Abstract
Elevated maternal plasma concentrations of homocysteine (Hcy) are associated with pregnancy complications and adverse neonatal outcomes. The postulate that we wish to advance here is that placental transport of Hcy, by competing with endogenous amino acids for transporter activity, may account for some of the damaging impacts of Hcy on placental metabolism and function as well as fetal development. In this article, we provide an overview of some recent studies characterising the transport mechanisms for Hcy across the microvillous plasma membrane (MVM) of the syncytiotrophoblast, the transporting epithelium of human placenta. Three Hcy transport systems have been identified, systems L, A and y+L. This was accomplished using a strategy of competitive inhibition to investigate the effects of Hcy on the uptake of well-characterised radiolabelled substrates for each transport system into isolated MVM vesicles. The reverse experiments were also performed, examining the effects of model substrates on [35S]L-Hcy uptake. This article describes the evidence for systems L, A and y+L involvement in placental Hcy transport and discusses the physiological implications of these findings with respect to placental function and fetal development.
Homocysteine in pregnancy and placental metabolism
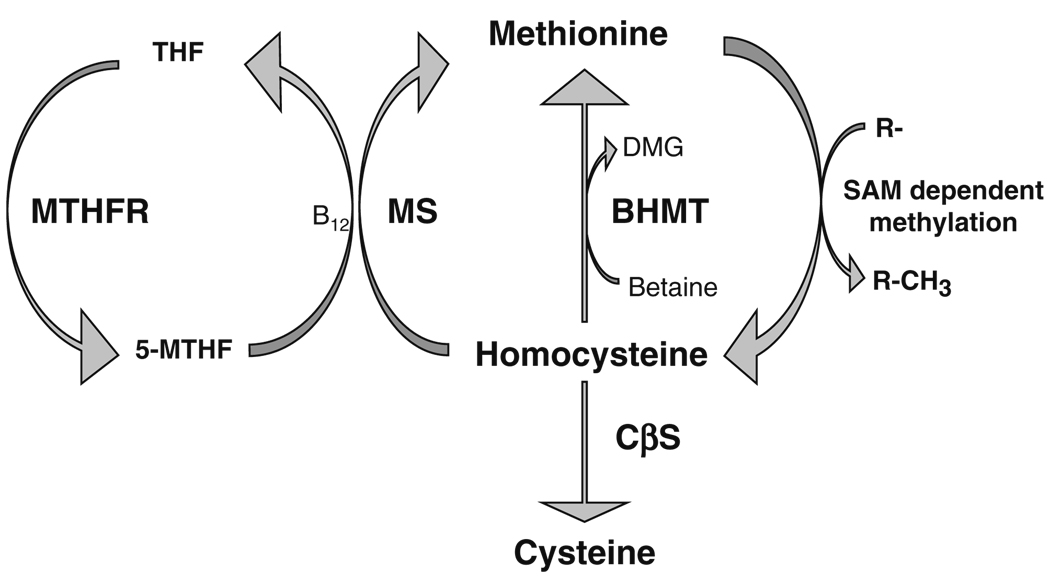
Homocysteine (Hcy), a thiol group-containing amino acid, is a metabolite generated by the methionine cycle (Selhub 1999: fig. 1). Once formed, there are three potential metabolic pathways available to Hcy as shown in Fig. 1: (1) remethylation to methionine using 5-methyltetrahydrofolate as methyl donor catalysed by the action of methionine synthase, (2) remethylation by betaine to generate methionine and dimethylglycine catalysed by betaine-homocysteine methyl-transferase (BHMT), or (3) transsulphuration to cysteine catalysed by cystathionine β-synthase (CβS). The relative expression and activity of these Hcy-metabolising enzymes in different tissues may be important in determining the scope of the cellular effects of Hcy (Finkelstein 1998). Interestingly, in human placenta, only one metabolic pathway seems to be prevalent. We have recently shown that the major metabolic pathway for Hcy metabolism in this tissue is likely to be the re-methylation of Hcy to methionine, as evidenced by the very low mRNA expression of CβS in human placenta, undetectable CβS and cystathionase activities and the absence of BHMT gene expression in this tissue (Gaull et al. 1972; Solanky et al. 2010).
Fig. 1.
Homocysteine metabolic pathways. Homocysteine can be metabolised by three metabolic pathways in tissues. (1) Remethylation to methionine using 5-methyltetrahydrofolate (5-MTHF) as methyl donor, catalysed by the vitamin B12-dependent action of methionine synthase (MS). THF is then re-cycled to form 5-MTHF, catalysed by 5,10-methylene tetrahydrofolate reductase (MTHFR). Methionine then acts as the precursor for the ATP-dependent synthesis of the primary methyl donor S-adenosylmethionine (SAM) which is involved in various methylation reactions, producing homocysteine and methylated acceptor(s). (2) Betaine can be utilized as an alternative methyl donor to generate methionine and dimethylglycine (DMG), catalysed by betaine-homocysteine methyltransferase (BHMT). (3) Homocysteine can enter the transsulphuration pathway being converted to cysteine through the action of cystathionine β-synthase (CβS)
Implicit in these observations is the concept that metabolism of Hcy in human placenta will be highly dependent on cellular folate availability, utilised by the vitamin B12-dependent enzyme methionine synthase as a substrate to catalyse the conversion of Hcy to methionine (Fig. 1). Maternal plasma Hcy concentration is influenced by maternal folate and vitamin B12 status (Malinow et al. 1998; Murphy et al. 2004; Refsum 2001), with an inverse relationship between these variables (Malinow et al. 1998; Molloy et al. 2002; Murphy et al. 2004; Yajnik et al. 2005). Raised maternal plasma concentrations of Hcy, whether arising from a maternal deficiency of these micronutrients, or perhaps a genetic susceptibility in the enzymes responsible for Hcy metabolism (Refsum et al. 1998; Selhub 1999), is associated with various vascular-related complications of pregnancy. These include pre-eclampsia, placental abruption, recurrent pregnancy loss, fetal growth restriction (FGR) and stillbirth (de la Calle et al. 2003; Ray and Laskin 1999; Vollset et al. 2000) as well as adverse outcomes for the baby such as neural tube defects and other congenital malformations (de la Calle et al. 2003; Vollset et al. 2000). As might be predicted, supplementation with folic acid and B group vitamins has proved beneficial in lowering maternal Hcy and improving pregnancy outcome (de la Calle et al. 2003), most notably in reducing the frequency of neural tube defects (MRC Vitamin Study Research Group 1991).
These phenomena strongly implicate the methionine cycle as a key metabolic locus of crucial importance for fetal development; a concept endorsed by the early embryonic lethality in mice mutants homozygous for methionine synthase gene ablation (Swanson et al. 2001) and the importance of methylation events in epigenetic modifications that occur during embryonic development (Dean et al. 2005). Perturbation of this metabolic pathway in placenta is consequently likely to have multiple influences, affecting both placental development and DNA methylation, with significant impacts on embryogenesis and fetal development (Kim et al. 2009; Pickell et al. 2009; Solanky et al. 2010).
