Table 2, and Rosa Borges et al., in preparation).
In this study, the assessment of MVC inhibition of HIV-1 infection may depend not only on protein expression but upon membrane carbohydrate and/or lipid profiles, particularly the expression of complex surface GSL, on the virus envelope and/or the target cell membrane. These profiles are most likely quite different for the plasma membranes of PBMC versus TZM-bl, and for the envelopes of pseudoviruses (293T endothelial cell-derived), as compared with primary isolates (PBMC-derived). Like PBMC, HeLa-derived cells also express both Gb3 (Shin et al., 2009) and GM3 (Markwell et al., 1984) although degree of expression has been shown to vary with degree of differentiation, passage number, and culture conditions (Markwell et al., 1984). It is known that mitogen activation increases cell surface expression of GSLs (Fantini et al., 1998a; Lund et al., 2006). T cell activation was shown to induce lipid raft reorganization (Alonso and Millan, 2001) and lipid rafts which are enriched in GSL have been implicated in HIV entry into macrophages (Carter et al., 2009). Gb3 expression on the surface of PBMC has been shown to correlate with susceptibility to HIV-1 infection (Lund et al., 2009). Combined, the foregoing observations support the hypothesis that differential membrane GSL profiles may be partially responsible for the differences in the MVC inhibitory capacities in the cell models employed. These data underscore the importance of the observation of primary isolate inhibition by the MVC using a primary cell model.
A recent report revealed that endotoxin contamination may explain some discrepant results between cell line and PBMC-based assays (Geonnotti et al., 2010). It was therefore essential to evaluate the role of endotoxin in relation to the MVC results in the PBMC assay. For several reasons, we conclude that endotoxin effects did not play a major role in MVC inhibition seen in the PBMC assay used in this study. The endotoxin mediated HIV-1 inhibition in certain PBMC assays is thought to occur primarily due to induction of chemokine expression by monocytic cells (Geonnotti et al., 2010; Verani et al., 1997). In the PBMC assay employed in these studies, the cells were first stimulated for 4 days with PHA. This procedure typically results in a loss of more than 90% of the monocytic cells from most PBMC sources. After PHA stimulation, the CD14+ cells range from 0.1–2.4% (as used in this assay) (Brown et al., manuscript in preparation). With 4-day PHA-stimulation, there appear to be very few cells available to produce sufficient chemokines in response to endotoxin. Secondly, the levels of MIP-1-alpha and beta measured in our PBMC supernatants were below the levels sufficient to mediate the significant HIV inhibitory effects (data not shown) reported by Geonnotti et al. (Geonnotti et al., 2010). Finally, the MVC inhibit X4 viruses, ruling out inhibition of these viruses by beta-chemokines.
Multivalent approaches utilizing the glycosphingolipid carbohydrates globotriose and 3’-sialyllactose suggest a novel, clade-independent approach to prevent HIV-1 entry in primary cells. However, the choice of the cell model system used for in vitro evaluation could have had a significant impact on conclusions regarding the potential utility of these compounds. These findings underscore the importance of the application of different inhibition assessment platforms so that viable anti-HIV-1 candidates are not overlooked. The identification of assay(s) that can provide in vivo correlates will also be an important element in the discovery and development of effective anti-HIV drugs and vaccines.
Research highlights.
Multivalent carbohydrates (MVCs) inhibited infection of PBMCs by HIV-1
MVCs inhibited infection by T cell line-adapted viruses
MVCs inhibited infection by primary isolates of HIV-1
MVCs inhibited Env-mediated membrane fusion
Acknowledgements
We thank Kara Lombardi, Erik Odom, Jason Newman, Charline Bermudez, Anita Gillis, and Maggie Wesberry for technical assistance with viral assays; Satinder S. Rawat at NCI-Frederick for help with initial studies; Shendra R. Miller and Mary Lee Ferguson for help with initial HIV-1 assays; Dr. Xuping Xia for help with NMR controls; Dr. Bruce Stanley for his help with MALDI-TOF MS analyses; Dr. Satoshi Koizumi at Kyowa Ltd., and Shawn DeFrees at NEOSE, Inc. for their help obtaining globotriose and 3’-sialyllactose, respectively. We also thank Dr. John Mascola for providing envelope clones and Drs. Christina Ochsenbauer and John Kappes for provision of HIV-1 IMC.
