Abstract
Sulforaphane (SF) is a well-known chemopreventive phytochemical and occurs in broccoli and to a lesser extent in other cruciferous vegetables, whereas 4-aminobiphenyl (ABP) is a major human bladder carcinogen and is present at significant levels in tobacco smoke. Here, we show that SF inhibits ABP-induced DNA damage in both human bladder cells in vitro and mouse bladder tissue in vivo, using dG-C8-ABP as a biomarker, which is the predominant ABP-DNA adduct formed in human bladder cells and tissues. SF activates NF-E2 related factor-2 (Nrf2), which is a well-recognized chemopreventive target and activates the Nrf2-regulated cytoprotective signaling pathway. Comparison between wild-type mice and mice without Nrf2 shows that Nrf2 activation is required by SF for inhibition of ABP-induced DNA damage. Moreover, Nrf2 activation by SF in the bladder occurs primarily in the epithelium, which is the principal site of bladder cancer development. These data, together with our recent observation that SF-enriched broccoli sprout extracts strongly inhibits N-butyl-N-(4-hydroxybutyl)nitrosamine-induced bladder cancer development, suggest that SF is a highly promising agent for bladder cancer prevention and provides a mechanistic insight into the repeated epidemiological observation that consumption of broccoli is inversely associated with bladder cancer risk and mortality.
Introduction
Aromatic amines through occupational exposure and cigarette smoking are well-known causes of human bladder cancer (1). The linkage between bladder cancer and 4-aminobiphenyl (ABP) from cigarette smoke is particularly strong. Cigarette smoke is both the main cause of human bladder cancer and the main source of human exposure to ABP (2–4). According to one study, up to 23 ng ABP is present in the smoke of each cigarette (5). Levels of ABP-DNA adducts were as much as eight times higher in bladder biopsies or exfoliated urothelial cells of smokers than those of non-smokers (6,7). ABP-DNA adducts were readily detected in a high percentage of human bladder cancer biopsies, and their levels in these specimens were significantly higher in smokers than in non-smokers (8,9). Tumorigenic transformation and neoplastic progression of human uroepithelial cells occurred after exposure to ABP or its metabolites (10), and higher levels of ABP-DNA adducts in bladder tumors were associated with more aggressive behavior of the tumors (8). Moreover, treatment of animals with ABP also caused formation of DNA adducts and tumors in the bladder (11,12).
Epidemiological studies have shown a significant inverse association between broccoli consumption and human bladder cancer risk (13,14). Our recent studies show that sulforaphane (SF), a key chemopreventive ingredient in broccoli (15), inhibits the growth of human bladder cancer cells (16) and that SF-enriched broccoli sprout extracts significantly inhibited bladder cancer development in a rat model induced by N-butyl-N-(4-hydroxybutyl)nitrosamine (BBN) (17). Moreover, several other studies also indicate that SF is selectively delivered to bladder tissue through urinary excretion (17–19). Thus, dietary SF may play an important role in bladder cancer prevention. We speculated that SF might protect bladder cells and tissues against ABP as SF is a well-known activator of NF-E2 related factor-2 (Nrf2), which transcriptionally activates many cytoprotective genes and has been shown to play a pivotal role in cellular defense against various chemical carcinogens, including BBN (20).
In this report, we show that SF inhibits ABP-induced DNA adduct formation in both human bladder cells in vitro and mouse bladder tissues in vivo. We also show that SF protects cells against ABP by activating Nrf2, a transcription factor which is critical for stimulation of a variety of cytoprotective genes involved in detoxification of carcinogens and oxidants and is a major chemopreventive target (21,22). Moreover, our data indicate that Nrf2 activation and stimulation of Nrf2 target gene in the bladder by SF occur primarily in the epithelium, the principal site of bladder cancer development.
Materials and methods
Chemicals
SF, ABP and N-hydroxy-N-acetyl-4-aminobiphenyl (N-OH-AABP) were purchased from LKT Laboratories (St Paul, MN), Sigma (St Louis, MO) and Midwest Research Institute (Kansas City, MO), respectively. Rat liver S9 (36–43 mg protein/ml) was purchased from Moltox (Boone, NC). Antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), including antibodies specific for the catalytic subunit and the regulatory subunit of glutamate cysteine ligase (GCSc and GCSm), NAD(P)H:quinone oxidoreductase 1 (NQO1) and Nrf2, from Alpha Diagnostic International (San Antonio, TX) for the antibody specific for the α isoform of glutathione S-transferase (GST) and from Millipore (Billerica, MA) for the antibody of glyceraldehyde 3-phosphate dehydrogenase. Another antibody against NQO1 purchased from Cell Signaling Technology (Danvers, MA) was used in some of the Western blot analyses.
