Abstract
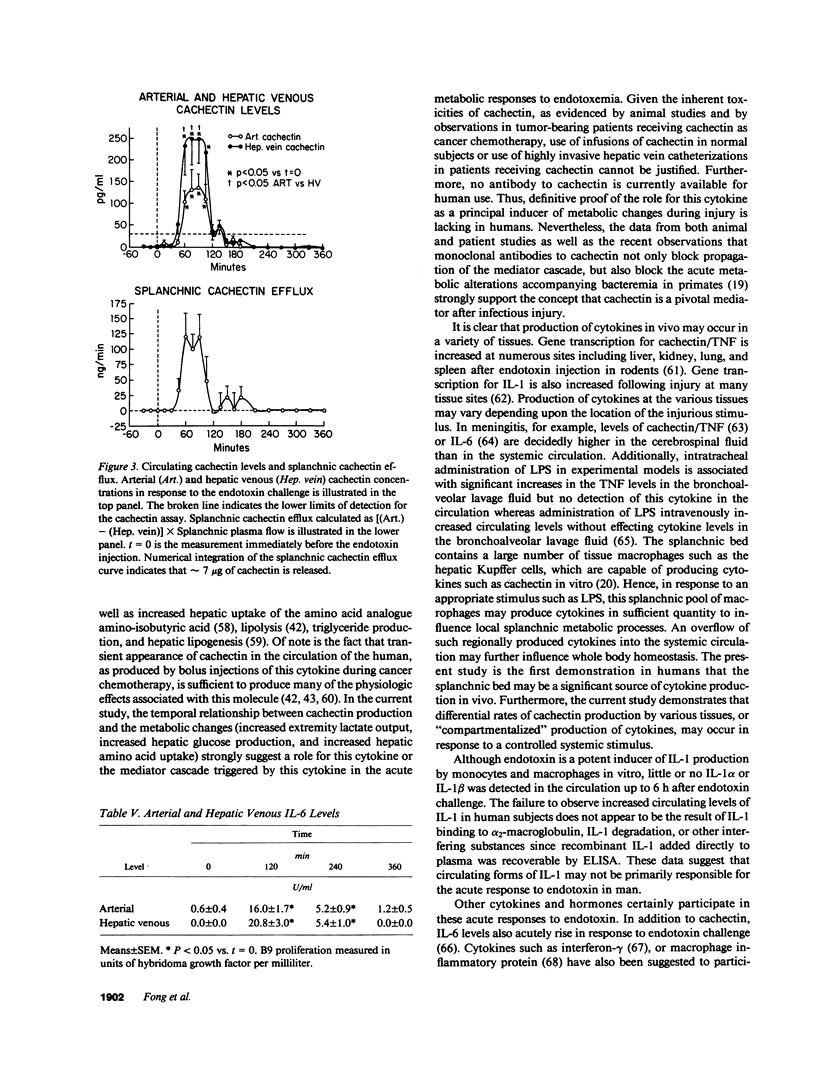
The in vivo alterations in organ-specific substrate processing and endogenous mediator production induced by endotoxin were investigated in healthy volunteers. An endotoxin bolus (20 U/kg) produced increased energy expenditure, hyperglycemia, hypoaminoacidemia, and an increase in circulating free fatty acids. These changes included increased peripheral lactate and free fatty acid output, along with increased peripheral uptake of glucose. Coordinately, there were increased splanchnic uptake of oxygen, lactate, amino acids, and free fatty acids, and increased splanchnic glucose output. There were no changes in circulating glucagon, or insulin and transient changes in epinephrine and cortisol were insufficient to explain the metabolic changes. Plasma cachectin levels peaked 90 min after the endotoxin infusion, and hepatic venous (HV) cachectin levels (peak 250 +/- 50 pg/ml) were consistently higher than arterial levels (peak 130 +/- 30 pg/ml, P less than 0.05 vs. HV). No interleukin 1 alpha or 1 beta was detected in the circulation. Circulating interleukin 6, measured by B.9 hybridoma proliferation, peaked 2 h after the endotoxin challenge (arterial, 16 +/- 2 U/ml; HV, 21 +/- 3 U/ml). The net cachectin efflux (approximately 7 micrograms) from the splanchnic organs demonstrates that these tissues are a major site for production of this cytokine. Hence, splanchnic tissues are likely influenced in a paracrine fashion by regional cachectin production and may also serve as a significant source for systemic appearance of this cytokine.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarden L. A., De Groot E. R., Schaap O. L., Lansdorp P. M. Production of hybridoma growth factor by human monocytes. Eur J Immunol. 1987 Oct;17(10):1411–1416. doi: 10.1002/eji.1830171004. [DOI] [PubMed] [Google Scholar]
- Aguilar-Parada E., Eisentraut A. M., Unger R. H. Effects of starvation on plasma pancreatic glucagon in normal man. Diabetes. 1969 Nov;18(11):717–723. doi: 10.2337/diab.18.11.717. [DOI] [PubMed] [Google Scholar]
- BEARN A. G., BILLING B., SHERLOCK S. The effect of adrenaline and noradrenaline on hepatic blood flow and splanchnic carbohydrate metabolism in man. J Physiol. 1951 Dec 28;115(4):430–441. doi: 10.1113/jphysiol.1951.sp004679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLAINEY J. D., ONEN K. H., WADE O. L. Amino-acids in hepatic venous and arterial blood investigated by paper chromatography. Lancet. 1956 Nov 24;271(6952):1075–1076. doi: 10.1016/s0140-6736(56)90209-4. [DOI] [PubMed] [Google Scholar]
- Bessey P. Q., Watters J. M., Aoki T. T., Wilmore D. W. Combined hormonal infusion simulates the metabolic response to injury. Ann Surg. 1984 Sep;200(3):264–281. doi: 10.1097/00000658-198409000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blick M., Sherwin S. A., Rosenblum M., Gutterman J. Phase I study of recombinant tumor necrosis factor in cancer patients. Cancer Res. 1987 Jun 1;47(11):2986–2989. [PubMed] [Google Scholar]
- CAESAR J., SHALDON S., CHIANDUSSI L., GUEVARA L., SHERLOCK S. The use of indocyanine green in the measurement of hepatic blood flow and as a test of hepatic function. Clin Sci. 1961 Aug;21:43–57. [PubMed] [Google Scholar]
