Abstract
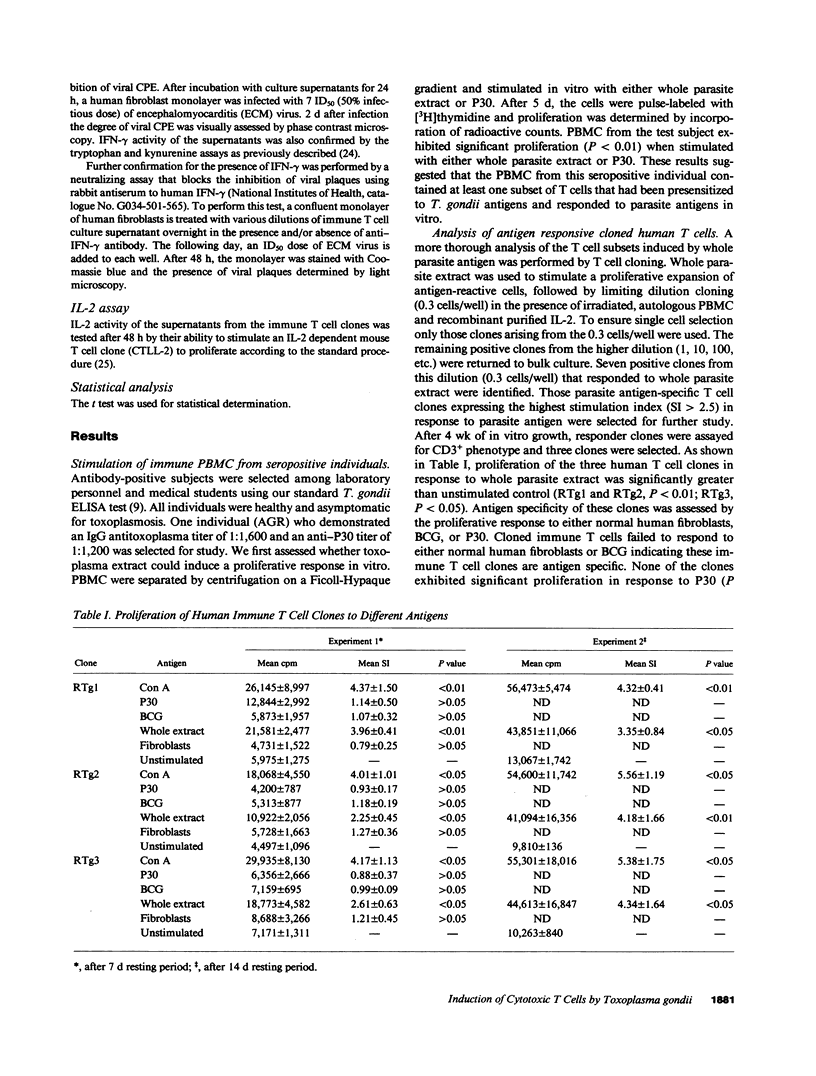
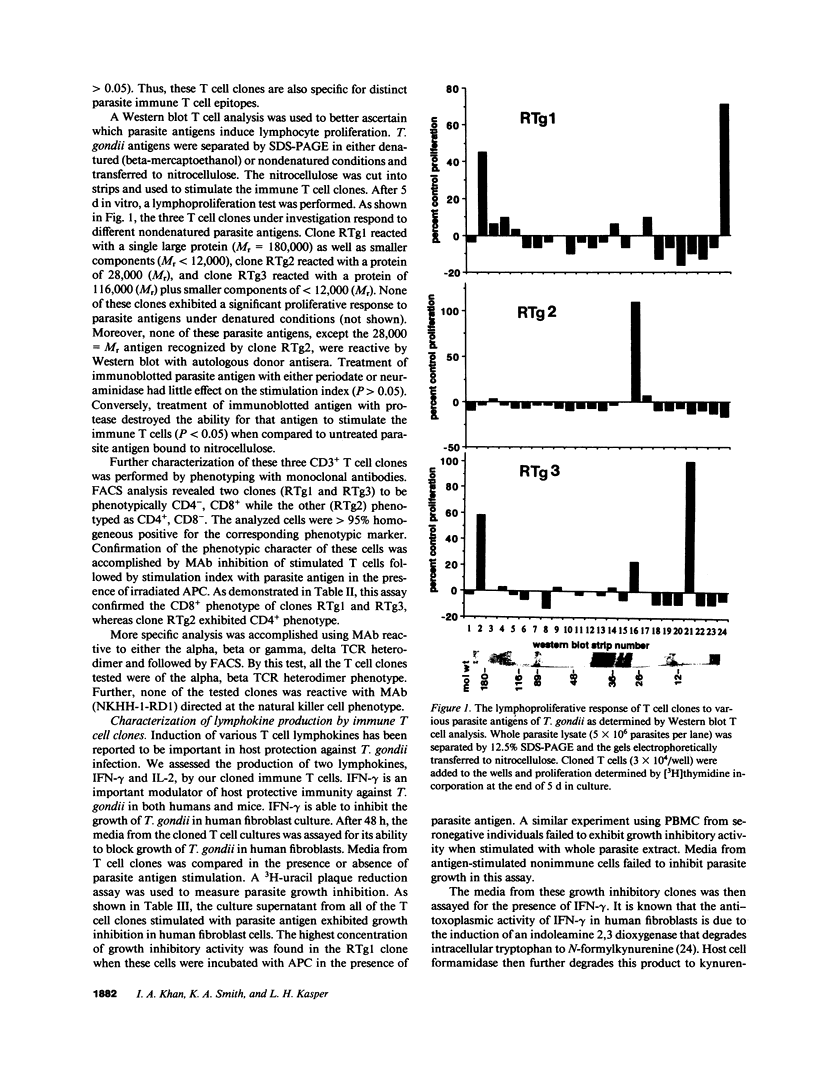
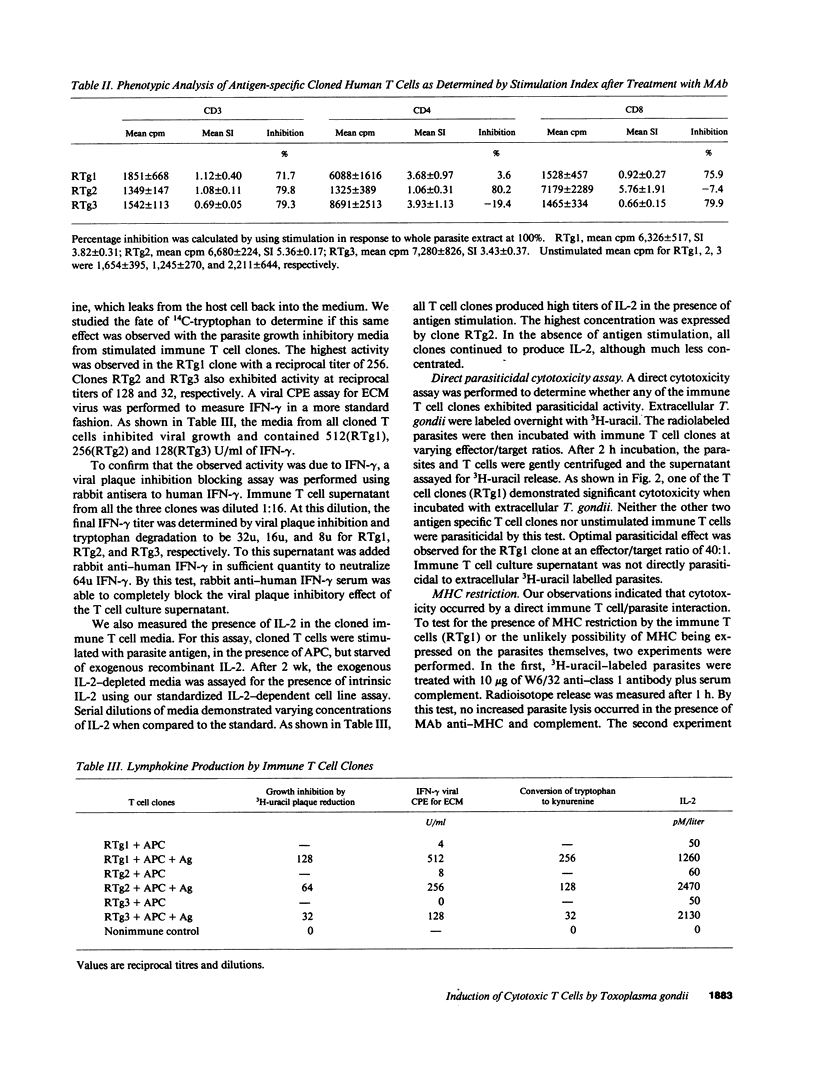
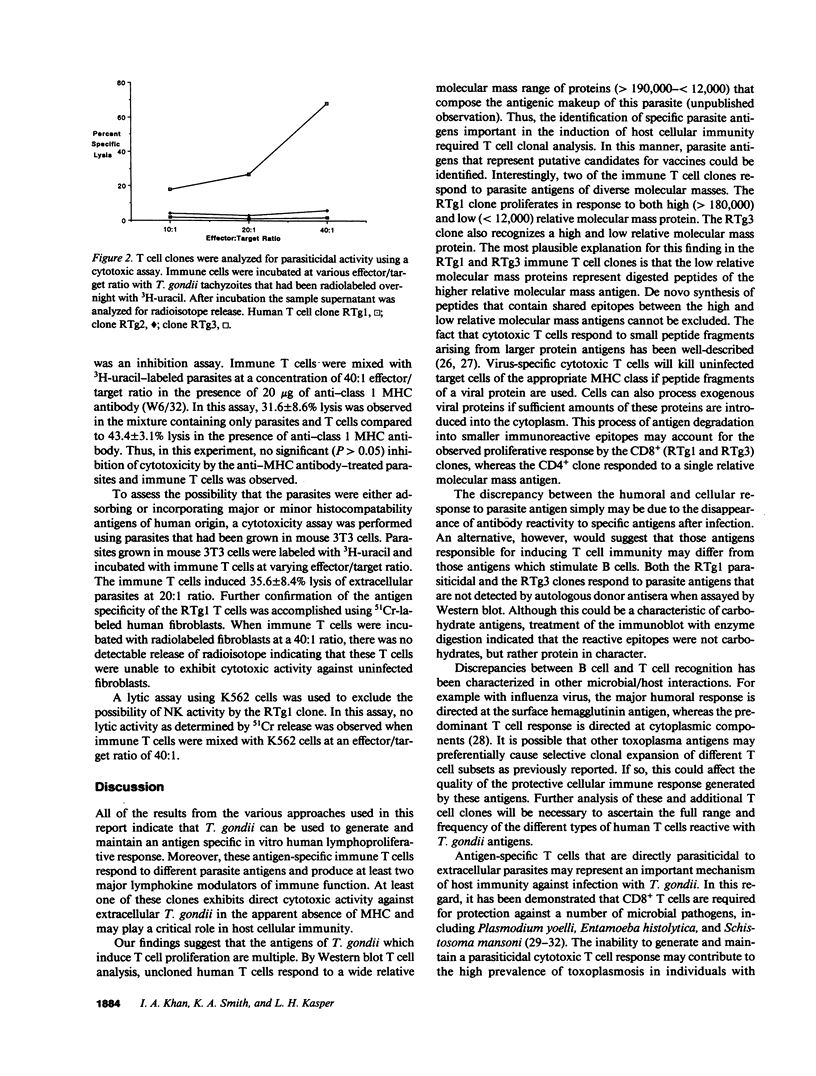
To further the understanding of the role of T cells in immunity to the parasite Toxoplasma gondii, antigen-specific T cell clones were generated using peripheral blood mononuclear cells from seropositive individuals. Whole parasites were used to stimulate a proliferative expansion of antigen-reactive cells, followed by limiting dilution cloning in the presence of irradiated, autologous PBMC and rIL-2. Three parasite antigen-specific T cell clones expressing the CD3+ phenotype were selected for further characterization. Phenotypic analysis with monoclonal antibodies revealed two clones reactive with CD8 (RTg1 and RTg3) while the other (RTg2) phenotyped as CD4+, CD8-. When tested in a proliferation assay using a panel of different T. gondii proteins, clone RTg1 reacted with a single large protein (Mr greater than 180,000) as well as smaller components (less than 12,000), clone RTg2 reacted with a protein of Mr = 28,000 and clone RTg3 reacted with a protein of 116,000 plus smaller components (less than 12,000). Only the 28,000 = Mr antigen recognized by RTg2 was reactive on Western blot with autologous donor antisera. All three clones produced IFN-gamma and IL-2 in varying amounts upon antigenic stimulation in the presence of irradiated APC. Moreover, one clone RTg1, exhibited direct parasite cytotoxicity, inhibiting extracellular T. gondii by greater than 70% when incubated at an effector/target ratio of 40:1. This clone was alpha, beta TCR heterodimer positive and exerted its cytotoxic parasiticidal activity in the apparent absence of MHC restriction. The results provide evidence for the existence of circulating antigen-specific cytotoxic T cells in normal humans who are toxoplasma antibody seropositive.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anichini A., Ress S., Strassmann G., Bach F. H. Inhibition of anti-class I cytotoxicity by anti-class II monoclonal antibodies (MoAb). II. Blocking of anti-class I CTL clones by anti-DR MoAb. Hum Immunol. 1985 Jun;13(2):139–143. doi: 10.1016/0198-8859(85)90020-5. [DOI] [PubMed] [Google Scholar]
