Abstract
The purpose of this study was to investigate the effect of carpal tunnel pressure on the gliding characteristics of flexor tendons within the carpal tunnel. Eight fresh human cadaver wrists and hands were used. A balloon was inserted into the carpal tunnel to elevate the pressure. The mean gliding resistance of the middle finger flexor digitorum superficialis tendon was measured with the following six conditions 1) as a baseline, before balloon insertion; 2) balloon with 0 mmHg pressure; 3) 30 mmHg; 4) 60 mmHg; 5) 90 mmHg; 6) 120 mmHg. The gliding resistance of flexor tendon gradually increased as the carpal tunnel pressure was elevated. At pressures above 60 mmHg, the increase in gliding resistance became significant compared to the baseline condition. This study helps us to understand the relationship between carpal tunnel pressure, which is elevated in the patient with carpal tunnel syndrome (CTS) and tendon gliding resistance, which is a component of the work of flexion. These findings suggest that patients with CTS may have to expend more energy to accomplish specific motions, which may in turn affect symptoms of hand pain, weakness and fatigue, seen commonly in such patients.
Keywords: Carpal Tunnel Syndrome, Flexor Tendon
INTRODUCTION
Carpal tunnel syndrome in one of the most common compression neuropathies, with an estimated lifetime risk of 10% and a prevalence of about 3% (1–3). It is generally accepted that repetitive hand motion is a risk factor for CTS (4–7).
While the etiology of CTS is in most cases idiopathic, it is known that CTS is a result of increased pressure within the carpal tunnel, which is a confined anatomic space bounded by the carpal bones on the dorsal side, the trapezium on the radial side, the hook of the hamate on the ulnar side and the flexor retinaculum on the volar side (8–10). However, while the effect of increase pressure on the median nerve has been well studied, the effect of pressure on tendon function has received little attention, even though tendons make up the majority of the cross section of the carpal tunnel contents. The purpose of this study was to investigate the effect of pressure changes on the gliding resistance of a representative tendon, the middle finger flexor digitorum superficialis (FDS), within the carpal tunnel in a human cadaver model. We hypothesized that the gliding resistance would increase with carpal tunnel pressure elevation.
MATERIAL AND METHODS
After IRB review and approval, eight human fresh frozen cadavers, amputated approximately 15 cm proximal to the wrist joint, were harvested and thawed at room temperature immediately prior to testing. The cadaver donors included 5 males and 3 females, with an average age of 76 years (range 40 – 91). There were 5 right and 3 left upper extremities. A medical record review was performed on each cadaver donor, to obtain demographic data and to be sure the individual did not have a recorded antemortem diagnosis of CTS. The flexor digitorum superficialis (FDS) tendons of the second, third and fourth digits were exposed proximal and distal to the flexor retinaculum, maintaining the carpal tunnel region intact. A 2 N load was attached to the proximal ends of each of the three FDS tendons by a cable, to maintain the tension on these three tendons. Marks were placed on the tendons and on the flexor retinaculum, a fixed reference point. Then the tendon excursion was measured from full flexion to full extension of all three fingers at the wrist fixed in the neutral position (0o extension). After the tendon excursions of all three fingers together were recorded, the tendons were dissected from their distal attachments, and the index, middle, ring and small fingers were amputated at the MCP joint level, leaving the flexor retinaculum intact. A custom-designed external fixator was used to fix the wrist in the neutral position. The specimen was then mounted on the testing apparatus by clamping the proximal end of the radius and ulna in a custom made clamping device (Figure 1). Load transducers were connected to the distal (F1) and proximal (F2) ends of the middle finger FDS tendon using a nylon cord. The proximal end of all three FDS tendons (index, middle and ring fingers) was connected to a mechanical actuator. Three 2-Newton loads were attached; one to each of the distal ends of the index, middle and ring finger FDS tendons. Three 1 N loads were attached, one to each of the distal ends of the index, middle and ring finger FDP tendons, in order to maintain a minimal level of tension in the FDP tendons.
Figure 1.