Cell study
Human bladder carcinoma RT4 cells were cultured as described previously (23). To induce DNA damage, RT4 cells (2–3 × 106 cells) were grown in each 10 cm dish with 10 ml medium for 24–48 h, followed by treatment with either ABP or N-OH-AABP for 3 h in 5 ml fresh medium per dish. In cases where cells were cotreated with ABP and S9, the medium also contained 6% S9 (vol/vol), 10 mM glucose-6-phosphate and 5 mM NADP. In experiments involving SF pretreatment, cells were first treated with SF or vehicle for 24 h before exposure to the carcinogens. All test agents were dissolved in dimethyl sulfoxide, and the final concentration of dimethyl sulfoxide in medium was ≤0.5%. Cells were harvested by trypsin treatment and low-speed centrifugation. Cell pellets were washed with ice-cold phosphate-buffered saline and used for DNA adduct analysis and Western blotting of Nrf2 and selected Nrf2-regulated cytoprotective proteins as described in the Quantification of dG-C8-ABP and Western blotting and immnohistochemistry sections below.
Animal study
Wild-type C57BL/6 mice and Nrf2-deficient C57BL/6 mice (male 6 weeks of age) were used. The wild-type mice were purchased from National Cancer Institute (Frederick, MD). The Nrf2-deficient mice were bred at our animal facility; the breeders were kindly provided by Dr Thomas W.Kensler (Johns Hopkins Bloomberg School of Public Health) (24). These animals were used in two experiments. In the first experiment, groups of three to four mice were randomized and treated with SF or vehicle by gavage once daily for 5 days. Three hours after the last SF dose, the mice were each administered a single dose of ABP by intraperitoneal injection and were killed 24 h after ABP treatment to collect the bladders for measurement of dG-C8-ABP. In the second experiment, groups of three to four mice were randomized and treated with SF or vehicle by gavage once daily for 5 days and were killed 24 h later to collect the bladders for measurement of selected Nrf2-regulated cytoprotective genes. All animal protocols and procedures were approved by the Roswell Park Cancer Institute Animal Care and Use Committee.
Quantification of dG-C8-ABP
Procedures of DNA isolation from bladder cells and tissues and the detailed protocol for the quantitative analysis of dG-C8-ABP by capillary liquid chromatography and nanoelectrospray ionization-tandem mass spectrometry have been described in a recent publication (25). This assay has a mass detection limit of 20 amol in 1.25 μg of DNA (five adducts in 109 nucleosides) with a linear range of 70 amol–70 fmol. The equation for the calibration line is Y = (0.100 ± 0.001) X + (0.05 ± 0.03), R2 = 0.99 ± 0.08, where Y is analyte/internal standard peak area ratio and X is femtomole dG-C8-ABP. For samples dosed beyond the range of the calibration line, the equation was linearly extrapolated.
Western blotting and immunohistochemistry
The relevant protocols have been previously published (26).
Statistical analysis
The numerical results are expressed as means ± SD. Unpaired two-tailed Student’s t-test was used for data analysis, with a P value <0.05 being considered significant (GraphPad Version 5.00; GraphPad Software, San Diego, CA).