- Durandy A., Fischer A., Charron D., Griscelli C. Specific binding of antigen onto human T lymphocytes. J Clin Invest. 1986 May;77(5):1557–1564. doi: 10.1172/JCI112471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellner J. J., Olds G. R., Lee C. W., Kleinhenz M. E., Edmonds K. L. Destruction of the multicellular parasite Schistosoma mansoni by T lymphocytes. J Clin Invest. 1982 Aug;70(2):369–378. doi: 10.1172/JCI110626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser W. E., Jr, Sharma S. D., Remington J. S. Augmentation of NK cell activity by soluble and particulate fractions of Toxoplasma gondii. J Immunol. 1983 Jul;131(1):458–463. [PubMed] [Google Scholar]
- Hauser W. E., Jr, Sharma S. D., Remington J. S. Natural killer cells induced by acute and chronic toxoplasma infection. Cell Immunol. 1982 May 15;69(2):330–346. doi: 10.1016/0008-8749(82)90076-4. [DOI] [PubMed] [Google Scholar]
- Hauser W. E., Jr, Tsai V. Acute toxoplasma infection of mice induces spleen NK cells that are cytotoxic for T. gondii in vitro. J Immunol. 1986 Jan;136(1):313–319. [PubMed] [Google Scholar]
- Kamiyama T., Hagiwara T. Augmented followed by suppressed levels of natural cell-mediated cytotoxicity in mice infected with Toxoplasma gondii. Infect Immun. 1982 May;36(2):628–636. doi: 10.1128/iai.36.2.628-636.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper L. H., Crabb J. H., Pfefferkorn E. R. Purification of a major membrane protein of Toxoplasma gondii by immunoabsorption with a monoclonal antibody. J Immunol. 1983 May;130(5):2407–2412. [PubMed] [Google Scholar]