- DOLE V. P., MEINERTZ H. Microdetermination of long-chain fatty acids in plasma and tissues. J Biol Chem. 1960 Sep;235:2595–2599. [PubMed] [Google Scholar]
- Dahn M. S., Lange P., Lobdell K., Hans B., Jacobs L. A., Mitchell R. A. Splanchnic and total body oxygen consumption differences in septic and injured patients. Surgery. 1987 Jan;101(1):69–80. [PubMed] [Google Scholar]
- Eigler N., Saccà L., Sherwin R. S. Synergistic interactions of physiologic increments of glucagon, epinephrine, and cortisol in the dog: a model for stress-induced hyperglycemia. J Clin Invest. 1979 Jan;63(1):114–123. doi: 10.1172/JCI109264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwyn D. H., Parikh H. C., Shoemaker W. C. Amino acid movements between gut, liver, and periphery in unanesthetized dogs. Am J Physiol. 1968 Nov;215(5):1260–1275. doi: 10.1152/ajplegacy.1968.215.5.1260. [DOI] [PubMed] [Google Scholar]
- Feingold K. R., Grunfeld C. Tumor necrosis factor-alpha stimulates hepatic lipogenesis in the rat in vivo. J Clin Invest. 1987 Jul;80(1):184–190. doi: 10.1172/JCI113046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felig P., Wahren J., Hendler R. Influence of physiologic hyperglucagonemia on basal and insulin-inhibited splanchnic glucose output in normal man. J Clin Invest. 1976 Sep;58(3):761–765. doi: 10.1172/JCI108523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong Y., Moldawer L. L., Marano M., Wei H., Barber A., Manogue K., Tracey K. J., Kuo G., Fischman D. A., Cerami A. Cachectin/TNF or IL-1 alpha induces cachexia with redistribution of body proteins. Am J Physiol. 1989 Mar;256(3 Pt 2):R659–R665. doi: 10.1152/ajpregu.1989.256.3.R659. [DOI] [PubMed] [Google Scholar]
- Fong Y., Moldawer L. L., Marano M., Wei H., Tatter S. B., Clarick R. H., Santhanam U., Sherris D., May L. T., Sehgal P. B. Endotoxemia elicits increased circulating beta 2-IFN/IL-6 in man. J Immunol. 1989 Apr 1;142(7):2321–2324. [PubMed] [Google Scholar]
- Fong Y., Tracey K. J., Moldawer L. L., Hesse D. G., Manogue K. B., Kenney J. S., Lee A. T., Kuo G. C., Allison A. C., Lowry S. F. Antibodies to cachectin/tumor necrosis factor reduce interleukin 1 beta and interleukin 6 appearance during lethal bacteremia. J Exp Med. 1989 Nov 1;170(5):1627–1633. doi: 10.1084/jem.170.5.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand R. A., Matthews D. E., Bier D. M., Sherwin R. S. Role of counterregulatory hormones in the catabolic response to stress. J Clin Invest. 1984 Dec;74(6):2238–2248. doi: 10.1172/JCI111650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUGGETT A. S., NIXON D. A. Use of glucose oxidase, peroxidase, and O-dianisidine in determination of blood and urinary glucose. Lancet. 1957 Aug 24;273(6991):368–370. doi: 10.1016/s0140-6736(57)92595-3. [DOI] [PubMed] [Google Scholar]
- Havel R. J., Kane J. P., Balasse E. O., Segel N., Basso L. V. Splanchnic metabolism of free fatty acids and production of triglycerides of very low density lipoproteins in normotriglyceridemic and hypertriglyceridemic humans. J Clin Invest. 1970 Nov;49(11):2017–2035. doi: 10.1172/JCI106422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfgott D. C., Tatter S. B., Santhanam U., Clarick R. H., Bhardwaj N., May L. T., Sehgal P. B. Multiple forms of IFN-beta 2/IL-6 in serum and body fluids during acute bacterial infection. J Immunol. 1989 Feb 1;142(3):948–953. [PubMed] [Google Scholar]