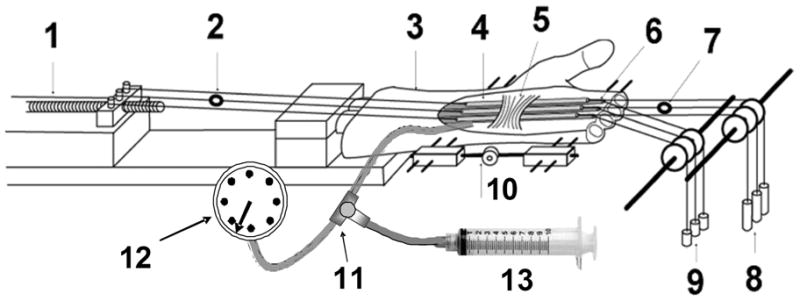
Testing Apparatus
1. Actuator with potentiometer;
2. Load transducer (F2) connected to the proximal end of the III FDS tendon;
3. Specimen;
4. Three Fingers (index, middle and ring);
5. Retianculum;
6. Three fingers (index, middle and ring) FDP tendons;
7. Load transducer (F1) connected to the distal end of the III FDS tendon;
8. Three 2-Newton weights attached to the FDS tendons;
9. Three 1-Newton weights attached to the FDP tendons;
10. External fixator to control the wrist angle;
11. Catheter with a three-stopcock;
12. Sphygmomanometer;
13. Syringe
The actuator pulled the FDP tendons proximally against the weight at a rate of 2.0 mm/sec. This movement of the tendon toward the actuator was regarded as flexion. The actuator movement was then reversed, causing the tendons to be pulled distally by the distal 2N load. This movement of the tendon toward the load was regarded as extension. In simulating making a fist motion, the excursion of the FDS tendon with the least excursion was used as the excursion for all three tendons. Therefore, during testing, all FDS tendons moved within a physiological range. The readings from the load transducers (F1, F2 in Figure 1) and the corresponding excursions were recorded by a computer workstation at a sampling rate of 20 Hz. After measurement of the baseline intact condition was done, a custom-made balloon was inserted beneath of the FDP tendons so that the interface between the retinaculum and FDS tendons was not interfered with and the balloon did not contact the FDS tendons. The proximal end of the balloon was connected by a catheter to a three-way stopcock (Terumo Terufusion Co., Ltd, Thailand). The other two ends of three-way stopcock were connected to a sphygmomanometer (Tycos, USA) and syringe. Saline solution was injected into the balloon to elevate the carpal tunnel pressure, which was measured by the sphygmomanometer. The FDS gliding resistance was measured in the following six conditions, 1) as a baseline, before the balloon insertion; 2) balloon with 0 mmHg pressure; 3) 30 mmHg; 4) 60 mmHg; 5) 90 mmHg; 6) 120 mmHg. All specimens were kept moist throughout testing by frequently spraying a saline mist on them.
Data Analysis
In each construct tested, the gliding resistance of the middle finger tendon was measured. Mean gliding resistance (MGR) was calculated based on the force values measured throughout the range of excursion by the following formula: (F2f-F1f) + (F1e-F2e)/2. As F1f = F1e = 2 Newton (the applied load), the MGR formula simplifies to (F2f–F2e)/2. The PGR was the measured peak force during flexion (F2f-F1f) (11).
Our sample size requirements were determined by a power calculation. Prior studies of gliding resistance of the extensor digitorum communis of the index finger at the wrist level (12) reported a normal gliding resistance of 0.06 +/−0.02 N in the wrist neutral position, while in 60° of wrist flexion, the friction increased to 0.17 +/−0.06 (12). Assuming similar variability in our data, a sample of 8 specimens would provide 80% power to detect a difference of 0.07 N between any two of the ten testing conditions (alpha = 0.05, beta = 0.20). This level of detection appeared to be reasonable from a clinical point of view.
Data obtained from the gliding tests were analyzed using one factor repeated ANOVA to assess whether there were measured differences among the different groups, followed by a Tukey-Kramer post-hoc test for individual comparisons. A p < 0.05 significance level was used in all cases.