Results and discussion
DNA damage by ABP in human bladder cells
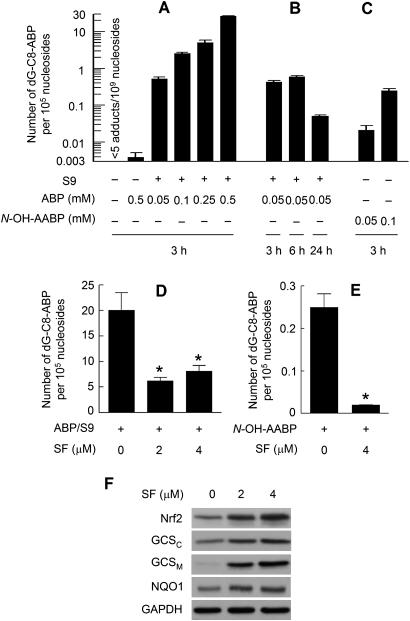
Dose-dependent DNA damage (up to 2100 adducts per 107 nucleosides) was detected in human bladder RT4 cells after exposure to ABP at 0.05, 0.1, 0.25 or 0.5 mM for 3 h in the presence of a rat liver S9 activation system, as measured by dG-C8-ABP (Figure 1A). dG-C8-ABP accounts for 80% of all ABP-DNA adducts formed in human bladder tissues in vivo (6,27). dG-C8-ABP forms rapidly but also appears to be repaired at a significantly high rate. For example, its level in RT4 cells after 3 h exposure to ABP (0.05 mM plus S9) increased >58-fold over the control, but increased only 1.4-fold over the next 3 h, and the adduct level after 24 h of exposure was only 11.7 and 8.6% of that detected at the 3 and 6 h exposure time points, respectively (Figure 1B). It is noteworthy that previous studies show that p53 is needed for repair of ABP-induced genomic DNA damage in human bladder cells (28) and that RT4 cells carry the normal p53 (29). However, ABP without S9 was more than four orders of magnitude weaker in inducing the formation of dG-C8-ABP in RT4 cells as only four adducts per 108 nucleosides were detected in cells after treatment with 0.5 mM ABP for 3 h, although the background level of dG-C8-ABP in these cells was >7.8-fold lower (Figure 1A). Similar results were seen in other bladder cell lines, including human bladder cancer UM-UC-3 cells and rat bladder cancer NBT-II cells (data not shown). In contrast, no S9 was required for N-OH-AABP, a metabolite of ABP, to cause significant and dose-dependent formation of dG-C8-ABP in RT4 cells (Figure 1C).
Fig. 1.
ABP-induced DNA damage in human bladder cells and the protective effect of SF. (A, B and C) RT4 cells were treated with ABP or AABP in the absence or presence of S9 for a desired time and then harvested for measurement of dG-C8-ABP levels by liquid chromatography-tandem mass spectrometry. (D and E) RT4 cells were pretreated with SF for 24 h and then exposed to ABP (0.5 mM) plus S9 or N-OH-AABP (0.1 mM) for 3 h before harvest for measurement of dG-C8-ABP levels. Each value is a mean ± SD (n = 3). Each value marked by an asterisk in the treatment groups is significantly different from the control (P < 0.05). (F) RT4 cells were treated with SF for 24 h and then harvested for measurement of Nrf2 and selected Phase 2 proteins by Western blotting. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) is a loading control. The data are representative of at least two experiments.
It is well established that ABP requires metabolic activation in order to damage DNA and is first metabolized to N-hydroxy-4-aminobiphenyl (N-OH-ABP) before being ultimately converted to the highly electrophilic arylnitrenium ion (30,31). N-OH-ABP was shown previously to cause DNA damage (mainly dG-C8-ABP formation) in human bladder cells (32). N-OH-AABP may also undergo conversion to N-OH-ABP in cell through deacetylation (33). Thus, bladder cells are probably deficient in enzyme(s) that catalyze the conversion of ABP to N-OH-ABP. Hepatic cytochrome P450 1A2 (Cyp1A2) was widely suggested to be the key enzyme catalyzing the formation of N-OH-ABP, but a subsequent study showed that knockout of Cyp1A2 gene in mice had no effect on ABP-DNA adduct formation in the bladder tissue (30).
SF inhibits dG-C8-ABP formation and activates the Nrf2 signaling pathway in human bladder cells
When RT4 cells were pretreated with SF at 2 or 4 μM for 24 h and then exposed to ABP (0.5 mM, with S9) for 3 h, dG-C8-ABP levels decreased by 70 and 59%, respectively, compared with control cells (Figure 1D). Thus, SF is highly effective in inhibiting ABP-induced genotoxicity. Interestingly, the low SF dose was somewhat more effective. It is unlikely that SF directly prevented S9 from activating ABP because SF was not present in the medium during cell exposure to ABP and S9. Indeed, SF pretreatment at 4 μM for 24 h also inhibited dG-C8-ABP formation by 92% in RT4 cells exposed to N-OH-AABP (0.1 mM, 3 h) in the absence of S9 (Figure 1E).