This work was supported in part by grant RO1 NS40231 from the National Institute of Allergy and Infectious Diseases (CLS), the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research (AP and RB), and by Cooperative Agreement no. DAMD W81XWH-04-2-0005 between the U.S. Army Medical Research and Material Command and the Henry M. Jackson Foundation for the Advancement of Military Medicine, working with the Division of AIDS, National Institute for Allergy and Infectious Diseases, National Institutes of Health.
The views expressed herein are the private opinions of the authors and are not to be considered as official or reflecting the views of the U.S. Army or the U.S Department of Defense. Use of trade names is for identification only and does not imply endorsement by the U.S. government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alonso MA, Millan J. The role of lipid rafts in signaling and membrane trafficking in T lymphocytes. J. Cell Sci. 2001;114:3957–3965. doi: 10.1242/jcs.114.22.3957. [DOI] [PubMed] [Google Scholar]
- Bagnarelli P, Fiorelli L, Vecchi M, Monachetti A, Menzo S, Clementi M. Analysis of the functional relationship between V3 loop and gp120 context with regard to human immunodeficiency virus coreceptor usage using naturally selected sequences and different viral backbones. Virology. 2003;307:328–340. doi: 10.1016/s0042-6822(02)00077-6. [DOI] [PubMed] [Google Scholar]
- Berger EA, Doms RW, Fenyo EM, Korber BT, Littman DR, Moore JP, Sattentau QJ, Schuitemaker H, Sodroski J, Weiss RA. A new classification for HIV-1. Nature. 1998;391:240. doi: 10.1038/34571. [DOI] [PubMed] [Google Scholar]
- Bomsel M. Transcytosis of infectious human immunodeficiency virus across a tight human epithelial cell line barrier. Nat. Med. 1997;3:42–47. doi: 10.1038/nm0197-42. [DOI] [PubMed] [Google Scholar]
- Bomsel M, Alfsen A. Entry of viruses through the epithelial barrier: pathogenic trickery. Nat. Rev. Mol. Cell Biol. 2003;4:57–68. doi: 10.1038/nrm1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomsel M, Heyman M, Hocini H, Lagaye S, Belec L, Dupont C, Desgranges C. Intracellular neutralization of HIV transcytosis across tight epithelial barriers by anti-HIV envelope protein dIgA or IgM. Immunity. 1998;9:277–287. doi: 10.1016/s1074-7613(00)80610-x. [DOI] [PubMed] [Google Scholar]
- Borkow G, Lapidot A. Multi-targeting the entrance door to block HIV-1. Curr Drug Targets Infect. Disord. 2005;5:3–15. doi: 10.2174/1568005053174645. [DOI] [PubMed] [Google Scholar]
- Brown BK, Darden JM, Tovanabutra S, Oblander T, Frost J, Sanders-Buell E, de Souza MS, Birx DL, McCutchan FE, Polonis VR. Biologic and genetic characterization of a panel of 60 human immunodeficiency virus type 1 isolates, representing clades A, B, C, D, CRF01_AE, and CRF02_AG, for the development and assessment of candidate vaccines. J Virol. 2005;79:6089–6101. doi: 10.1128/JVI.79.10.6089-6101.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown BK, Karasavvas N, Beck Z, Matyas GR, Birx DL, Polonis VR, Alving CR. Monoclonal antibodies to phosphatidylinositol phosphate neutralize human immunodeficiency virus type 1: role of phosphate-binding subsites. J Virol. 2007;81(4):2087–2091. doi: 10.1128/JVI.02011-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown BK, Wieczorek L, Sanders-Buell E, Rosa Borges A, Robb ML, Birx DL, Michael NL, McCutchan FE, Polonis VR. Cross-clade neutralization patterns among HIV-1 strains from the six major clades of the pandemic evaluated and compared in two different models. Virology. 2008;375(2):529–538. doi: 10.1016/j.virol.2008.02.022. [DOI] [PubMed] [Google Scholar]