RESULTS
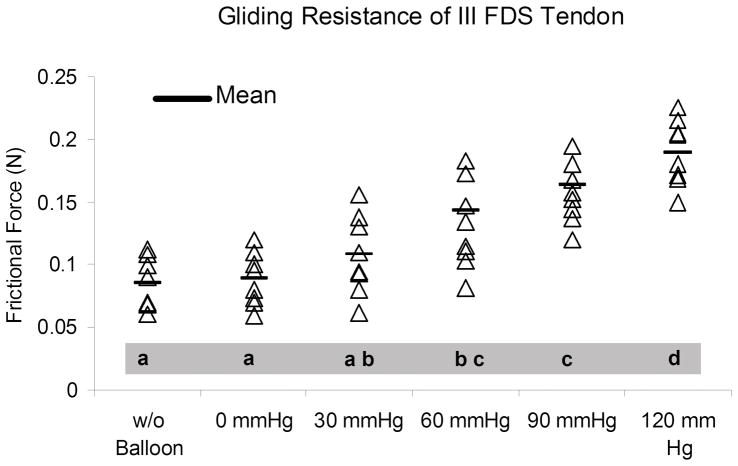
The mean gliding resistance of the middle FDS tendon at baseline and with the balloon set at 0 mmHg was significantly lower than that of the 60, 90 and 120 mmHg groups (p < 0.05). There was no significant difference among baseline, 0 and 30 mmHg groups, and no significant difference was also obtained between 60 and 90 mmHg groups. The mean gliding resistance of the 30 mmHg group was significantly lower than the 90 and 120 mmHg groups (p < 0.05). The mean gliding resistance in 120 mmHg was significantly higher than all the other groups (p < 0.05) (Figure 2).
Figure 2.
Mean gliding resistance of the middle FDS tendon in all six conditions. Different letter indicates significant difference (p < 0.05) (a < b < c < d. If a same letter appears, there is no significant difference. For example, there is no difference between a and ab, ab and bc, bc and c).
DISCUSSION
It is generally accepted that repetitive hand motion is one of the risk factors that evoke carpal tunnel syndrome, the most common compression neuropathy (7; 13–15). Subsynovial connective tissue (SSCT) fibrosis is often observed as a pathological feature in carpal tunnel syndrome (16–19). SSCT is the major structure surrounding the tendons and nerve within the carpal tunnel and forms a unique gliding environment (20–23).
While the etiology of CTS is usually unknown, one hypothesis (17) is that injury to the SSCT may be a cause, with subsequent changes in SSCT permeability (24) and stiffness (25) leading to progressive SSCT thickening, increased carpal tunnel pressure and, finally, carpal tunnel syndrome.
Given the possible etiology of the carpal tunnel syndrome described above, a thorough knowledge of the tendon kinematics in the carpal tunnel region becomes valuable. One recent study by Zhao et al. measured the gliding resistance of flexor tendons in this region and demonstrated that gliding resistance was increased with the wrist in flexion (26). Some clinical studies have shown that carpal tunnel pressure is also increased with wrist flexion (8; 27; 28). In the current study, we have demonstrated that the carpal tunnel pressure directly affects the gliding resistance of flexor tendons, i.e. the gliding resistance increases when the pressure is increased
Although an increase in carpal tunnel pressure is a characteristic of carpal tunnel syndrome, the increase in pressure varies according to hand motion, activity and position, in addition to the measurement methods chosen (10; 29–33). The carpal tunnel pressure in the patients with carpal tunnel syndrome typically exceeds a threshold of 30 mmHg (34–38), but levels of 100 mmHg or more can be attained at 90 degrees of wrist dorsiflexion (8). More recently, Goss and Agee have reported that carpel tunnel pressure could reach as much as 1000 mmHg with forceful gripping (39). The pressure increments in the current study therefore are clinically relevant. When the carpal tunnel pressure rose above 60 mm Hg, the gliding resistance was significantly increased compared to the baseline condition. These finding suggest that patients with elevated carpal tunnel pressure, as is found in patients with CTS, may suffer some impairment of tendon function, as well as the obvious nerve pathology.