The inhibitory effect of SF on ABP was associated with activation of the Nrf2 signaling pathway. Nrf2 is an essential transcriptional activator of a variety of genes involved in many aspects of cytoprotection, including carcinogen-detoxifying Phase 2 genes, and is itself activated by environmental and endogenous signals through inhibition of Keap1-mediated Nrf2 ubiquitination (34,35). A previous study showed that Nrf2 knockout in mice resulted in increased bladder cancer development induced by BBN (20). In the present study, treatment of RT4 cells with SF at 2 and 4 μM for 24 h led to a significant increase in Nrf2 level (Figure 1F), which is consistent with previous reports showing that SF activates Nrf2 by inhibiting its ubiquitination (36). As expected, Nrf2 elevation by SF in RT4 cells was accompanied by increased expression of Nrf2-regulated Phase 2 genes, as shown by both the catalytic and the regulatory units of GCS (GCSC and GCSM) and NQO1 (Figure 1F), indicating activation of the Nrf2 signaling pathway. Although these genes were assessed as biomakers of Nrf2 transactivation activity and may not necessarily mediate the inhibitory effect of SF on ABP, GCS may contribute in part to the detoxification of the electrophilic nitrenium ion generated from ABP and N-OH-AABP as it is the key enzyme for the biosynthesis of glutathione, which plays a major role in cellular electrophile scavenging. It is widely recognized that UDP-glucuronosyltransferase (UGT) and N-acetyltransferase (NAT) modulate the genotoxicity of ABP by catalyzing the conjugation of the latter with endogenous ligands glucuronide and acetyl-CoA. But no change in their expression levels, including UGT1A, UGT2B, NAT1 and NAT2, were detected in SF-treated RT4 cells as measured by Western blotting (results not shown).
Nrf2 is essential for SF to inhibit ABP-DNA adduct formation
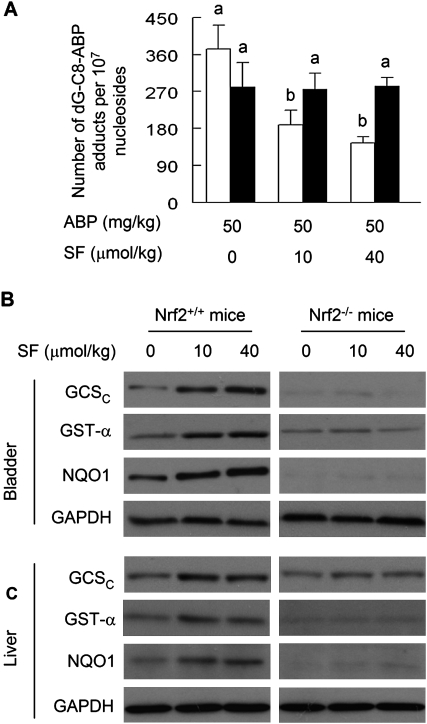
To assess the effect of SF on ABP in vivo, both wild-type mice and Nrf2 knockout mice were administered SF at 0, 10 and 40 μmol/kg by gavage once daily for 5 days. A single intraperitoneal dose of ABP at 50 mg/kg was given 3 h after the last SF dose. The mice were killed 24 h after ABP treatment, and the bladders were processed for measurement of DNA damage. A preliminary experiment was performed to identify the ABP dose (result not shown). Although the background level of dG-C8-ABP in the bladder tissues was below the detection limit of five adducts per 109 nucleosides, its level increased to 371 ± 58 adducts per 107 nucleosides in the wild-type mice and 281 ± 59 adducts per 107 nucleosides in the Nrf2 knockout mice after ABP treatment (Figure 2A). Surprisingly, in the absence of SF pretreatment, the adduct level was 33% higher in the wild-type mice than in the Nrf2 knockout mice. The reason is not known, but such difference disappeared when in a subsequent experiment the ABP dose was reduced 10-fold. In that case, the adduct levels in bladder tissue were 13 ± 4 adducts per 107 nucleosides in the wild-type mice and 11 ± 1 adducts per 107 nucleosides in the Nrf2 knockout mice, 24 h after a single intraperitoneal dose of ABP at 5 mg/kg.
Fig. 2.