During pregnancy, the maternal plasma concentration of total Hcy (tHcy), comprising reduced and oxidised forms of Hcy, is lowered to reach a nadir at about 20 weeks in the second trimester. This decrease occurs independently of folic acid supplementation (Murphy et al. 2002, 2004) leading to the suggestion that this is a physiological adaptation to pregnancy, perhaps modulated by endocrine factors (Murphy et al. 2002). Others concur with this view, speculating that this trend may assist in the regulation of haemostasis (Holmes 2003). Maternal tHcy is higher than fetal plasma tHcy concentration and is a major determinant of the latter, with a significant, positive correlation between these two variables (Malinow et al. 1998; Molloy et al. 2002; Murphy et al. 2004). Importantly, the concentration of maternal tHcy at pre-conception and throughout pregnancy is significantly correlated with fetal cord blood tHcy concentration measured at birth (Murphy et al. 2004). The parallelism between maternal and fetal tHcy concentrations could most simplistically be explained if Hcy was transported across the placental exchange barrier (Fig. 2). Such a concept is strengthened by the evidence that there is a decreasing concentration gradient of plasma tHcy from maternal vein to umbilical vein to umbilical artery (Malinow et al. 1998). Although this could reflect transfer of Hcy across the placenta by diffusion down its electrochemical gradient through the paracellular pathway (Sibley 2009), carrier-mediated transport mechanisms may also play an important role, as they do for amino acids. This premise is founded on the observation that fetal cord tHcy concentrations, whilst significantly related to maternal tHcy concentrations, are differentially influenced by maternal folate and vitamin B12 status (Molloy et al. 2002), suggesting the existence of a route of transplacental transfer for Hcy that is regulated, most plausibly a transcellular pathway.
Fig. 2.
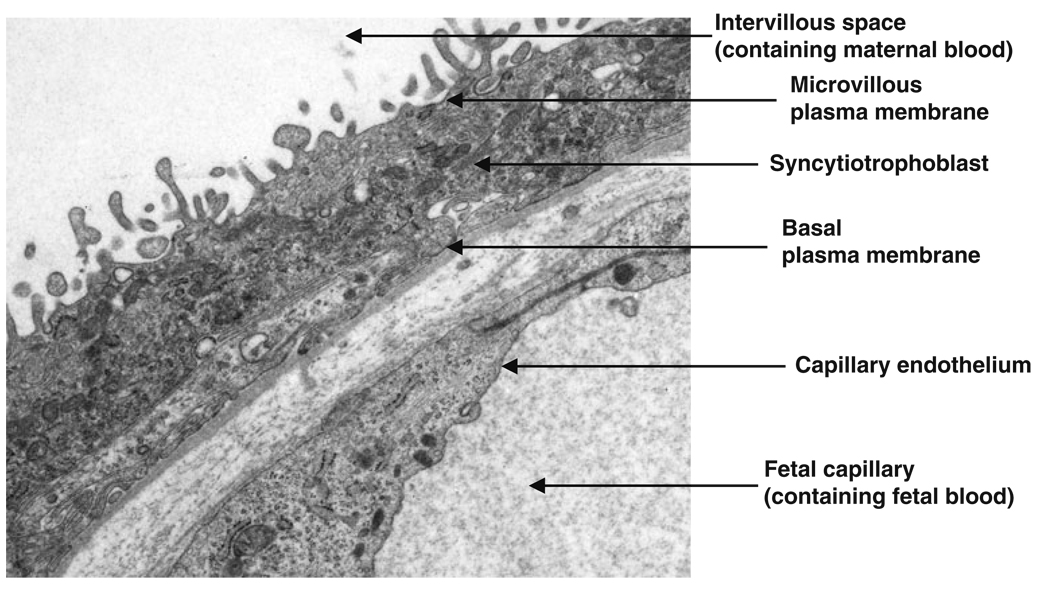
The exchange barrier of human placenta. Electron micrograph showing the two cellular layers involved in transplacental exchange: the transporting epithelium of the syncytiotrophoblast and fetal capillary endothelium. The syncytiotrophoblast has two plasma membranes, a maternal-facing microvillous plasma membrane which is in direct contact with maternal blood in the intervillous space and a basal plasma membrane which faces the fetal capillary containing fetal blood. Image kindly supplied by Dr Carolyn Jones
In human placenta, maternofetal exchange occurs across two cellular layers separating maternal and fetal blood: a transporting epithelium, the syncytiotrophobast, and endothelium lining the fetal capillary (Fig. 2). The latter forms an extensive microvascular bed of continuous endothelial cells with intercellular spaces that restrict permeability to large hydrophilic solutes, but is not thought to be restrictive to relatively small solutes such as amino acids (Firth and Leach 1996). The primary barrier to maternofetal exchange of amino acids therefore resides in the properties of the maternal-facing microvillous plasma membrane (MVM) and fetal-facing basal plasma membrane (BM) of the syncytiotrophoblast (Fig. 2). Different classes of amino acid transporters are distributed to these syncytiotrophoblast plasma membranes and mediate amino acid transport, providing amino acids for fetal metabolism and biosynthetic processes as well as fetal protein accretion (Cleal and Lewis 2008; Jansson 2001).
With this knowledge, and in the light of the maternal-fetal Hcy interrelationships detailed above, some fundamental questions need to be addressed: when the concentration of tHcy is elevated in maternal plasma resulting in hyper-homocysteinaemia (HHcy), what is happening at the placental interface? Is Hcy transported by the placenta? If so, what are the mechanisms? We have recently been investigating some of these issues (Tsitsiou et al. 2009), and this article presents an overview of our current findings, and their potential impact is discussed in the context of placental function and fetal development.
Placental transport of homocysteine
Based on the biochemical similarity of L-Hcy to L-methionine, our initial speculation was that these two amino acids would share common transport mechanisms mediated by amino acid transporters intrinsic to the syncytiotrophoblast plasma membranes of human placenta. To elucidate the mechanisms involved, our experimental approach was, therefore, to study three candidate transport systems which (1) utilise L-methionine as a well-characterised substrate, (2) whose transport characteristics are well defined, and (3) are known to be functionally active in human placenta (Cleal and Lewis 2008; Tsitsiou et al. 2009). As shown in Table 1, the neutral amino acid transporters systems L, A and y+L fulfil these criteria.
Table 1.