- Kees U., Krammer P. H. Most influenza A virus-specific memory cytotoxic T lymphocytes react with antigenic epitopes associated with internal virus determinants. J Exp Med. 1984 Feb 1;159(2):365–377. doi: 10.1084/jem.159.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan I. A., Eckel M. E., Pfefferkorn E. R., Kasper L. H. Production of gamma interferon by cultured human lymphocytes stimulated with a purified membrane protein (P30) from Toxoplasma gondii. J Infect Dis. 1988 May;157(5):979–984. doi: 10.1093/infdis/157.5.979. [DOI] [PubMed] [Google Scholar]
- Khan I. A., Smith K. A., Kasper L. H. Induction of antigen-specific parasiticidal cytotoxic T cell splenocytes by a major membrane protein (P30) of Toxoplasma gondii. J Immunol. 1988 Nov 15;141(10):3600–3605. [PubMed] [Google Scholar]
- Kumar S., Miller L. H., Quakyi I. A., Keister D. B., Houghten R. A., Maloy W. L., Moss B., Berzofsky J. A., Good M. F. Cytotoxic T cells specific for the circumsporozoite protein of Plasmodium falciparum. Nature. 1988 Jul 21;334(6179):258–260. doi: 10.1038/334258a0. [DOI] [PubMed] [Google Scholar]
- Lamb J. R., Young D. B. A novel approach to the identification of T-cell epitopes in Mycobacterium tuberculosis using human T-lymphocyte clones. Immunology. 1987 Jan;60(1):1–5. [PMC free article] [PubMed] [Google Scholar]
- Levy R. M., Bredesen D. E., Rosenblum M. L. Neurological manifestations of the acquired immunodeficiency syndrome (AIDS): experience at UCSF and review of the literature. J Neurosurg. 1985 Apr;62(4):475–495. doi: 10.3171/jns.1985.62.4.0475. [DOI] [PubMed] [Google Scholar]
- Luft B. J., Kansas G., Engleman E. G., Remington J. S. Functional and quantitative alterations in T lymphocyte subpopulations in acute toxoplasmosis. J Infect Dis. 1984 Nov;150(5):761–767. doi: 10.1093/infdis/150.5.761. [DOI] [PubMed] [Google Scholar]
- Luft B. J., Pedrotti P. W., Engleman E. G., Remington J. S. Induction of antigen-specific suppressor T cells during acute infection with Toxoplasma gondii. J Infect Dis. 1987 May;155(5):1033–1037. doi: 10.1093/infdis/155.5.1033. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Rubin B. Y., Masur H., Roberts R. B. Impaired production of lymphokines and immune (gamma) interferon in the acquired immunodeficiency syndrome. N Engl J Med. 1984 Apr 5;310(14):883–889. doi: 10.1056/NEJM198404053101404. [DOI] [PubMed] [Google Scholar]