There are several limitations to our study. A cadaver model cannot perfectly mimic in vivo conditions. Especially, the artificially increased pressure by balloon inflation is different from the in vivo situation, in which hydrostatic pressure builds up within a closed compartment and combines with complex soft tissue pathological changes, such as fibrosis, edema and tissue hypertrophy. Second, the gliding resistance of the FDP tendons and the other FDS tendons was not measured. The middle finger FDS tendon was chosen for the assessment because it is closest to the median nerve. Third, the gliding resistance was measured only in the wrist in neutral position with a 2N tension on the tendon. Other wrist positions or loads were not tested. The neutral position was selected because gliding resistance in the wrist neutral position is low, compared to wrist flexion or extension (26). We selected the a 2 N load as it is similar to the load recorded in vivo during unrestricted active finger motion (40). Increased loading, which may simulate different levels of forceful hand work, may present different gliding characteristics. Finally, the gliding resistance was measured with a relatively slow velocity and at room temperature. The effects of these non-physiological conditions are unknown.
In summary, this study revealed for the first time the relationship between flexion tendon gliding resistance and carpal tunnel pressure. We demonstrated that the middle finger FDS gliding resistance increased when the carpal tunnel pressure increased, especially when the pressure exceeded 60 mmHg, a pressure commonly noted in the CTS patient. Even this small amount of increased gliding resistance may affect tendon function over time. For example, patients with CTS may have to expend more energy to accomplish specific motions, which may in turn affect symptoms of hand pain, weakness and fatigue, seen commonly in such patients.
In conclusion, this study helps us to understand the relationship between carpal tunnel pressure, which is elevated in the patient with CTS and tendon gliding resistance, which is a component of the work of flexion. This understanding may in turn shed some light on the way in which tendon function might be compromised in patients with CTS.
Acknowledgments
This study was funded by grants from NIH (NIAMS AR49823) and Mayo Foundation.
References
- 1.Stevens JC. AAEM minimonograph #26: the electrodiagnosis of carpal tunnel syndrome. Muscle Nerve. 1997;1320(12):1164–8. 1477–1486. doi: 10.1002/(sici)1097-4598(199712)20:12<1477::aid-mus1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 2.Papanicolaou GD, McCabe SJ, Firrell J. The prevalence and characteristics of nerve compression symptoms in the general population. J Hand Surg [Am] 2001;26:460–466. doi: 10.1053/jhsu.2001.24972. [DOI] [PubMed] [Google Scholar]
- 3.Atroshi I, Gummesson C, Johnsson R, et al. Prevalence of carpal tunnel syndrome in a general population. JAMA. 1999;263282(2):236–7. 153–158. doi: 10.1001/jama.282.2.153. [DOI] [PubMed] [Google Scholar]
- 4.English CJ, Maclaren WM, Court-Brown C, et al. Relations between upper limb soft tissue disorders and repetitive movements at work. Am J Ind Med. 1995;1127(3):343–58. 75–90. doi: 10.1002/ajim.4700270108. [DOI] [PubMed] [Google Scholar]
- 5.Mackinnon SE, Novak CB. Repetitive strain in the workplace. J Hand Surg [Am] 1997;22:2–18. doi: 10.1016/S0363-5023(05)80174-1. [DOI] [PubMed] [Google Scholar]
- 6.Latko WA, Armstrong TJ, Franzblau A, et al. Cross-sectional study of the relationship between repetitive work and the prevalence of upper limb musculoskeletal disorders. Am J Ind Med. 1999;36:248–259. doi: 10.1002/(sici)1097-0274(199908)36:2<248::aid-ajim4>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 7.Szabo RM. Carpal tunnel syndrome as a repetitive motion disorder. Clin Orthop. 1998;120–6(427):78–89. [PubMed] [Google Scholar]