- Carter GC, Bernstone L, Sangani D, Bee JW, Harder T, James W. HIV entry in macrophages is dependent on intact lipid rafts. Virology. 2009;386:192–202. doi: 10.1016/j.virol.2008.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhry V, Zhang M-Y, Harris I, Sidorov IA, Vu B, Dimitrov AS, Fouts T, Dimitrov DS. Increased efficacy of HIV-1 neutralization by antibodies at low CCR5 surface concentration. Biochem. Biophys. Res. Commun. 2006;348:1107–1115. doi: 10.1016/j.bbrc.2006.07.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAIDS. DAIDS Virology Manual for HIV Laboratories, Vol. January 1997, Division of AIDS, National Institute of Allergy and Infections Diseases. National Institutes of Health; 1997. [Google Scholar]
- Degroote S, Wolthoorn J, van Meer G. The cell biology of glycosphingolipids. Semin Cell Dev Biol. 2004;15:375–387. doi: 10.1016/j.semcdb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Delezay O, Hammache D, Fantini J, Yahi N. SPC3, a V3 loop-derived synthetic peptide inhibitor of HIV-1 infection, binds to cell surface glycosphingolipids. Biochemistry. 1996;35:15663–15671. doi: 10.1021/bi961205g. [DOI] [PubMed] [Google Scholar]
- Elofsson M, Walse B, Kihlberg J. Building Blocks for Glycopeptide Synthesis: Glycosylation of 3-mercaptopropionic acid and Fmoc Amino Acids with Unprotected Carboxyl Groups. Tetrahedron Letters. 1991;32:7613–7616. [Google Scholar]
- Fantini J, Hammache D, Delezay O, Pieroni G, Tamalet C, Yahi N. Sulfatide inhibits HIV-1 entry into CD4−/CXCR4+ cells. Virology. 1998a;246:211–220. doi: 10.1006/viro.1998.9216. [DOI] [PubMed] [Google Scholar]
- Fantini J, Maresca M, Hammache D, Yahi N, Delezay O. Glycosphingolipid (GSL) microdomains as attachment platforms for host pathogens and their toxins on intestinal epithelial cells: activation of signal transduction pathways and perturbations of intestinal absorption and secretion. Glycoconj J. 2000;17:173–179. doi: 10.1023/a:1026580905156. [DOI] [PubMed] [Google Scholar]
- Fantini J, Tamalet C, Hammache D, Tourres C, Duclos N, Yahi N. HIV-1-induced perturbations of glycosphingolipid metabolism are cell-specific and can be detected at early stages of HIV-1 infection. J Acquir. Immune Defic. Syndr. Hum. Retrovirol. 1998b;19:221–229. doi: 10.1097/00042560-199811010-00003. [DOI] [PubMed] [Google Scholar]
- Fantini J, Yahi N, Mabrouk K, Van Rietschoten J, Rochat H, Sabatier JM. Multi-branched peptides based on the HIV-1 V3 loop consensus motif inhibit HIV-1 and HIV-2 infection in CD4+ and CD4− cells. C. R. Acad. Sci. III. 1993;316:1381–1387. [PubMed] [Google Scholar]
- Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- Fenyo EM, Heath A, Dispinseri S, Holmes H, Lusso P, Zolla-Pazner S, Donners H, Heyndrickx L, Alcami J, Bongertz V, Jassoy C, Malnati M, Montefiori D, Moog C, Morris L, Osmanov S, Polonis V, Sattentau Q, Schuitemaker H, Sutthent R, Wrin T, Scarlatti G. International network for comparison of HIV neutralization assays: the NeutNet report. PLoS One. 2009;4(2):e4505. doi: 10.1371/journal.pone.0004505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard AL, Pfeiffer SE. Oligodendrocyte progenitors isolated directly from developing telencephalon at a specific phenotypic stage: myelinogenic potential in a defined environment. Development. 1989;106(1):119–132. doi: 10.1242/dev.106.1.119. [DOI] [PubMed] [Google Scholar]
- Geonnotti AR, Bilska M, Yuan X, Ochsenbauer C, Edmonds TG, Kappes JC, Liao HX, Haynes BF, Montefiori DC. Differential inhibition of human immunodeficiency virus type 1 in peripheral blood mononuclear cells and TZM-bl cells by endotoxin-mediated chemokine and gamma interferon production. AIDS Res. Hum. Retroviruses. 2010;26(3):279–291. doi: 10.1089/aid.2009.0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon CJ, Muesing MA, Proudfoot AEI, Power CA, Moore JP, Trkola A. Enhancement of human immunodeficiency virus type 1 infection by the CC-chemokine RANTES is independent of the mechanism of virus-cell fusion. J. Virol. 1999;73:684–694. doi: 10.1128/jvi.73.1.684-694.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray GR. Antibodies to carbohydrates: preparation of antigens by coupling carbohydrates to proteins by reductive amination with cyanoborohydride. Methods Enzymol. 1978;50:155–160. doi: 10.1016/0076-6879(78)50014-1. [DOI] [PubMed] [Google Scholar]
- Griggi T, Bauer R, Garofalo T, Kukel S, Lenti L, Massetti AP, Muller C, Sorice M, Pontieri GM. Autoantibodies against ganglioside GM3 represent a portion of anti-lymphocyte antibodies in AIDS patients. Scand. J. Immunol. 1994;40(1):77–82. doi: 10.1111/j.1365-3083.1994.tb03436.x. [DOI] [PubMed] [Google Scholar]
- Hammache D, Pieroni G, Maresca M, Ivaldi S, Yahi N, Fantini J. Reconstitution of sphingolipid-cholesterol plasma membrane microdomains for studies of virus-glycolipid interactions. Methods Enzymol. 2000;312:495–506. doi: 10.1016/s0076-6879(00)12934-9. [DOI] [PubMed] [Google Scholar]
- Hammache D, Pieroni G, Yahi N, Delezay O, Koch N, Lafont H, Tamalet C, Fantini J. Specific interaction of HIV-1 and HIV-2 surface envelope glycoproteins with monolayers of galactosylceramide and ganglioside GM3. J. Biol. Chem. 1998a;273:7967–7971. doi: 10.1074/jbc.273.14.7967. [DOI] [PubMed] [Google Scholar]
- Hammache D, Yahi N, Maresca M, Pieroni G, Fantini J. Human erythrocyte glycosphingolipids as alternative cofactors for human immunodeficiency virus type 1 (HIV-1) entry: evidence for CD4-induced interactions between HIV-1 gp120 and reconstituted membrane microdomains of glycosphingolipids (Gb3 and GM3) J. Virol. 1999;73:5244–5248. doi: 10.1128/jvi.73.6.5244-5248.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammache D, Yahi N, Pieroni G, Ariasi F, Tamalet C, Fantini J. Sequential interaction of CD4 and HIV-1 gp120 with a reconstituted membrane patch of ganglioside GM3: implications for the role of glycolipids as potential HIV-1 fusion cofactors. Biochem. Biophys. Res. Commun. 1998b;246:117–122. doi: 10.1006/bbrc.1998.8531. [DOI] [PubMed] [Google Scholar]
- Harouse JM, Bhat S, Spitalnik SL, Laughlin M, Stefano K, Silberberg DH, Gonzalez-Scarano F. Inhibition of entry of HIV-1 in neural cell lines by antibodies against galactosyl ceramide. Science. 1991;253:320–323. doi: 10.1126/science.1857969. [DOI] [PubMed] [Google Scholar]