Transport mechanisms for L-methionine distributed to both the microvillous plasma membrane and the basal plasma membrane of human placenta
| Transporter | System A | System L | System y+L |
|---|---|---|---|
| Characteristics and mode of amino acid transport |
Na+-dependent co-transporter | Na+-independent exchanger | Na+-independent (cationic amino acids) and Na+-dependent (neutral amino acids) exchanger |
| Amino acid preference |
Small, short chain neutral amino acids |
Neutral amino acids with bulky side chains |
Both neutral and cationic amino acids |
| Molecular components |
Monomeric with three isoforms: SNAT1 (SLC38A1) SNAT2 (SLC38A2) SNAT4 (SLC38A4) |
Heterodimeric comprising CD98 linked to either of two light chain variants: LAT1 (SLC7A5) or LAT2 (SLC7A8) |
Heterodimeric comprising CD98 linked to either of two light chain variants: y+LAT1 (SLC7A7) or y+LAT2 (SLC7A6) |
| Amino acid specificity |
Alanine, glycine, serine, MeAIB, methionine |
Leucine, isoleucine, valine, phenylalanine, BCH, methionine |
Arginine, lysine, leucine, glutamine, methionine |
These three amino acid transporters are localised to both MVM and BM of human placenta (Cleal and Lewis 2008; Jansson 2001). System A is a monomeric transporter comprising three highly homologous isoforms of the sodium-coupled neutral amino acid transporter (SNAT) family (Table 1). In contrast, systems L and y+L form heterodimeric complexes consisting of a heavy chain, CD98, linked to light chain variants which confer functional activity and substrate specificity (Table 1). Whilst CD98 is found localised to both MVM and BM (Ayuk et al. 2000), the distribution of the LAT1 light chain only is well described, being particularly enriched in MVM (Okamoto et al. 2002). Progress in defining the distribution of the other light chains in human placenta has really been hampered by poor antibody specificity to these polytopic membrane proteins.
Our hypothesis was, therefore, that these three transport mechanisms, systems L, A and y+L, would support Hcy as a substrate. To test this hypothesis, we focussed on the first plasma membrane barrier to maternofetal amino acid transfer, MVM of the syncytiotrophoblast (Fig. 2). This plasma membrane can be isolated in a relatively pure form by magnesium precipitation and differential centrifugation, and once encouraged to vesiculate, by application of a shear force to form intact vesicles, provides a useful model of enclosed plasma membrane vesicles across which transport mechanisms can be characterised. This is most commonly achieved using radiolabelled tracers placed in the extra-vesicular buffer to measure influx across the plasma membrane or within the intravesicular space to measure efflux mechanisms (Glazier et al. 1988; Glazier and Sibley 2006). Hence, we employed MVM plasma membrane vesicles, isolated from term, human placenta as a model to investigate whether (1) unlabelled L-Hcy inhibited the uptake of well-characterised radiolabelled substrates for each transport system and (2) unlabelled model substrates for each transport system inhibited radiolabelled L-Hcy uptake. The ability of L-Hcy to inhibit uptake of amino acid substrate into MVM vesicles and vice versa was taken as evidence that L-Hcy shared a common transport pathway (Tsitsiou et al. 2009).
Placental transport mechanisms for homocysteine
System L
For system L, an amino acid exchanger, [35S]L-methionine was used as a model substrate for this transporter (Ganapathy et al. 1986; Johnson and Smith 1988). A concentration-dependent inhibition of substrate uptake by L-Hcy was observed, comparable in magnitude to the classic system L inhibitor 2-aminobicyclo[2.2.1]heptane-2-carboxylic acid (BCH) as well as other model substrates (Tsitsiou et al. 2009). In the reciprocal experiment, BCH significantly inhibited radiolabelled [35S]L-Hcy uptake in a dose-dependent manner, achieving a maximal inhibition of ~69% with 20 mM BCH (Tsitsiou et al. 2009). Together these data strongly supported the concept that system L transports L-Hcy across the MVM. It is not possible to delineate whether such Hcy transport is LAT1- or LAT2-mediated as L-methionine is a preferred substrate for both LAT1 and LAT2 light chains (Kanai et al. 1998; Meier et al. 2002; Pineda et al. 1999) and there is evidence of both activities in MVM (Kudo and Boyd 2001; Lewis et al. 2007). The affinity of these two light chains for the same amino acid can be dissimilar (Rossier et al. 1999). It is interesting that such a high proportion of Hcy transport appears to be utilising a system L-mediated mechanism in MVM, as this transporter also appears to make a major contribution to cellular Hcy uptake in several other cell types (Büdy et al. 2006; Ewadh et al. 1990; Jiang et al. 2007; Limpach et al. 2000; Naggar et al. 2003).
As an amino acid exchanger, system L mediates both the uptake of amino acids into the placenta across MVM as well as efflux of amino acids across BM into the fetal circulation (Cleal and Lewis 2008). This property of system L at the BM locus therefore has the capacity to qualitatively alter the composition of fetal plasma by exchanging syncytiotrophoblast amino acids for those in fetal plasma (Cleal et al. 2007; Cleal and Lewis 2008). However, mechanisms additional to system L also transport L-leucine across this plasma membrane (Cleal et al. 2007). The identity of these remain to be fully elucidated, but it has been postulated that these comprise non-exchange mechanisms; facilitated diffusion mechanisms governed by the substrate concentration gradient generated within the syncytiotrophoblast and postulated to be mediated by TAT1 (system T) and/or LAT4 (Cleal et al. 2007). LAT4 seems a particularly appealing candidate to engage in L-Hcy transport at the BM locus based on the studies described here, as it supports L-methionine and BCH, in addition to L-leucine, as substrates (Bodoy et al. 2005; Cleal and Lewis 2008). Thus, the balance of L-Hcy influx across MVM, syncytiotrophoblast production and metabolism of L-Hcy, and efflux of L-Hcy across BM will be important determinants in the delivery of L-Hcy to the fetus.
System A
One accumulative amino acid transporter that plays a key role in mediating the influx of neutral amino acids into the syncytiotrophoblast, driven by the inward Na+-gradient across MVM, is system A. To investigate whether system A is implicated in L-Hcy uptake across MVM we utilised 14C-labelled α-(methylamino)isobutyric acid (MeAIB) as a well-characterised and paradigm substrate for this transport mechanism (Mackenzie and Erickson 2004). 14C-MeAIB has been extensively used to measure system A activity in placental MVM vesicles (Desforges et al. 2009; Jansson 2001; Jansson et al. 2002; Johnson and Smith 1988; Mahendran et al. 1993). Uptake of 14C-MeAIB at initial rate was inhibited in a concentration-dependent manner by L-Hcy, in a manner similar to L-methionine serving as a positive control (Tsitsiou et al. 2009). At high concentration (20 mM), the magnitude of inhibition of 14C-MeAIB uptake by L-Hcy was comparable to that of other model substrates (Tsitsiou et al. 2009). In the reverse experiment, a high concentration of MeAIB (20mM) inhibited [35S]L-Hcy uptake by ~21% and exhibited Na+-dependency (Tsitsiou et al. 2009). Collectively, these observations are consistent with the notion that system A is involved in the uptake of L-Hcy by the placenta.