- Pfefferkorn E. R., Guyre P. M. Inhibition of growth of Toxoplasma gondii in cultured fibroblasts by human recombinant gamma interferon. Infect Immun. 1984 May;44(2):211–216. doi: 10.1128/iai.44.2.211-216.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferkorn E. R., Rebhun S., Eckel M. Characterization of an indoleamine 2,3-dioxygenase induced by gamma-interferon in cultured human fibroblasts. J Interferon Res. 1986 Jun;6(3):267–279. doi: 10.1089/jir.1986.6.267. [DOI] [PubMed] [Google Scholar]
- Salata R. A., Cox J. G., Ravdin J. I. The interaction of human T-lymphocytes and Entamoeba histolytica: killing of virulent amoebae by lectin-dependent lymphocytes. Parasite Immunol. 1987 Mar;9(2):249–261. doi: 10.1111/j.1365-3024.1987.tb00504.x. [DOI] [PubMed] [Google Scholar]
- Salata R. A., Martinez-Palomo A., Murray H. W., Conales L., Trevino N., Segovia E., Murphy C. F., Ravdin J. I. Patients treated for amebic liver abscess develop cell-mediated immune responses effective in vitro against Entamoeba histolytica. J Immunol. 1986 Apr 1;136(7):2633–2639. [PubMed] [Google Scholar]
- Sethi K. K., Brandis H. Characteristics of soluble T-cell derived factors(s) which can induce non-immune murine macrophages to exert anti-Toxoplasma activity. Z Immunitatsforsch Immunobiol. 1978 Jun;154(3):226–242. [PubMed] [Google Scholar]
- Sharma S. D., Hofflin J. M., Remington J. S. In vivo recombinant interleukin 2 administration enhances survival against a lethal challenge with Toxoplasma gondii. J Immunol. 1985 Dec;135(6):4160–4163. [PubMed] [Google Scholar]
- Shirahata T., Shimizu K. Production and properties of immune interferon from spleen cell cultures of Toxoplasma-infected mice. Microbiol Immunol. 1980;24(11):1109–1120. doi: 10.1111/j.1348-0421.1980.tb02915.x. [DOI] [PubMed] [Google Scholar]