- 8.Gelberman RH, Hergenroeder PT, Hargens AR, et al. The carpal tunnel syndrome. A study of carpal canal pressures. J Bone Joint Surg [Am] 1981;63:380–383. [PubMed] [Google Scholar]
- 9.Okutsu I, Ninomiya S, Hamanaka I, et al. Measurement of pressure in the carpal canal before and after endoscopic management of carpal tunnel syndrome. J Bone Joint Surg [Am] 1989;71:679–683. [PubMed] [Google Scholar]
- 10.Sekiya H, Sugimoto N, Kariya Y, Hoshino Y. Carpal tunnel pressure in patients with carpal tunnel syndrome due to long-term hemodialysis. Int Orthop. 2002;26(5):274–277. doi: 10.1007/s00264-002-0366-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao C, Amadio PC, Zobitz ME, An KN. Gliding characteristics of tendon repair in canine flexor digitorum profundus tendons. J Orthop Res. 2001;19:580–586. doi: 10.1016/S0736-0266(00)00055-3. [DOI] [PubMed] [Google Scholar]
- 12.Kutsumi K, Amadio PC, Zhao C, et al. Gliding resistance of the extensor pollicis brevis tendon and abductor pollicis longus tendon within the first dorsal compartment in fixed wrist positions. J Orthop Res. 2005;23:243–248. doi: 10.1016/j.orthres.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 13.Morse LH. Repetitive motion musculoskeletal problems in the microelectronics industry. Occup Med. 1986;1:167–174. [PubMed] [Google Scholar]
- 14.Tanaka S, Wild DK, Seligman PJ, et al. Prevalence and work-relatedness of self-reported carpal tunnel syndrome among U.S. workers: analysis of the Occupational Health Supplement data of 1988 National Health Interview Survey. Am J Ind Med. 1995;27:451–470. doi: 10.1002/ajim.4700270402. [DOI] [PubMed] [Google Scholar]
- 15.Amadio PC. Repetitive stress injury. J Bone & Joint Surg [Am] 2001;83:136–137. doi: 10.2106/00004623-200101000-00018. discussion 138–141. [DOI] [PubMed] [Google Scholar]
- 16.Fuchs PC, Nathan PA, Myers LD. Synovial histology in carpal tunnel syndrome. J Hand Surg [Am] 1991;16:753–758. doi: 10.1016/0363-5023(91)90208-s. [DOI] [PubMed] [Google Scholar]
- 17.Lluch AL. Thickening of the synovium of the digital flexor tendons: cause or consequence of the carpal tunnel syndrome? J Hand Surg [Br] 1992;17:209–212. doi: 10.1016/0266-7681(92)90091-f. [DOI] [PubMed] [Google Scholar]
- 18.Chell J, Stevens A, Davis TR. Work practices and histopathological changes in the tenosynovium and flexor retinaculum in carpal tunnel syndrome in women. J Bone Joint Surg [Br] 1999;81:868–870. doi: 10.1302/0301-620x.81b5.9453. [DOI] [PubMed] [Google Scholar]
- 19.Ettema AM, Amadio PC, Zhao C, et al. A histological and immunohistochemical study of the subsynovial connective tissue in idiopathic carpal tunnel syndrome. J Bone Joint Surg [Am] 2004;86:1458–1466. doi: 10.2106/00004623-200407000-00014. [DOI] [PubMed] [Google Scholar]
- 20.Guimberteau JC. New ideas in hand flexor tendon surgery. Institut Aquitain De La Main; 2001. [Google Scholar]
- 21.Yoshii Y, Zhao C, Zhao KD, et al. The effect of wrist position on the relative motion of tendon, nerve, and subsynovial connective tissue within the carpal tunnel in a human cadaver model. J Orthop Res. 2008;26:1153–1158. doi: 10.1002/jor.20640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshii Y, Zhao C, Henderson J, et al. Effects of carpal tunnel release on the relative motion of tendon, nerve, and subsynovial connective tissue in a human cadaver model. Clin Biomech. 2008;23:1121–1127. doi: 10.1016/j.clinbiomech.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oh S, Belohlavek M, Zhao C, et al. Detection of differential gliding characteristics of the flexor digitorum superficialis tendon and subsynovial connective tissue using color Doppler sonographic imaging. J Ultrasound Med. 2007;26:149–155. doi: 10.7863/jum.2007.26.2.149. [DOI] [PubMed] [Google Scholar]