From these observations, it was not possible to elucidate which SNAT isoform is involved in the transport of L-Hcy across MVM. Immunolocalisation studies have demonstrated that all three SNAT isoforms, SNAT1, 2 and 4, are localised to MVM (Desforges et al. 2006), allowing for the possibility that more than one SNAT isoform contributes. We favour the involvement of SNAT1 and/or SNAT2 based on their relatively high affinity for L-methionine and their markedly higher affinity for MeAIB as compared to SNAT4 (Hatanaka et al. 2000, 2001; Wang et al. 2000). As for other transporter isoforms exhibiting a high degree of homology, dissection of their relative contribution is difficult due to their overlapping functional properties. It is of interest that L-Hcy transport across MVM of human placenta utilises system A as this phenomenon is apparent in other cell types (Büdy et al. 2006; Hultberg 2004; Naggar et al. 2003), but this trend is not found ubiquitously (Ewadh et al. 1990; Jiang et al. 2007).
System y+L
Another Na+-dependent amino acid transporter involved in the influx of neutral amino acids into the syncytiotrophoblast is system y+L. This transport mechanism has a dual mode of transport, transporting both cationic and neutral amino acids, the latter only showing Na+-dependency (Table 1). These characteristics of system y+L were exploited to examine whether L-Hcy inhibited the uptake of [3H]L-arginine into MVM vesicles in a Na+-dependent manner (Tsitsiou et al. 2009). We found evidence of a Ki shift for neutral amino acid inhibition when Na+ was removed from the extravesicular buffer and replaced with K+(Tsitsiou et al. 2009); a feature considered consistent with the positive identification of system y+L activity (Devés et al. 1998; Devés and Boyd 1998). The reverse experiment, using unlabelled L-arginine to inhibit [35S]L-Hcy uptake into MVM vesicles, is confounded by the presence of the high capacity cationic amino acid transport system y+ in MVM (Ayuk et al. 2000; Speake et al. 2003), which may reduce the efficacy of L-arginine to inhibit mediation of [35S]L-Hcy uptake by system y+L (Tsitsiou et al. 2009). Again, functional delineation of which y+LAT light chain might be involved in L-Hcy transport is not easily accomplished. Both y+LAT light chains are expressed in human placenta at the mRNA level (Dye et al. 2004) and share a similar substrate specificity for neutral amino acids such as L-methionine (Bröer et al. 2000; Pfeiffer et al. 1999), but their distribution remains undetermined due to a lack of targeting antibodies. Measurements of L-Hcy transport following knockdown of individual y+LAT chains may go some way to resolving this issue.
Our observation that system y+L mediates L-Hcy transport across MVM of human placenta is interesting as this also appears to be the case in platelets, where Hcy-inhibits a leucine-sensitive, arginine-transporting component (Brunini et al. 2003; Leoncini et al. 2003), suggesting this might be an L-Hcy influx mechanism more broadly. This concept has not been well explored but has physiological significance, as by limiting L-arginine provision, the precursor for nitric oxide synthesis, L-Hcy impairs cellular nitric oxide production (Leoncini et al. 2003; Mutus et al. 2001; Upchurch et al. 1997). This has particular relevance as regards placental function, as nitric oxide derived from the fetal endothelium induces vasodilation within the fetoplacental circulation to maintain low vascular resistance (Sladek et al. 1997). It is therefore worthy of comment that in pregnancy pathologies associated with maternal HHcy such as pre-eclampsia and FGR, altered nitric oxide synthesis and/or biological action(s) is associated with abberant blood flow (Escudero and Sobrevia 2008).
Overview and physiological implications
This article highlights that there are three potential influx mechanisms for L-Hcy in human placenta, systems L, A and y+L, supporting our starting hypothesis that L-Hcy would utilise the same transport moieties that support L-methionine as substrate (Tsitsiou et al. 2009). The use of multiple transport systems to import L-Hcy is not unique to the placenta; other cell types mirror this trend (Büdy et al. 2006; Hultberg 2004; Jiang et al. 2007; Naggar et al. 2003). The molecular identity of the transport systems involved in cellular L-Hcy uptake, their relative affinity for L-Hcy and contribution to total L-Hcy uptake appears to be dictated by cell type, perhaps influencing cellular susceptibility to an environment where HHcy prevails or serving to confer a functional advantage through L-Hcy/endogenous amino acid interactions. It is interesting that all three amino acid transporters appear to mediate L-Hcy influx across MVM, as the activity of systems A and y+L may well influence that of system L by providing amino acid substrates for system L-mediated exchange (Verrey 2003).
Our evidence is that system L is likely to be the major mechanism to mediate L-Hcy influx across MVM (Tsitsiou et al. 2009). Further, we have speculated that the kinetic discrimination of two Na+-independent mechanisms for [35S]L-Hcy uptake into MVM vesicles with high (~72 µM) and low (9.7 mM) affinity transport components represents CD98/LAT1- and CD98/LAT2-mediated activity, respectively (Tsitsiou et al. 2009). This notion would accord with the generally higher substrate affinity of LAT1 and its high abundance in MVM. Thus, system L activity in MVM would be particularly susceptible to fluctuations in maternal Hcy status. This is of key importance as system L provides essential amino acids for both fetal development and placental metabolism. Placental supply of these only just meets fetal demand for protein synthesis (Chien et al. 1993), so such an interaction is plausibly likely to impact on fetal growth. The predisposition to FGR and a lower birthweight in mothers with HHcy (Vollset et al. 2000) is certainly consistent with such a concept.
Whilst the studies described here indicate that Hcy is transported across the MVM, is there corroborative evidence that L-Hcy is taken up by placental tissue/cells? We have addressed this issue by investigating whether L-Hcy inhibits Na+-dependent [14C]MeAIB uptake into placental villous fragments, a model whereby the cellular architecture is maintained and the syncytiotrophoblast remains intact (Greenwood and Sibley 2006). L-Hcy (10 mM) reduced [14C]MeAIB accumulation by placental villous fragments by ~85% (Tsitsiou et al. 2008) inferring that L-Hcy must be taken up into the syncytiotrophoblast. Although some tissue accumulation may reflect uptake of tracer into other cellular compartments, tracer uptake is likely to be largely reflective of tracer movement across MVM into the syncytiotrophoblast (Greenwood and Sibley 2006). Previous studies by others, showing that treatment of cytotrophoblast cells with exogenous Hcy (progenitor cells which differentiate into syncytiotrophoblast-like structures in culture; Greenwood et al. 1996) results in apoptosis and an associated reduction in human chorionic gonadotropin secretion (Di Simone et al. 2003), further argues for the existence of uptake mechanisms for Hcy in this cell type. Attenuation of these effects by folic acid addition (Di Simone et al. 2004) implicates intracellular folate concentration as a key regulator of Hcy’s action in this cell type.