The inhibition of ABP-induced DNA damage by SF in the bladder and the role of Nrf2. (A) Wild-type C57BL/6 mice (open bar) and Nrf2-knocked out C57BL/6 mice (filled bar) were treated with vehicle or SF by gavage once a day for 5 days. Three hours after the last SF dose, each mouse was given a single intraperitoneal dose of ABP. The mice were killed 24 h later for measurement of dG-C8-ABP in bladder tissues. Each value is a mean ± SD (n = 3–4). Values not marked with the same letter are significantly different at P < 0.05. (B) Wild-type C57BL/6 mice and Nrf2 knocked out C57BL/6 mice were treated with vehicle or SF by gavage once a day for 5 days. The mice were killed 24 h later for measurement of selected Phase 2 proteins by Western blotting. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) is a loading control. The data are representative of mice in each group.
SF caused significant inhibition of dG-C8-ABP formation in the bladder in the wild-type mice, achieving 50 and 61% inhibition at 10 and 40 μmol/kg dose levels, respectively, but was totally ineffective in the Nrf2 knockout mice (Figure 2A). Thus, Nrf2 is required by SF for inhibition of ABP-induced DNA damage in the bladder. Although the effect of SF on ABP-induced bladder tumorigenesis could not be determined, due to very low incidence of bladder cancer in ABP-treated animals (12,37), we recently showed that feeding SF-enriched broccoli sprout extracts significantly inhibited BBN-induced bladder cancer development in the rat (17).
As expected, SF, under the treatment conditions described above (10 and 40 μmol/kg/day for 5 days), significantly induced multiple Nrf2-targeted Phase 2 genes in the bladder, including GST, GCS and NQO1, in the wild-type mice but not in the Nrf2 knockout mice (Figure 2B). These results show that SF activates the Nrf2 cytoprotective signaling pathway in the bladder and are consistent with the results shown in human bladder cells (Figure 1F), although it is not known if any of these particular Phase 2 genes directly mediate the inhibitory effect of SF. ABP is known to be metabolized mainly in the liver. Hepatic levels of GST, GCS and NQO1 were also significantly elevated in SF-treated wild-type mice but not in the Nrf2 knockout mice (Figure 2C). As in RT4 cells, however, neither UGT nor NAT in the bladder and liver appears to be modulated by SF at the doses tested (results not shown).
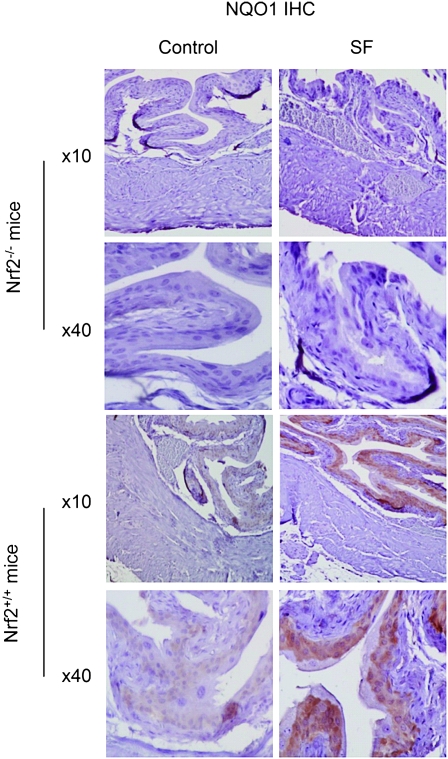
We further investigated the tissue site in the bladder where the Nrf2 signaling pathway is activated by SF. NQO1 was chosen as the biomarker of Nrf2 transactivation activity and was measured by immunohistochemistry. NQO1 exists mainly in the cytoplasm and is transcriptionally activated by Nrf2. We focused on the bladders removed from both wild-type mice and Nrf2 knockout mice, which were treated with either the vehicle or SF at 40 μmol/kg as described above. Low level of NQO1 was detected in the bladder epithelia of control wild-type mice, but it was undetectable anywhere in the bladders of control Nrf2-deficient mice (Figure 3). SF caused significant NQO1 induction in the bladder epithelium, but not in other sites of bladder, in the wild-type mice. SF failed to induce NQO1 in the epithelium or other sites in the bladders of Nrf2-deficient mice. These results together with those shown in Figure 2B demonstrate that SF elicits Nrf2-mediated cytoprotective functions specifically in the epithelium. This finding is highly significant because the epithelium is the principal site of bladder cancer development in humans (2). This finding is also in accordance with previous studies showing that SF is selectively delivered to the bladder through urinary excretion (17–19) as the bladder epithelium is directly exposed to urine.
Fig. 3.