- Harrison AL, Olsson ML, Jones RB, Ramkumar S, Sakac D, Binnington B, Henry S, Lingwood CA, Branch DR. A synthetic globotriaosylceramide analogue inhibits HIV-1 infection in vitro by two mechanisms. Glycoconj. J. pmid. 2010:20582467. doi: 10.1007/s10719-010-9297-y. [DOI] [PubMed] [Google Scholar]
- Hartley O, Klasse PJ, Sattentau QJ, Moore JP. V3: HIV's switch-hitter. AIDS Res. Hum. Retroviruses. 2005;21:171–189. doi: 10.1089/aid.2005.21.171. [DOI] [PubMed] [Google Scholar]
- Hatch SC, Archer J, Gummuluru S. Glycosphingolipid composition of human immunodeficiency virus type 1 (HIV-1) particles is a crucial determinant for dendritic cell-mediated HIV-1 trans-infection. J. Virol. 2009;83:3496–3506. doi: 10.1128/JVI.02249-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes BF, Montefiori DC. Aiming to induce broadly reactive neutralizing antibody responses with HIV-1 vaccine candidates. Expert Rev. Vaccines. 2006;5:579–595. doi: 10.1586/14760584.5.4.579. [DOI] [PubMed] [Google Scholar]
- Hug P, Lin HM, Korte T, Xiao X, Dimitrov DS, Wang JM, Puri A, Blumenthal R. Glycosphingolipids promote entry of a broad range of human immunodeficiency virus type 1 isolates into cell lines expressing CD4, CXCR4, and/or CCR5. J. Virol. 2000;74:6377–6385. doi: 10.1128/jvi.74.14.6377-6385.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonak ZL, Clark RK, Matour D, Trulli S, Craig R, Henri E, Lee EV, Greig R, Debouck C. A human lymphoid recombinant cell line with functional human immunodeficiency virus type 1 envelope. AIDS Res Hum Retroviruses. 1993;9(1):23–32. doi: 10.1089/aid.1993.9.23. [DOI] [PubMed] [Google Scholar]
- Kensinger RD, Catalone BJ, Krebs FC, Wigdahl B, Schengrund CL. Novel polysulfated galactose-derivatized dendrimers as binding antagonists of human immunodeficiency virus type 1 infection. Antimicrob. Agents Chemother. 2004a;48:1614–1623. doi: 10.1128/AAC.48.5.1614-1623.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensinger RD, Yowler BC, Benesi AJ, Schengrund CL. Synthesis of novel, multivalent glycodendrimers as ligands for HIV-1 gp120. Bioconjug. Chem. 2004b;15:349–358. doi: 10.1021/bc034156a. [DOI] [PubMed] [Google Scholar]
- Kitov PI, Sadowska JM, Mulvey G, Armstrong GD, Ling H, Pannu NS, Read RJ, Bundle DR. Shiga-like toxins are neutralized by tailored multivalent carbohydrate ligands. Nature. 2000;403:669–672. doi: 10.1038/35001095. [DOI] [PubMed] [Google Scholar]
- Koff WC. Accelerating HIV vaccine development. Nature. 2010;464(7286):161–162. doi: 10.1038/464161a. [DOI] [PubMed] [Google Scholar]
- Kwong PD, Wyatt R, Robinson J, Sweet RW, Sodroski J, Hendrickson WA. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature. 1998;393:648–659. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louder MK, Sambor A, Chertova E, Hunte T, Barrett S, Ojong F, Sanders-Buell E, Zolla-Pazner S, McCutchan FE, Roser JD, Gabuzda D, Lifson JD, Mascola JR. HIV-1 envelope pseudotyped viral vectors and infectious molecular clones expressing the same envelope glycoprotein have a similar neutralization phenotype, but culture in peripheral blood mononuclear cells is associated with decreased neutralization sensitivity. Virology. 2005;339:226–238. doi: 10.1016/j.virol.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Lund N, Branch DR, Mylvaganam M, Chark D, Ma XZ, Sakac D, Binnington B, Fantini J, Puri A, Blumenthal R, Lingwood CA. A novel soluble mimic of the glycolipid, globotriaosyl ceramide inhibits HIV infection. Aids. 2006;20:333–343. doi: 10.1097/01.aids.0000206499.78664.58. [DOI] [PubMed] [Google Scholar]