It has recently been demonstrated that folic acid is taken up by cytotrophoblast cells by three transporters: folate receptor α, proton-coupled folate transporter and reduced folate carrier (Keating et al. 2009), agreeing well with our observations in placental tissue implicating these transporters in placental folate transport and showing that these three folate transporters are localised to MVM (Solanky et al. 2010), where they exhibit functional activity (Yasuda et al. 2008). The protection afforded by folic acid to the damaging effects of Hcy on cytotrophoblast function (Di Simone et al. 2004) is compatible with the concept of an increased intracellular conversion of Hcy to methionine, thereby reducing the potential for Hcy-related toxicity. This again serves to emphasise the key role of methionine cycle activity in placental Hcy metabolism and regulation of placental homeostasis.
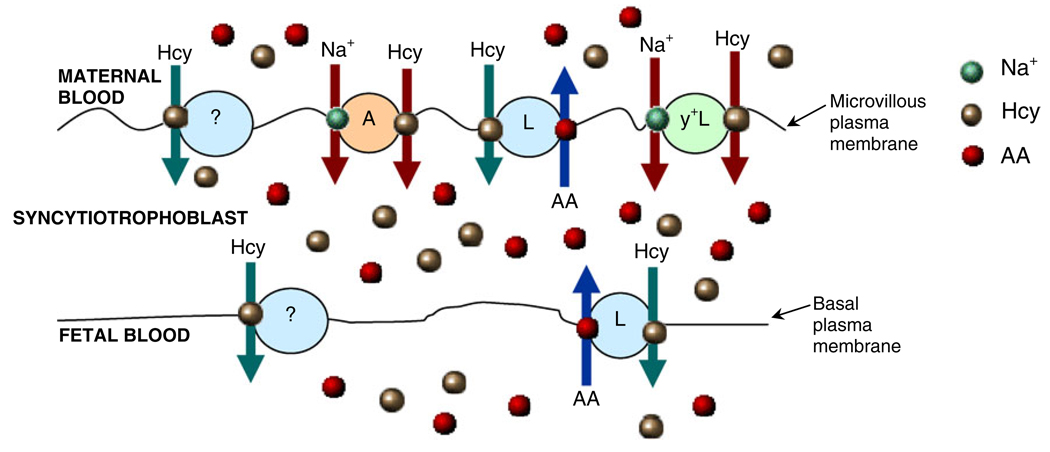
Overall, our studies suggest that L-Hcy can potentially be transported by, or accumulate within, the syncytio-trophoblast (Tsitsiou et al. 2008, 2009). This is depicted in Fig. 3 which, based on the collective evidence detailed here, also provides a scheme for the maternofetal transfer of L-Hcy. This scenario allows for several ensuing possibilities and Hcy could impact on syncytiotrophoblast function in several ways. By competing with other endogenous substrates of amino acid transporters, the provision of amino acids to the developing fetus could be reduced, placental metabolism of Hcy may be perturbed, especially if folate and vitamin B12 availability is suboptimal, apoptosis may be induced and functional integrity compromised. Additionally, it should be highlighted that systems L and A activities in MVM are reduced in pregnancies compromised by FGR (Jansson and Powell 2007; Sibley 2009), so maternal HHcy would serve to further limit amino acid supply. Indeed, the observation that the human placenta transports Hcy may provide a novel mechanism that could underscore the link between FGR and the predisposition to cardiovascular disease later in life (Jansson 2009). This is an exciting postulate that is certainly worthy of future investigation.
Fig. 3.
Maternofetal transfer of homocysteine. Schematic representation of the involvement of transport mechanisms systems L, A and y+L in the maternofetal transfer of Hcy across the human placenta. The scheme includes the possibility that as yet uncharacterised mechanisms may also be involved, denoted as carrier ‘?’. AA Amino acid
Acknowledgements
The work described in this article was supported by the Medical Research Council (MRC) (G0500647; J.D.G., S.W.D’S., C.P.S.) and a MRC Doctoral Training Studentship (E.T.). This work was also supported by Grant HL52234 from the National Heart, Lung and Blood Institute of the National Institutes of Health (D.W.J.). The Maternal and Fetal Health Research Group is supported by the Manchester NIHR Biomedical Research Centre. We would like to acknowledge that Professor Jonathan Clayden and Mr Lee Mullen (Department of Chemistry, University of Manchester) synthesised the L-Hcy used in our studies, and we extend our grateful thanks to them. We are also grateful to Dr Carolyn Jones for providing the electron micrograph of the placental barrier.
Abbreviations
- BCH
2-aminobicyclo[2.2.1]heptane-2-carboxylic acid
- BM
Basal plasma membrane
- FGR
Fetal growth restriction
- Hcy
Homocysteine
- HHcy
Hyperhomocysteinemia
- MeAIB
α-(methylamino)isobutyric acid
- MVM
Microvillous plasma membrane
- SNAT
Sodium-coupled neutral amino acid transporter
- tHcy
Total Hcy
Footnotes
Competing interest: None declared.
Conflict of interests: None to declare.
Presented at the 7th International Conference on Homocysteine Metabolism, Prague, June 21–25, 2009
Contributor Information
Eleni Tsitsiou, Maternal and Fetal Health Research Group, School of Biomedicine, University of Manchester, Manchester Academic Health Science Centre, St Mary’s Hospital, Oxford Road, Manchester M13 9WL, UK.
Colin P. Sibley, Maternal and Fetal Health Research Group, School of Biomedicine, University of Manchester, Manchester Academic Health Science Centre, St Mary’s Hospital, Oxford Road, Manchester M13 9WL, UK
Stephen W. D’Souza, Maternal and Fetal Health Research Group, School of Biomedicine, University of Manchester, Manchester Academic Health Science Centre, St Mary’s Hospital, Oxford Road, Manchester M13 9WL, UK
Otilia Catanescu, Department of Cell Biology, Lerner Research Institute, Cleveland Clinic, Cleveland, OH, USA.
Donald W. Jacobsen, Department of Cell Biology, Lerner Research Institute, Cleveland Clinic, Cleveland, OH, USA Department of Molecular Medicine, Cleveland Clinic Lerner College of Medicine, Case Western Reserve University, Cleveland, OH, USA.