- Hesse D. G., Tracey K. J., Fong Y., Manogue K. R., Palladino M. A., Jr, Cerami A., Shires G. T., Lowry S. F. Cytokine appearance in human endotoxemia and primate bacteremia. Surg Gynecol Obstet. 1988 Feb;166(2):147–153. [PubMed] [Google Scholar]
- Hochstein H. D., Mills D. F., Outschoorn A. S., Rastogi S. C. The processing and collaborative assay of a reference endotoxin. J Biol Stand. 1983 Oct;11(4):251–260. doi: 10.1016/s0092-1157(83)80013-4. [DOI] [PubMed] [Google Scholar]
- Imamura M., Clowes G. H., Jr Hepatic blood flow and oxygen consumption in starvation, sepsis and septic shock. Surg Gynecol Obstet. 1975 Jul;141(1):27–34. [PubMed] [Google Scholar]
- Jepson M. M., Pell J. M., Bates P. C., Millward D. J. The effects of endotoxaemia on protein metabolism in skeletal muscle and liver of fed and fasted rats. Biochem J. 1986 Apr 15;235(2):329–336. doi: 10.1042/bj2350329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIBLER R. F., TAYLOR W. J., MYERS J. D. THE EFFECT OF GLUCAGON ON NET SPANCHNIC BALANCES OF GLUCOSE, AMINO ACID NITROGEN, UREA, KETONES, ANS OXYGEN IN MAN. J Clin Invest. 1964 May;43:904–915. doi: 10.1172/JCI104976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney J. S., Masada M. P., Eugui E. M., Delustro B. M., Mulkins M. A., Allison A. C. Monoclonal antibodies to human recombinant interleukin 1 (IL 1)beta: quantitation of IL 1 beta and inhibition of biological activity. J Immunol. 1987 Jun 15;138(12):4236–4242. [PubMed] [Google Scholar]
- Kurzrock R., Rohde M. F., Quesada J. R., Gianturco S. H., Bradley W. A., Sherwin S. A., Gutterman J. U. Recombinant gamma interferon induces hypertriglyceridemia and inhibits post-heparin lipase activity in cancer patients. J Exp Med. 1986 Oct 1;164(4):1093–1101. doi: 10.1084/jem.164.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. D., Zentella A., Vine W., Pekala P. H., Cerami A. Effect of endotoxin-induced monokines on glucose metabolism in the muscle cell line L6. Proc Natl Acad Sci U S A. 1987 May;84(9):2590–2594. doi: 10.1073/pnas.84.9.2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P. L. Single-column system for accelerated amino acid analysis of physiological fluids using five lithium buffers. Biochem Med. 1974 Jun;10(2):107–121. doi: 10.1016/0006-2944(74)90013-1. [DOI] [PubMed] [Google Scholar]
- Leist T. P., Frei K., Kam-Hansen S., Zinkernagel R. M., Fontana A. Tumor necrosis factor alpha in cerebrospinal fluid during bacterial, but not viral, meningitis. Evaluation in murine model infections and in patients. J Exp Med. 1988 May 1;167(5):1743–1748. doi: 10.1084/jem.167.5.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathison J. C., Wolfson E., Ulevitch R. J. Participation of tumor necrosis factor in the mediation of gram negative bacterial lipopolysaccharide-induced injury in rabbits. J Clin Invest. 1988 Jun;81(6):1925–1937. doi: 10.1172/JCI113540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan M. W., Artiss J. D., Strandbergh D. R., Zak B. A peroxidase-coupled method for the colorimetric determination of serum triglycerides. Clin Chem. 1983 Mar;29(3):538–542. [PubMed] [Google Scholar]