Immunohistochemical staining of NQO1. The bladder tissues were obtained from wild-type C57BL/6 mice and their Nrf2-deficient counterparts. The mice were treated with either vehicle or SF at 40 μmol/kg as described in the Figure 2B legend. Representative images of bladder staining of NQO1 showing the entire bladder wall (low magnification images ×10) or the epithelium (high magnification ×40).
Summary
ABP is a major human bladder carcinogen and cigarette smoke is a major source of human exposure. We have shown that SF is highly effective in blocking ABP-induced DNA damage in human bladder cells in vitro and in mouse bladder tissue in vivo. These findings not only provide further evidence of the chemopreventive activity of SF against bladder cancer but also offer a mechanistic insight into the epidemiological reports that consumption of broccoli is inversely associated with bladder cancer risk and bladder cancer mortality in humans (13,14,38), since SF is considered the key chemopreventive ingredient in broccoli. Although the SF doses used in the animal study (10–40 μmol/kg body weight) are probably >10 times higher than the amount that is typically consumed by a human (∼1000 μmol SF per kg fresh broccoli) (39), the animals were exposed to far greater level of ABP (50 mg/kg) than humans normally are because ABP is present at low nanogram levels in the smoke of each cigarette.
SF activates the Nrf2 signaling pathway in both human bladder cancer cells in vitro and mouse bladder tissues in vivo, and Nrf2 is essential for the chemopreventive activity of SF. In vivo, SF is specifically active in the epithelium, which is the principal site of bladder cancer development. However, we have not been able to determine the molecular mechanism by which Nrf2 inhibits ABP-caused DNA damage or to identify the exact Nrf2-regulated gene(s) that mediate the anti-ABP activity of SF. Although metabolic activation and inhibition of ABP have been well studied, much remains unknown as to the enzymes that participate in ABP activation and subsequent inactivation. Identification of these enzymes in future studies will facilitate elucidation of the protective mechanism of Nrf2 against ABP.
Funding
National Cancer Institute (R01CA69390, R01CA112231, R01CA120533).
Acknowledgments
We thank Dr Thomas W.Kensler (Johns Hopkins University Bloomberg School of Public Health) for providing the breeders of Nrf2-deficient C57BL/6 mice.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- ABP
4-aminobiphenyl
- BBN
N-butyl-N-(4-hydroxybutyl)nitrosamine
- GCS
glutamate cysteine ligase
- GST
glutathione S-transferase
- NAT
N-acetyltransferase
- N-OH-AABP
N-hydroxy-N-acetyl-4-aminobiphenyl
- N-OH-ABP
N-hydroxy-4-aminobiphenyl
- NQO1
NAD(P)H:quinone oxidoreductase 1
- Nrf2
NF-E2 related factor-2
- SF
sulforaphane
- UGT
UDP-glucuronosyltransferase
References
- 1.Vineis P. Epidemiology of cancer from exposure to arylamines. Environ. Health Perspect. 1994;102(suppl. 6):7–10. doi: 10.1289/ehp.94102s67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Negri E, et al. Epidemiology and prevention of bladder cancer. Eur. J. Cancer Prev. 2001;10:7–14. doi: 10.1097/00008469-200102000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Smith CJ, et al. IARC carcinogens reported in cigarette mainstream smoke and their calculated log P values. Food Chem. Toxicol. 2003;41:807–817. doi: 10.1016/s0278-6915(03)00021-8. [DOI] [PubMed] [Google Scholar]
- 4.Stabbert R, et al. Analysis of aromatic amines in cigarette smoke. Rapid Commun. Mass Spectrom. 2003;17:2125–2132. doi: 10.1002/rcm.1161. [DOI] [PubMed] [Google Scholar]
- 5.Moir D, et al. A comparison of mainstream and sidestream marijuana and tobacco cigarette smoke produced under two machine smoking conditions. Chem. Res. Toxicol. 2008;21:494–502. doi: 10.1021/tx700275p. [DOI] [PubMed] [Google Scholar]