Jocelyn D. Glazier, Maternal and Fetal Health Research Group, School of Biomedicine, University of Manchester, Manchester Academic Health Science Centre, St Mary’s Hospital, Oxford Road, Manchester M13 9WL, UK. j.glazier@manchester.ac.uk
References
- Ayuk PT, Sibley CP, Donnai P, et al. Development and polarization of cationic amino acid transporters and regulators in the human placenta. Am J Physiol Cell Physiol. 2000;278:C1162–C1171. doi: 10.1152/ajpcell.2000.278.6.C1162. [DOI] [PubMed] [Google Scholar]
- Bodoy S, Martin L, Zorzano A, et al. Identification of LAT4, a novel amino acid transporter with system L activity. J Biol Chem. 2005;280:12002–12011. doi: 10.1074/jbc.M408638200. [DOI] [PubMed] [Google Scholar]
- Bröer A, Wagner CA, Lang F, et al. The heterodimeric amino acid transporter 4F2hc/y+LAT2 mediates arginine efflux in exchange with glutamine. Biochem J. 2000;349:787–795. doi: 10.1042/bj3490787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunini TM, Yaqoob MM, Novaes Malagris LE, et al. Increased nitric oxide synthesis in uraemic platelets is dependent on L-arginine transport via system y(+)L. Pflugers Arch. 2003;445:547–550. doi: 10.1007/s00424-002-0978-7. [DOI] [PubMed] [Google Scholar]
- Büdy B, O'Neill R, DiBello PM, et al. Homocysteine transport by human aortic endothelial cells: identification and properties of import systems. Arch Biochem Biophys. 2006;446:119–130. doi: 10.1016/j.abb.2005.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien PF, Smith K, Watt PW, et al. Protein turnover in the human fetus studied at term using stable isotope tracer amino acids. Am J Physiol. 1993;265:E31–E35. doi: 10.1152/ajpendo.1993.265.1.E31. [DOI] [PubMed] [Google Scholar]
- Cleal JK, Lewis RM. The mechanisms and regulation of placental amino acid transport to the human foetus. J Neuroendocrinol. 2008;20:419–426. doi: 10.1111/j.1365-2826.2008.01662.x. [DOI] [PubMed] [Google Scholar]
- Cleal JK, Brownbill P, Godfrey KM, et al. Modification of fetal plasma amino acid composition by placental amino acid exchangers in vitro. J Physiol. 2007;582:871–882. doi: 10.1113/jphysiol.2007.130690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Calle M, Usandizaga R, Sancha M, et al. Homocysteine, folic acid and B-group vitamins in obstetrics and gynaecology. Eur J Obstet Gynecol Reprod Biol. 2003;107:125–134. doi: 10.1016/s0301-2115(02)00305-6. [DOI] [PubMed] [Google Scholar]
- Dean W, Lucifero D, Santos F. DNA methylation in mammalian development and disease. Birth Defects Res C Embryo Today. 2005;75:98–111. doi: 10.1002/bdrc.20037. [DOI] [PubMed] [Google Scholar]
- Desforges M, Lacey HA, Glazier JD, et al. SNAT4 isoform of system A amino acid transporter is expressed in human placenta. Am J Physiol Cell Physiol. 2006;290:C305–C312. doi: 10.1152/ajpcell.00258.2005. [DOI] [PubMed] [Google Scholar]
- Desforges M, Mynett KJ, Jones RL, et al. The SNAT4 isoform of the system A amino acid transporter is functional in human placental microvillous plasma membrane. J Physiol. 2009;587:61–72. doi: 10.1113/jphysiol.2008.161331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devés R, Boyd CA. Transporters for cationic amino acids in animal cells: discovery, structure, and function. Physiol Rev. 1998;78:487–545. doi: 10.1152/physrev.1998.78.2.487. [DOI] [PubMed] [Google Scholar]
- Devés R, Angelo S, Rojas AM. System y+L: the broad scope and cation modulated amino acid transporter. Exp Physiol. 1998;83:211–220. doi: 10.1113/expphysiol.1998.sp004105. [DOI] [PubMed] [Google Scholar]
- Di Simone N, Maggiano N, Caliandro D, et al. Homocysteine induces trophoblast cell death with apoptotic features. Biol Reprod. 2003;69:1129–1134. doi: 10.1095/biolreprod.103.015800. [DOI] [PubMed] [Google Scholar]
- Di Simone N, Riccardi P, Maggiano N, et al. Effect of folic acid on homocysteine-induced trophoblast apoptosis. Mol Hum Reprod. 2004;10:665–669. doi: 10.1093/molehr/gah091. [DOI] [PubMed] [Google Scholar]
- Dye JF, Vause S, Johnston T, et al. Characterization of cationic amino acid transporters and expression of endothelial nitric oxide synthase in human placental microvascular endothelial cells. FASEB J. 2004;18:125–127. doi: 10.1096/fj.02-0916fje. [DOI] [PubMed] [Google Scholar]
- Escudero C, Sobrevia L. A hypothesis for preeclampsia: adenosine and inducible nitric oxide synthase in human placental microvascular endothelium. Placenta. 2008;29:469–483. doi: 10.1016/j.placenta.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Ewadh MJ, Tudball N, Rose FA. Homocysteine uptake by human umbilical vein endothelial cells in culture. Biochim Biophys Acta. 1990;1054:263–266. doi: 10.1016/0167-4889(90)90097-w. [DOI] [PubMed] [Google Scholar]
- Finkelstein JD. The metabolism of homocysteine: pathways and regulation. Eur J Pediatr. 1998;157 Suppl 2:S40–S44. doi: 10.1007/pl00014300. [DOI] [PubMed] [Google Scholar]
- Firth JA, Leach L. Not trophoblast alone: a review of the contribution of the fetal microvasculature to transplacental exchange. Placenta. 1996;17:89–96. doi: 10.1016/s0143-4004(96)80001-4. [DOI] [PubMed] [Google Scholar]
- Ganapathy ME, Leibach FH, Mahesh VB, et al. Characterization of tryptophan transport in human placental brush-border membrane vesicles. Biochem J. 1986;238:201–208. doi: 10.1042/bj2380201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaull G, Sturman JA, Raiha NC. Development of mammalian sulfur metabolism: absence of cystathionase in human fetal tissues. Pediatr Res. 1972;6:538–547. doi: 10.1203/00006450-197206000-00002. [DOI] [PubMed] [Google Scholar]
- Glazier JD, Sibley CP. In vitro methods for studying human placental amino acid transport: placental plasma membrane vesicles. Meth Mol Med. 2006;122:241–252. doi: 10.1385/1-59259-989-3:241. [DOI] [PubMed] [Google Scholar]
- Glazier JD, Jones CJ, Sibley CP. Purification and Na+ uptake by human placental microvillus membrane vesicles prepared by three different methods. Biochim Biophys Acta. 1988;945:127–134. doi: 10.1016/0005-2736(88)90475-0. [DOI] [PubMed] [Google Scholar]