- 24.Osamura N, Zhao C, Zobitz ME, et al. Permeability of the subsynovial connective tissue in the human carpal tunnel: a cadaver study. Clin Biomech. 2007;22:524–528. doi: 10.1016/j.clinbiomech.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Osamura N, Zhao C, Zobitz ME, et al. Evaluation of the material properties of the subsynovial connective tissue in carpal tunnel syndrome. Clin Biomech. 2007;22:999–1003. doi: 10.1016/j.clinbiomech.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao C, Ettema AM, Osamura N, et al. Gliding characteristics between flexor tendons and surrounding tissues in the carpal tunnel: a biomechanical cadaver study. J Orthop Res. 2007;25:185–190. doi: 10.1002/jor.20321. [DOI] [PubMed] [Google Scholar]
- 27.Kumar K, Deshpande S, Jain M, Nayak MG. Evaluation of various fibro-osseous tunnel pressures (carpal, cubital and tarsal) in normal human subjects. Indian J Physiol Pharmacol. 1988;32:139–145. [PubMed] [Google Scholar]
- 28.MacDermid JC, Doherty T. Clinical and electrodiagnostic testing of carpal tunnel syndrome: a narrative review. J Orthop Sports Phys Ther. 2004;34:565–588. doi: 10.2519/jospt.2004.34.10.565. [DOI] [PubMed] [Google Scholar]
- 29.Luchetti R, Schoenhuber R, Nathan P. Correlation of segmental carpal tunnel pressures with changes in hand and wrist positions in patients with carpal tunnel syndrome and controls. J Hand Surg [Br] 1998;23:598–602. doi: 10.1016/s0266-7681(98)80009-0. [DOI] [PubMed] [Google Scholar]
- 30.Keir PJ, Bach JM, Rempel D. Effects of computer mouse design and task on carpal tunnel pressure. Ergonomics. 1999;42:1350–1360. doi: 10.1080/001401399184992. [DOI] [PubMed] [Google Scholar]
- 31.Okutsu I, Hamanaka I, Chiyokura Y, et al. Intraneural median nerve pressure in carpal tunnel syndrome. J Hand Surg [Br] 2001;26:155–156. doi: 10.1054/jhsb.2000.0534. [DOI] [PubMed] [Google Scholar]
- 32.Yoshida A, Okutsu I. Relationship of carpal canal contents volume to carpal canal pressure in carpal tunnel syndrome patients. J Hand Surg [Br] 2004;29:277–280. doi: 10.1016/j.jhsb.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 33.Fuller DA, Barrett M, Marburger RK, Hirsch R. Carpal canal pressures after volar plating of distal radius fractures. J Hand Surg [Br] 2006;31:236–239. doi: 10.1016/j.jhsb.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 34.Werner CO, Elmqvist D, Ohlin P. Pressure and nerve lesion in the carpal tunnel. Acta Orthop Scand. 1983;54:312–316. doi: 10.3109/17453678308996576. [DOI] [PubMed] [Google Scholar]
- 35.Luchetti R, Schoenhuber R, De Cicco G, et al. Carpal-tunnel pressure. Acta Orthop Scand. 1989;60:397–399. doi: 10.3109/17453678909149305. [DOI] [PubMed] [Google Scholar]
- 36.Peterson T, Dresing K, Schmidt G. [Measuring pressure in the carpal tunnel in distal radius fracture] Unfallchirurg. 1993;96:217–223. [PubMed] [Google Scholar]
- 37.Murata K, Yajima H, Maegawa N, et al. Investigation of segmental carpal tunnel pressure in patients with idiopathic carpal tunnel syndrome--is it necessary to release the distal aponeurotic portion of the flexor retinaculum in endoscopic carpal tunnel release surgery? Hand Surg. 2007;12:205–209. doi: 10.1142/S0218810407003559. [DOI] [PubMed] [Google Scholar]
- 38.Amadio PC, Amadio PC. What’s new in hand surgery. J Bone Joint Surg [Am] 2009;91:496–502. doi: 10.2106/JBJS.H.01697. [DOI] [PubMed] [Google Scholar]
- 39.Goss BC, Agee JM. Dynamics of intracarpal tunnel pressure in patients with carpal tunnel syndrome (CTS). 63rd Annual Meeting of the American Society for Surgery of the Hand (ASSH) Abstract Clinical Paper 06; Sep, 2008. < http://www.assh.org/Professionals/Education/AnnualMeeting/archive/Documents/draft03.pdf>. [DOI] [PubMed] [Google Scholar]
- 40.Schuind F, Garcia-Elias M, Cooney WP, 3rd, An KN. Flexor tendon forces: in vivo measurements. J Hand Surg [Am] 1992;17:291–298. doi: 10.1016/0363-5023(92)90408-h. [DOI] [PubMed] [Google Scholar]