- 6.Talaska G, et al. Smoking related carcinogen-DNA adducts in biopsy samples of human urinary bladder: identification of N-(deoxyguanosin-8-yl)-4-aminobiphenyl as a major adduct. Proc. Natl Acad. Sci. USA. 1991;88:5350–5354. doi: 10.1073/pnas.88.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Talaska G, et al. Detection of carcinogen-DNA adducts in exfoliated urothelial cells of cigarette smokers: association with smoking, hemoglobin adducts, and urinary mutagenicity. Cancer Epidemiol. Biomarkers Prev. 1991;1:61–66. [PubMed] [Google Scholar]
- 8.Airoldi L, et al. Determinants of 4-aminobiphenyl-DNA adducts in bladder cancer biopsies. Carcinogenesis. 2002;23:861–866. doi: 10.1093/carcin/23.5.861. [DOI] [PubMed] [Google Scholar]
- 9.Curigliano G, et al. Immunohistochemical quantitation of 4-aminobiphenyl-DNA adducts and p53 nuclear overexpression in T1 bladder cancer of smokers and nonsmokers. Carcinogenesis. 1996;17:911–916. doi: 10.1093/carcin/17.5.911. [DOI] [PubMed] [Google Scholar]
- 10.Bookland EA, et al. Tumorigenic transformation and neoplastic progression of human uroepithelial cells after exposure in vitro to 4-aminobiphenyl or its metabolites. Cancer Res. 1992;52:1606–1614. [PubMed] [Google Scholar]
- 11.Block NL, et al. The initiation, progress, and diagnosis of dog bladder cancer induced by 4-aminobiphenyl. Invest. Urol. 1978;16:50–54. [PubMed] [Google Scholar]
- 12.Poirier MC, et al. DNA adduct formation and tumorigenesis in mice during the chronic administration of 4-aminobiphenyl at multiple dose levels. Carcinogenesis. 1995;16:2917–2921. doi: 10.1093/carcin/16.12.2917. [DOI] [PubMed] [Google Scholar]
- 13.Michaud DS, et al. Fruit and vegetable intake and incidence of bladder cancer in a male prospective cohort. J. Natl Cancer Inst. 1999;91:605–613. doi: 10.1093/jnci/91.7.605. [DOI] [PubMed] [Google Scholar]
- 14.Tang L, et al. Consumption of raw cruciferous vegetables is inversely associated with bladder cancer risk. Cancer Epidemiol. Biomarkers Prev. 2008;17:938–944. doi: 10.1158/1055-9965.EPI-07-2502. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, et al. Discovery and development of sulforaphane as a cancer chemopreventive phytochemical. Acta Pharmacol. Sin. 2007;28:1343–1354. doi: 10.1111/j.1745-7254.2007.00679.x. [DOI] [PubMed] [Google Scholar]
- 16.Tang L, et al. Dietary isothiocyanates inhibit the growth of human bladder carcinoma cells. J. Nutr. 2004;134:2004–2010. doi: 10.1093/jn/134.8.2004. [DOI] [PubMed] [Google Scholar]
- 17.Munday R, et al. Inhibition of urinary bladder carcinogenesis by broccoli sprouts. Cancer Res. 2008;68:1593–1600. doi: 10.1158/0008-5472.CAN-07-5009. [DOI] [PubMed] [Google Scholar]
- 18.Kassahun K, et al. Biotransformation of the naturally occurring isothiocyanate sulforaphane in the rat: identification of phase I metabolites and glutathione conjugates. Chem. Res. Toxicol. 1997;10:1228–1233. doi: 10.1021/tx970080t. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, et al. Induction of GST and NQO1 in cultured bladder cells and in the urinary bladders of rats by an extract of broccoli (Brassica oleracea italica) sprouts. J. Agric. Food Chem. 2006;54:9370–9376. doi: 10.1021/jf062109h. [DOI] [PubMed] [Google Scholar]
- 20.Iida K, et al. Nrf2 is essential for the chemopreventive efficacy of oltipraz against urinary bladder carcinogenesis. Cancer Res. 2004;64:6424–6431. doi: 10.1158/0008-5472.CAN-04-1906. [DOI] [PubMed] [Google Scholar]
- 21.Kwak MK, et al. Chemoprevention through the Keap1-Nrf2 signaling pathway by phase 2 enzyme inducers. Mutat Res. 2004;555:133–148. doi: 10.1016/j.mrfmmm.2004.06.041. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, et al. A strategy for cancer prevention: stimulation of the Nrf2-ARE signaling pathway. Mol. Cancer Ther. 2004;3:885–893. [PubMed] [Google Scholar]