- Greenwood SL, Sibley CP. In vitro methods for studying human placental amino acid transport placental villous fragments. Meth Mol Med. 2006;122:253–264. doi: 10.1385/1-59259-989-3:253. [DOI] [PubMed] [Google Scholar]
- Greenwood SL, Clarson LH, Sides MK, et al. Membrane potential difference and intracellular cation concentrations in human placental trophoblast cells in culture. J Physiol. 1996;492:629–640. doi: 10.1113/jphysiol.1996.sp021333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatanaka T, Huang W, Wang H, et al. Primary structure, functional characteristics and tissue expression pattern of human ATA2, a subtype of amino acid transport system A. Biochim Biophys Acta. 2000;1467:1–6. doi: 10.1016/s0005-2736(00)00252-2. [DOI] [PubMed] [Google Scholar]
- Hatanaka T, Huang W, Ling R, et al. Evidence for the transport of neutral as well as cationic amino acids by ATA3, a novel and liver-specific subtype of amino acid transport system A. Biochim Biophys Acta. 2001;1510:10–17. doi: 10.1016/s0005-2736(00)00390-4. [DOI] [PubMed] [Google Scholar]
- Holmes VA. Changes in haemostasis during normal pregnancy: does homocysteine play a role in maintaining homeostasis? Proc Nutr Soc. 2003;62:479–493. doi: 10.1079/pns2003251. [DOI] [PubMed] [Google Scholar]
- Hultberg B. Extracellular concentration of homocysteine in human cell lines is influenced by specific inhibitors of cyst(e)ine transport. Clin Chem Lab Med. 2004;42:378–383. doi: 10.1515/CCLM.2004.067. [DOI] [PubMed] [Google Scholar]
- Jansson T. Amino acid transporters in the human placenta. Pediatr Res. 2001;49:141–147. doi: 10.1203/00006450-200102000-00003. [DOI] [PubMed] [Google Scholar]
- Jansson T. Novel mechanism causing restricted fetal growth: does maternal homocysteine impair placental amino acid transport? J Physiol. 2009;587:4123. doi: 10.1113/jphysiol.2009.178327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson T, Powell TL. Role of the placenta in fetal programming: underlying mechanisms and potential interventional approaches. Clin Sci (Lond) 2007;113:1–13. doi: 10.1042/CS20060339. [DOI] [PubMed] [Google Scholar]
- Jansson T, Ylven K, Wennergren M, et al. Glucose transport and system A activity in syncytiotrophoblast microvillous and basal plasma membranes in intrauterine growth restriction. Placenta. 2002;23:392–399. doi: 10.1053/plac.2002.0826. [DOI] [PubMed] [Google Scholar]
- Jiang X, Yang F, Brailoiu E, et al. Differential regulation of homocysteine transport in vascular endothelial and smooth muscle cells. Arterioscler Thromb Vasc Biol. 2007;27:1976–1983. doi: 10.1161/ATVBAHA.107.148544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LW, Smith CH. Neutral amino acid transport systems of microvillous membrane of human placenta. Am J Physiol. 1988;254:C773–C780. doi: 10.1152/ajpcell.1988.254.6.C773. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Segawa H, Miyamoto K, et al. Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98) J Biol Chem. 1998;273:23629–23632. doi: 10.1074/jbc.273.37.23629. [DOI] [PubMed] [Google Scholar]
- Keating E, Goncalves P, Campos I, et al. Folic acid uptake by the human syncytiotrophoblast: interference by pharmacotherapy, drugs of abuse and pathological conditions. Reprod Toxicol. 2009;28:511–520. doi: 10.1016/j.reprotox.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Kim JM, Hong K, Lee JH, et al. Effect of folate deficiency on placental DNA methylation in hyperhomocysteinemic rats. J Nutr Biochem. 2009;20:172–176. doi: 10.1016/j.jnutbio.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Kudo Y, Boyd CA. Characterisation of L-tryptophan transporters in human placenta: a comparison of brush border and basal membrane vesicles. J Physiol. 2001;531:405–416. doi: 10.1111/j.1469-7793.2001.0405i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leoncini G, Pascale R, Signorello MG. Effects of homocysteine on l-arginine transport and nitric oxide formation in human platelets. Eur J Clin Investig. 2003;33:713–719. doi: 10.1046/j.1365-2362.2003.01203.x. [DOI] [PubMed] [Google Scholar]
- Lewis RM, Glazier J, Greenwood SL, et al. L-serine uptake by human placental microvillous membrane vesicles. Placenta. 2007;28:445–452. doi: 10.1016/j.placenta.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Limpach A, Dalton M, Miles R, et al. Homocysteine inhibits retinoic acid synthesis: a mechanism for homocysteine-induced congenital defects. Exp Cell Res. 2000;260:166–174. doi: 10.1006/excr.2000.5000. [DOI] [PubMed] [Google Scholar]
- Mackenzie B, Erickson JD. Sodium-coupled neutral amino acid (System N/A) transporters of the SLC38 gene family. Pflugers Arch. 2004;447:784–795. doi: 10.1007/s00424-003-1117-9. [DOI] [PubMed] [Google Scholar]
- Mahendran D, Donnai P, Glazier JD, et al. Amino acid (system A) transporter activity in microvillous membrane vesicles from the placentas of appropriate and small for gestational age babies. Pediatr Res. 1993;34:661–665. doi: 10.1203/00006450-199311000-00019. [DOI] [PubMed] [Google Scholar]
- Malinow MR, Rajkovic A, Duell PB, et al. The relationship between maternal and neonatal umbilical cord plasma homocyst (e)ine suggests a potential role for maternal homocyst(e)ine in fetal metabolism. Am J Obstet Gynecol. 1998;178:228–233. doi: 10.1016/s0002-9378(98)80005-7. [DOI] [PubMed] [Google Scholar]
- Meier C, Ristic Z, Klauser S, et al. Activation of system L heterodimeric amino acid exchangers by intracellular substrates. EMBO J. 2002;21:580–589. doi: 10.1093/emboj/21.4.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy AM, Mills JL, McPartlin J, et al. Maternal and fetal plasma homocysteine concentrations at birth: the influence of folate, vitamin B12, and the 5, 10-methylenetetrahydrofolate reductase 677C–>T variant. Am J Obstet Gynecol. 2002;186:499–503. doi: 10.1067/mob.2002.121105. [DOI] [PubMed] [Google Scholar]
- MRC Vitamin Study Research Group. Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. Lancet. 1991;338:131–137. [PubMed] [Google Scholar]