- Moldawer L. L., Andersson C., Gelin J., Lundholm K. G. Regulation of food intake and hepatic protein synthesis by recombinant-derived cytokines. Am J Physiol. 1988 Mar;254(3 Pt 1):G450–G456. doi: 10.1152/ajpgi.1988.254.3.G450. [DOI] [PubMed] [Google Scholar]
- Morrison D. C., Ryan J. L. Endotoxins and disease mechanisms. Annu Rev Med. 1987;38:417–432. doi: 10.1146/annurev.me.38.020187.002221. [DOI] [PubMed] [Google Scholar]
- Mészáros K., Lang C. H., Bagby G. J., Spitzer J. J. Tumor necrosis factor increases in vivo glucose utilization of macrophage-rich tissues. Biochem Biophys Res Commun. 1987 Nov 30;149(1):1–6. doi: 10.1016/0006-291x(87)91596-8. [DOI] [PubMed] [Google Scholar]
- Nelson S., Bagby G. J., Bainton B. G., Wilson L. A., Thompson J. J., Summer W. R. Compartmentalization of intraalveolar and systemic lipopolysaccharide-induced tumor necrosis factor and the pulmonary inflammatory response. J Infect Dis. 1989 Feb;159(2):189–194. doi: 10.1093/infdis/159.2.189. [DOI] [PubMed] [Google Scholar]
- Nordenström J., Carpentier Y. A., Askanazi J., Robin A. P., Elwyn D. H., Hensle T. W., Kinney J. M. Free fatty acid mobilization and oxidation during total parenteral nutrition in trauma and infection. Ann Surg. 1983 Dec;198(6):725–735. doi: 10.1097/00000658-198312000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton L., Moore G., Eiseman B. Liver failure in the postoperative patient: the role of sepsis and immunologic deficiency. Surgery. 1975 Jul;78(1):6–13. [PubMed] [Google Scholar]
- Passon P. G., Peuler J. D. A simplified radiometric assay for plasma norepinephrine and epinephrine. Anal Biochem. 1973 Feb;51(2):618–631. doi: 10.1016/0003-2697(73)90517-4. [DOI] [PubMed] [Google Scholar]
- Revhaug A., Michie H. R., Manson J. M., Watters J. M., Dinarello C. A., Wolff S. M., Wilmore D. W. Inhibition of cyclo-oxygenase attenuates the metabolic response to endotoxin in humans. Arch Surg. 1988 Feb;123(2):162–170. doi: 10.1001/archsurg.1988.01400260042004. [DOI] [PubMed] [Google Scholar]
- Scuderi P., Sterling K. E., Lam K. S., Finley P. R., Ryan K. J., Ray C. G., Petersen E., Slymen D. J., Salmon S. E. Raised serum levels of tumour necrosis factor in parasitic infections. Lancet. 1986 Dec 13;2(8520):1364–1365. doi: 10.1016/s0140-6736(86)92007-6. [DOI] [PubMed] [Google Scholar]
- Silen M. L., Hesse D. G., Felsen D., Moldawer L. L., Calvano S. E., Seniuk S., Cerami A., Lowry S. F. Cachectin/tumor necrosis factor production by fetal and newborn rat hepatic macrophages. J Pediatr Surg. 1989 Jan;24(1):34–38. doi: 10.1016/s0022-3468(89)80296-9. [DOI] [PubMed] [Google Scholar]
- Simmons P. S., Miles J. M., Gerich J. E., Haymond M. W. Increased proteolysis. An effect of increases in plasma cortisol within the physiologic range. J Clin Invest. 1984 Feb;73(2):412–420. doi: 10.1172/JCI111227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeldner J. S., Slone D. Critical variables in the radioimmunoassay of serum insulin using the double antibody technic. Diabetes. 1965 Dec;14(12):771–779. doi: 10.2337/diab.14.12.771. [DOI] [PubMed] [Google Scholar]
- Starnes H. F., Jr, Warren R. S., Jeevanandam M., Gabrilove J. L., Larchian W., Oettgen H. F., Brennan M. F. Tumor necrosis factor and the acute metabolic response to tissue injury in man. J Clin Invest. 1988 Oct;82(4):1321–1325. doi: 10.1172/JCI113733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takács L., Kovacs E. J., Smith M. R., Young H. A., Durum S. K. Detection of IL-1 alpha and IL-1 beta gene expression by in situ hybridization. Tissue localization of IL-1 mRNA in the normal C57BL/6 mouse. J Immunol. 1988 Nov 1;141(9):3081–3095. [PubMed] [Google Scholar]