- 23.Tang L, et al. The principal urinary metabolites of dietary isothiocyanates, N-acetylcysteine conjugates, elicit the same anti-proliferative response as their parent compounds in human bladder cancer cells. Anticancer Drugs. 2006;17:297–305. doi: 10.1097/00001813-200603000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Shin S, et al. Role of Nrf2 in prevention of high-fat diet-induced obesity by synthetic triterpenoid CDDO-imidazolide. Eur. J. Pharmacol. 2009;620:138–144. doi: 10.1016/j.ejphar.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Randall KL, et al. An improved liquid chromatography-tandem mass spectrometry method for the quantification of 4-aminobiphenyl DNA adducts in urinary bladder cells and tissues. J. Chromatogr. A. 2010;1217:4135–4143. doi: 10.1016/j.chroma.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paonessa JD, et al. 5,6-Dihydrocyclopenta[c][1,2]-dithiole-3(4H)-thione is a promising cancer chemopreventive agent in the urinary bladder. Chem. Biol. Interact. 2009;180:119–126. doi: 10.1016/j.cbi.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beland FA, et al. Arylamine-DNA adducts in vitro and in vivo: their role in bacterial mutagenesis and urinary bladder carcinogenesis. Environ. Health Perspect. 1983;49:125–134. doi: 10.1289/ehp.8349125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swaminathan S, et al. Human urinary bladder epithelial cells lacking wild-type p53 function are deficient in the repair of 4-aminobiphenyl-DNA adducts in genomic DNA. Mutat. Res. 2002;499:103–117. doi: 10.1016/s0027-5107(01)00271-8. [DOI] [PubMed] [Google Scholar]
- 29.Sanchez-Carbayo M, et al. Molecular profiling of bladder cancer using cDNA microarrays: defining histogenesis and biological phenotypes. Cancer Res. 2002;62:6973–6980. [PubMed] [Google Scholar]
- 30.Tsuneoka Y, et al. 4-Aminobiphenyl-induced liver and urinary bladder DNA adduct formation in Cyp1a2(−/−) and Cyp1a2(+/+) mice. J. Natl Cancer Inst. 2003;95:1227–1237. doi: 10.1093/jnci/djg025. [DOI] [PubMed] [Google Scholar]
- 31.Trinidad A, et al. Purification of hepatic polymorphic arylamine N-acetyltransferase from homozygous rapid acetylator inbred hamster: identity with polymorphic N-hydroxyarylamine-O-acetyltransferase. Cancer Res. 1990;50:7942–7949. [PubMed] [Google Scholar]
- 32.Hatcher JF, et al. Detection of deoxyadenosine-4-aminobiphenyl adduct in DNA of human uroepithelial cells treated with N-hydroxy-4-aminobiphenyl following nuclease P1 enrichment and 32P-postlabeling analysis. Carcinogenesis. 1995;16:295–301. doi: 10.1093/carcin/16.2.295. [DOI] [PubMed] [Google Scholar]
- 33.Feng Z, et al. 4-Aminobiphenyl is a major etiological agent of human bladder cancer: evidence from its DNA binding spectrum in human p53 gene. Carcinogenesis. 2002;23:1721–1727. doi: 10.1093/carcin/23.10.1721. [DOI] [PubMed] [Google Scholar]
- 34.Zhang DD. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab. Rev. 2006;38:769–789. doi: 10.1080/03602530600971974. [DOI] [PubMed] [Google Scholar]
- 35.Dinkova-Kostova AT, et al. The role of Keap1 in cellular protective responses. Chem. Res. Toxicol. 2005;18:1779–1791. doi: 10.1021/tx050217c. [DOI] [PubMed] [Google Scholar]
- 36.Zhang DD, et al. Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol. Cell. Biol. 2003;23:8137–8151. doi: 10.1128/MCB.23.22.8137-8151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poirier MC, et al. DNA adduct measurements and tumor incidence during chronic carcinogen exposure in rodents. Environ. Health Perspect. 1994;102(suppl. 6):161–165. doi: 10.1289/ehp.94102s6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang L, et al. Intake of cruciferous vegetables modifies bladder cancer survival. Cancer Epidemiol. Biomarkers Prev. 2010;19:1806–1811. doi: 10.1158/1055-9965.EPI-10-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fahey JW, et al. Broccoli sprouts: an exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc. Natl Acad. Sci. USA. 1997;94:10367–10372. doi: 10.1073/pnas.94.19.10367. [DOI] [PMC free article] [PubMed] [Google Scholar]