- Lund N, Olsson ML, Ramkumar S, Sakac D, Yahalom V, Levene C, Hellberg A, Ma XZ, Binnington B, Jung D, Lingwood CA, Branch DR. The human Pk histo-blood group antigen provides protection against HIV-1 infection. Blood. 2009;113(20):4980–4991. doi: 10.1182/blood-2008-03-143396. [DOI] [PubMed] [Google Scholar]
- Magerus-Chatinet A, Yu H, Garcia S, Ducloux E, Terris B, Bomsel M. Galactosyl ceramide expressed on dendritic cells can mediate HIV-1 transfer from monocyte derived dendritic cells to autologous T cells. Virology. 2007;362(1):67–74. doi: 10.1016/j.virol.2006.11.035. [DOI] [PubMed] [Google Scholar]
- Magnusson G, Nouri G, Dahmen J, Frejd T, Lave T. BF3-Etherate Induced Formation of 2,2,2,-Trichloroethyl Glycopyranosides. Selective Visualization of Carbohydrate Derivatives on TLC Plates. Acta Chemica Scandinavica B. 1981;35:213–216. [Google Scholar]
- Mangeney M, Richard Y, Coulaud D, Tursz T, Wiels J. CD77: an antigen of germinal center B cells entering apoptosis. Eur. J. Immunol. 1991;21:1131–1140. doi: 10.1002/eji.1830210507. [DOI] [PubMed] [Google Scholar]
- Mann AM, Rusert P, Berlinger L, Kuster H, Gunthard HF, Trkola A. HIV sensitivity to neutralization is determined by target and virus producer cell properties. AIDS. 2009;23(13):1659–1667. doi: 10.1097/QAD.0b013e32832e9408. [DOI] [PubMed] [Google Scholar]
- Markwell MA, Fredman P, Svennerholm L. Receptor ganglioside content of three hosts for Sendai virus. MDBK, HeLa, and MDCK cells. Biochim. Biophys. Acta. 1984;775:7–16. doi: 10.1016/0005-2736(84)90228-1. [DOI] [PubMed] [Google Scholar]
- Mascola JR. Methods in Molecular Medicine. In: Michael N, Kim JH, editors. HIV Protocols. Totowa, New Jersey: Humana Press; 1999. p. 317. [Google Scholar]
- Mascola JR, D’Souza P, Gilbert P, Hahn BH, Haigwood NL, Morris L, Petropoulos CJ, Polonis VR, Sarzotti M, Montefiori DC. Recommendations for the design and use of standard virus panels to assess neutralizing antibody responses elicited by candidate human immunodeficiency virus type 1 vaccines. J. Virol. 2005;79:10103–10107. doi: 10.1128/JVI.79.16.10103-10107.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnell JA, Saag MS. Novel drug classes: entry inhibitors [enfuvirtide, chemokine (C-C motif) receptor 5 antagonists] Curr. Opin. HIV AIDS. 2009;4:513–517. doi: 10.1097/COH.0b013e328331d3d0. [DOI] [PubMed] [Google Scholar]
- Misasi R, Sorice M, Griggi T, d'Agostino F, Garofalo T, Masala C, Pontieri GM, Lenti L. GM3 as a target of anti-lymphocytic ganglioside antibodies in AIDS patients. Clin. Immunol. Immunopathol. 1993;67(3 Pt 1):216–223. doi: 10.1006/clin.1993.1068. [DOI] [PubMed] [Google Scholar]
- Montefiori D. Evaluating neutralizing antibodies against HIV, SIV, and SHIV in luciferase reporter gene assays. In: Coicko R, editor. Current protocols in immunolgy. New York, New York: John Wiley & Sons; 2004. pp. 12.11.1–12.11.15. [DOI] [PubMed] [Google Scholar]