- Murphy MM, Scott JM, McPartlin JM, et al. The pregnancy-related decrease in fasting plasma homocysteine is not explained by folic acid supplementation, hemodilution, or a decrease in albumin in a longitudinal study. Am J Clin Nutr. 2002;76:614–619. doi: 10.1093/ajcn/76.3.614. [DOI] [PubMed] [Google Scholar]
- Murphy MM, Scott JM, Arija V, et al. Maternal homocysteine before conception and throughout pregnancy predicts fetal homocysteine and birth weight. Clin Chem. 2004;50:1406–1412. doi: 10.1373/clinchem.2004.032904. [DOI] [PubMed] [Google Scholar]
- Mutus B, Rabini RA, Staffolani R, et al. Homocysteine-induced inhibition of nitric oxide production in platelets: a study on healthy and diabetic subjects. Diabetologia. 2001;44:979–982. doi: 10.1007/s001250100581. [DOI] [PubMed] [Google Scholar]
- Naggar H, Fei YJ, Ganapathy V, et al. Regulation of reduced-folate transporter-1 (RFT-1) by homocysteine and identity of transport systems for homocysteine uptake in retinal pigment epithelial (RPE) cells. Exp Eye Res. 2003;77:687–697. doi: 10.1016/j.exer.2003.08.013. [DOI] [PubMed] [Google Scholar]
- Okamoto Y, Sakata M, Ogura K, et al. Expression and regulation of 4F2hc and hLAT1 in human trophoblasts. Am J Physiol Cell Physiol. 2002;282:C196–C204. doi: 10.1152/ajpcell.2002.282.1.C196. [DOI] [PubMed] [Google Scholar]
- Pfeiffer R, Rossier G, Spindler B, et al. Amino acid transport of y+L-type by heterodimers of 4F2hc/CD98 and members of the glycoprotein-associated amino acid transporter family. EMBO J. 1999;18:49–57. doi: 10.1093/emboj/18.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickell L, Li D, Brown K, et al. Methylenetetrahydrofolate reductase deficiency and low dietary folate increase embryonic delay and placental abnormalities in mice. Birth Defects Res A Clin Mol Teratol. 2009;85:531–541. doi: 10.1002/bdra.20575. [DOI] [PubMed] [Google Scholar]
- Pineda M, Fernandez E, Torrents D, et al. Identification of a membrane protein, LAT-2, that Co-expresses with 4F2 heavy chain, an L-type amino acid transport activity with broad specificity for small and large zwitterionic amino acids. J Biol Chem. 1999;274:19738–19744. doi: 10.1074/jbc.274.28.19738. [DOI] [PubMed] [Google Scholar]
- Ray JG, Laskin CA. Folic acid and homocyst(e)ine metabolic defects and the risk of placental abruption, pre-eclampsia and spontaneous pregnancy loss: a systematic review. Placenta. 1999;20:519–529. doi: 10.1053/plac.1999.0417. [DOI] [PubMed] [Google Scholar]
- Refsum H. Folate, vitamin B12 and homocysteine in relation to birth defects and pregnancy outcome. Br J Nutr. 2001;85 Suppl 2:S109–S113. [PubMed] [Google Scholar]
- Refsum H, Ueland PM, Nygard O, et al. Homocysteine and cardiovascular disease. Annu Rev Med. 1998;49:31–62. doi: 10.1146/annurev.med.49.1.31. [DOI] [PubMed] [Google Scholar]
- Rossier G, Meier C, Bauch C, et al. LAT2, a new basolateral 4F2hc/CD98-associated amino acid transporter of kidney and intestine. J Biol Chem. 1999;274:34948–34954. doi: 10.1074/jbc.274.49.34948. [DOI] [PubMed] [Google Scholar]
- Selhub J. Homocysteine metabolism. Annu Rev Nutr. 1999;19:217–246. doi: 10.1146/annurev.nutr.19.1.217. [DOI] [PubMed] [Google Scholar]
- Sibley CP. Understanding placental nutrient transfer—why bother? New biomarkers of fetal growth. J Physiol. 2009;587:3431–3440. doi: 10.1113/jphysiol.2009.172403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladek SM, Magness RR, Conrad KP. Nitric oxide and pregnancy. Am J Physiol. 1997;272:R441–R463. doi: 10.1152/ajpregu.1997.272.2.R441. [DOI] [PubMed] [Google Scholar]
- Solanky N, Requena Jimenez A, D'Souza SW, et al. Expression of folate transporters in human placenta and implications for homocysteine metabolism. Placenta. 2010;31:134–143. doi: 10.1016/j.placenta.2009.11.017. [DOI] [PubMed] [Google Scholar]
- Speake PF, Glazier JD, Ayuk PT, et al. L-Arginine transport across the basal plasma membrane of the syncytiotrophoblast of the human placenta from normal and preeclamptic pregnancies. J Clin Endocrinol Metab. 2003;88:4287–4292. doi: 10.1210/jc.2003-030067. [DOI] [PubMed] [Google Scholar]
- Swanson DA, Liu ML, Baker PJ, et al. Targeted disruption of the methionine synthase gene in mice. Mol Cell Biol. 2001;21:1058–1065. doi: 10.1128/MCB.21.4.1058-1065.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsitsiou E, Greenwood SL, Sibley CP, et al. Homocysteine inhibition of system A amino acid transporter activity in human placenta. Reprod Sci. 2008;15:91A. [Google Scholar]
- Tsitsiou E, Sibley CP, D'Souza SW, et al. Homocysteine transport by systems L, A and y+L across the microvillous plasma membrane of human placenta. J Physiol. 2009;587:4001–4013. doi: 10.1113/jphysiol.2009.173393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upchurch GR, Jr, Welch GN, Fabian AJ, et al. Homocyst(e) ine decreases bioavailable nitric oxide by a mechanism involving glutathione peroxidase. J Biol Chem. 1997;272:17012–17017. doi: 10.1074/jbc.272.27.17012. [DOI] [PubMed] [Google Scholar]
- Verrey F. System L: heteromeric exchangers of large, neutral amino acids involved in directional transport. Pflugers Arch. 2003;445:529–533. doi: 10.1007/s00424-002-0973-z. [DOI] [PubMed] [Google Scholar]
- Vollset SE, Refsum H, Irgens LM, et al. Plasma total homocysteine, pregnancy complications, and adverse pregnancy outcomes: the Hordaland Homocysteine study. Am J Clin Nutr. 2000;71:962–968. doi: 10.1093/ajcn/71.4.962. [DOI] [PubMed] [Google Scholar]
- Wang H, Huang W, Sugawara M, et al. Cloning and functional expression of ATA1, a subtype of amino acid transporter A, from human placenta. Biochem Biophys Res Commun. 2000;273:1175–1179. doi: 10.1006/bbrc.2000.3061. [DOI] [PubMed] [Google Scholar]
- Yajnik CS, Deshpande SS, Panchanadikar AV, et al. Maternal total homocysteine concentration and neonatal size in India. Asia Pac J Clin Nutr. 2005;14:179–181. [PubMed] [Google Scholar]
- Yasuda S, Hasui S, Yamamoto C, et al. Placental folate transport during pregnancy. Biosci Biotechnol Biochem. 2008;72:2277–2284. doi: 10.1271/bbb.80112. [DOI] [PubMed] [Google Scholar]