- Shirahata T., Shimizu K., Suzuki N. Effects of immune lymphocyte products and serum antibody on the multiplication of Toxoplasma in murine peritoneal macrophages. Z Parasitenkd. 1976 Mar 31;49(1):11–23. doi: 10.1007/BF00445014. [DOI] [PubMed] [Google Scholar]
- Siliciano R. F., Hemesath T. J., Pratt J. C., Dintzis R. Z., Dintzis H. M., Acuto O., Shin H. S., Reinherz E. L. Direct evidence for the existence of nominal antigen binding sites on T cell surface Ti alpha-beta heterodimers of MHC-restricted T cell clones. Cell. 1986 Oct 24;47(2):161–171. doi: 10.1016/0092-8674(86)90439-3. [DOI] [PubMed] [Google Scholar]
- Sklenar I., Jones T. C., Alkan S., Erb P. Association of symptomatic human infection with Toxoplasma gondii with imbalance of monocytes and antigen-specific T cell subsets. J Infect Dis. 1986 Feb;153(2):315–324. doi: 10.1093/infdis/153.2.315. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Orellana M. A., Schreiber R. D., Remington J. S. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science. 1988 Apr 22;240(4851):516–518. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- Townsend A. R., Rothbard J., Gotch F. M., Bahadur G., Wraith D., McMichael A. J. The epitopes of influenza nucleoprotein recognized by cytotoxic T lymphocytes can be defined with short synthetic peptides. Cell. 1986 Mar 28;44(6):959–968. doi: 10.1016/0092-8674(86)90019-x. [DOI] [PubMed] [Google Scholar]
- Weiss W. R., Sedegah M., Beaudoin R. L., Miller L. H., Good M. F. CD8+ T cells (cytotoxic/suppressors) are required for protection in mice immunized with malaria sporozoites. Proc Natl Acad Sci U S A. 1988 Jan;85(2):573–576. doi: 10.1073/pnas.85.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yewdell J. W., Bennink J. R., Hosaka Y. Cells process exogenous proteins for recognition by cytotoxic T lymphocytes. Science. 1988 Feb 5;239(4840):637–640. doi: 10.1126/science.3257585. [DOI] [PubMed] [Google Scholar]