- Moody MA, Liao H-X, Alam SM, Scearce RM, Plonk MK, Kozink DM, Drinker MS, Zhang R, Xia S-M, Sutherland LL, Tomaras GD, Giles IP, Kappes JC, Ochsenbauer-Jambor C, Edmonds TG, Soares M, Barbero G, Forthal DN, Landucci G, Chang C, King SW, Kavlie A, Denny TN, Hwang K-K, Chen PP, Thorpe PE, Montefiori DC, Haynes BF. Anti-phospholipid human monoclonal antibodies inhibit CCR5-tropic HIV-1 and induce β-chemokines. J. Exp. Med. 2010 doi: 10.1084/jem.20091281. DOI: 10.1084/jem.20091281jem.20091281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss J, Fishman PH, Manganiello VC, Vaughan M, Brady RO. Functional incorporation of ganglioside into intact cells: induction of choleragen responsiveness. Proc. Natl. Acad. Sci. U S A. 1976;73(4):1034–1037. doi: 10.1073/pnas.73.4.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehete PN, Vela EM, Hossain MM, Sarkar AK, Yahi N, Fantini J, Sastry KJ. A post-CD4-binding step involving interaction of the V3 region of viral gp120 with host cell surface glycosphingolipids is common to entry and infection by diverse HIV-1 strains. Antiviral Res. 2002;56:233–251. doi: 10.1016/s0166-3542(02)00130-4. [DOI] [PubMed] [Google Scholar]
- Nguyen CH, Giri B, Collins G, Taub DD. Dynamic reorganization of chemokine receptors, cholesterol, lipid rafts, and adhesion molecules to sites of CD4 engagement. Exp. Cell Res. 2005;304:559–569. doi: 10.1016/j.yexcr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- Poirot E, Zhang X, Whittaker NF, Kovac P. Syntheses of the L-manno and some other analogs of the terminal determinants of the O-PS of Vibrio cholerae O:1. Carbohydr. Res. 2001;330:7–20. doi: 10.1016/s0008-6215(00)00265-2. [DOI] [PubMed] [Google Scholar]
- Polonis VR, Brown BK, Rosa Borges A, Zolla-Pazner S, Dimitrov DS, Zhang MY, Barnett SW, Ruprecht RM, Scarlatti G, Fenyo EM, Montefiori DC, McCutchan FE, Michael NL. Recent advances in the characterization of HIV-1 neutralization assays for standardized evaluation of the antibody response to infection and vaccination. Virology. 2008;375(2):315–320. doi: 10.1016/j.virol.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Polonis VR, Schuitemaker H, Bunnik EM, Brown BK, Scarlatti G. Impact of host cell variation on the neutralization of HIV-1 in vitro. Curr. Opin. HIV AIDS. 2009;4(5):400–407. doi: 10.1097/COH.0b013e32832edc50. [DOI] [PubMed] [Google Scholar]
- Puri A, Hug P, Jernigan K, Barchi J, Kim HY, Hamilton J, Wiels J, Murray GJ, Brady RO, Blumenthal R. The neutral glycosphingolipid globotriaosylceramide promotes fusion mediated by a CD4-dependent CXCR4-utilizing HIV type 1 envelope glycoprotein. Proc. Natl. Acad. Sci. USA. 1998;95:14435–14440. doi: 10.1073/pnas.95.24.14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri A, Hug P, Jernigan K, Rose P, Blumenthal R. Role of glycosphingolipids in HIV-1 entry: requirement of globotriosylceramide (Gb3) in CD4/CXCR4-dependent fusion. Biosci. Rep. 1999;19:317–325. doi: 10.1023/a:1020554509642. [DOI] [PubMed] [Google Scholar]
- Reimer CB, Black CM, Holman RC, Wells TW, Ramirez RM, Sa-Ferreira JA, Nicholson JK, McDougal JS. Hypergammaglobulinemia associated with human immunodeficiency virus infection. Monogr. Allergy. 1988;23:83–96. [PubMed] [Google Scholar]
- Rusert P, Mann A, Huber M, von Wyl V, Gunthard HF, Trkola A. Divergent effects of cell environment on HIV entry inhibitor activity. AIDS. 2009;23(11):1319–1327. doi: 10.1097/qad.0b013e32832d92c2. [DOI] [PubMed] [Google Scholar]
- Sattentau QJ, Moore JP, Vignaux F, Traincard F, Poignard P. Conformational changes induced in the envelope glycoproteins of the human and simian immunodeficiency viruses by soluble receptor binding. J. Virol. 1993;67:7383–7393. doi: 10.1128/jvi.67.12.7383-7393.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schengrund CL. "Multivalent" saccharides: development of new approaches for inhibiting the effects of glycosphingolipid-binding pathogens. Biochem. Pharmacol. 2003;65:699–707. doi: 10.1016/s0006-2952(02)01553-8. [DOI] [PubMed] [Google Scholar]
- Shin IS, Ishii S, Shin JS, Sung KI, Park BS, Jang HY, Kim BW. Globotriaosylceramide (Gb3) content in HeLa cells is correlated to Shiga toxin-induced cytotoxicity and Gb3 synthase expression. BMB Rep. 2009;42:310–314. doi: 10.5483/bmbrep.2009.42.5.310. [DOI] [PubMed] [Google Scholar]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387(6633):569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Sorice M, Garofalo T, Misasi R, Longo A, Mattei V, Sale P, Dolo V, Gradini R, Pavan A. Evidence for cell surface association between CXCR4 and ganglioside GM3 after gp120 binding in SupT1 lymphoblastoid cells. FEBS Lett. 2001;506:55–60. doi: 10.1016/s0014-5793(01)02830-7. [DOI] [PubMed] [Google Scholar]
- Sorice M, Garofalo T, Misasi R, Longo A, Mikulak J, Dolo V, Pontieri GM, Pavan A. Association between GM3 and CD4-Ick complex in human peripheral blood lymphocytes. Glycoconj. J. 2000;17:247–252. doi: 10.1023/a:1026501609699. [DOI] [PubMed] [Google Scholar]
- Sorice M, Longo A, Garofalo T, Mattei V, Misasi R, Pavan A. Role of GM3-enriched microdomains in signal transduction regulation in T lymphocytes. Glycoconj. J. 2004;20:63–70. doi: 10.1023/B:GLYC.0000018018.29488.c6. [DOI] [PubMed] [Google Scholar]
- Verani A, Scarlatti G, Comar M, Tresoldi E, Polo S, Giacca M, Lusso P, Siccardi AG, Vercelli D. C-C chemokines released by lipopolysaccharide (LPS)-stimulated human macrophages suppress HIV-1 infection in both macrophages and T cells. J. Exp. Med. 1997;185(5):805–816. doi: 10.1084/jem.185.5.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Decker JM, Liu H, Zhang Z, Arani RB, Kilby JM, Saag MS, Wu X, Shaw GM, Kappes JC. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob. Agents Chemother. 2002;46(6):1896–1905. doi: 10.1128/AAC.46.6.1896-1905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfrom ML, Thompson A. Acetylation. In: Whistler RL, Wolfrom ML, BeMiller JN, editors. Methods in Carbohydrate Chemistry. Vol. II. New York: Academic Press; 1963. pp. 211–213. [Google Scholar]
- Woller EK, Cloninger MJ. Mannose functionalization of a sixth generation dendrimer. Biomacromolecules. 2001;2:1052–1054. doi: 10.1021/bm015560k. [DOI] [PubMed] [Google Scholar]
- Yohe HC, Wallace PK, Berenson CS, Ye S, Reinhold BB, Reinhold VN. The major gangliosides of human peripheral blood monocytes/macrophages: absence of ganglio series structures. Glycobiology. 2001;11:831–841. doi: 10.1093/glycob/11.10.831. [DOI] [PubMed] [Google Scholar]
- Zemplen G. Degradation of the reducing bioses. I. Direct determination of the constitution of cellobiose. Ber. 59B. 1926:1254–1266. [Google Scholar]