- Tracey K. J., Beutler B., Lowry S. F., Merryweather J., Wolpe S., Milsark I. W., Hariri R. J., Fahey T. J., 3rd, Zentella A., Albert J. D. Shock and tissue injury induced by recombinant human cachectin. Science. 1986 Oct 24;234(4775):470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- Tracey K. J., Fong Y., Hesse D. G., Manogue K. R., Lee A. T., Kuo G. C., Lowry S. F., Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987 Dec 17;330(6149):662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- Tracey K. J., Lowry S. F., Fahey T. J., 3rd, Albert J. D., Fong Y., Hesse D., Beutler B., Manogue K. R., Calvano S., Wei H. Cachectin/tumor necrosis factor induces lethal shock and stress hormone responses in the dog. Surg Gynecol Obstet. 1987 May;164(5):415–422. [PubMed] [Google Scholar]
- Ulich T. R., Guo K., del Castillo J. Endotoxin-induced cytokine gene expression in vivo. I. Expression of tumor necrosis factor mRNA in visceral organs under physiologic conditions and during endotoxemia. Am J Pathol. 1989 Jan;134(1):11–14. [PMC free article] [PubMed] [Google Scholar]
- Waage A., Halstensen A., Espevik T. Association between tumour necrosis factor in serum and fatal outcome in patients with meningococcal disease. Lancet. 1987 Feb 14;1(8529):355–357. doi: 10.1016/s0140-6736(87)91728-4. [DOI] [PubMed] [Google Scholar]
- Wahren J., Felig P., Hagenfeldt L. Effect of protein ingestion on splanchnic and leg metabolism in normal man and in patients with diabetes mellitus. J Clin Invest. 1976 Apr;57(4):987–999. doi: 10.1172/JCI108375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren R. S., Donner D. B., Starnes H. F., Jr, Brennan M. F. Modulation of endogenous hormone action by recombinant human tumor necrosis factor. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8619–8622. doi: 10.1073/pnas.84.23.8619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren R. S., Starnes H. F., Jr, Gabrilove J. L., Oettgen H. F., Brennan M. F. The acute metabolic effects of tumor necrosis factor administration in humans. Arch Surg. 1987 Dec;122(12):1396–1400. doi: 10.1001/archsurg.1987.01400240042007. [DOI] [PubMed] [Google Scholar]
- Wilmore D. W., Goodwin C. W., Aulick L. H., Powanda M. C., Mason A. D., Jr, Pruitt B. A., Jr Effect of injury and infection on visceral metabolism and circulation. Ann Surg. 1980;192(4):491–504. doi: 10.1097/00000658-198010000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmore D. W., Long J. M., Mason A. D., Jr, Skreen R. W., Pruitt B. A., Jr Catecholamines: mediator of the hypermetabolic response to thermal injury. Ann Surg. 1974 Oct;180(4):653–669. doi: 10.1097/00000658-197410000-00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmore J. H., Davis J. A., Norton A. C. An automated system for assessing metabolic and respiratory function during exercise. J Appl Physiol. 1976 Apr;40(4):619–624. doi: 10.1152/jappl.1976.40.4.619. [DOI] [PubMed] [Google Scholar]
- Wolff J. E., Bergman E. N., Williams H. H. Net metabolism of plasma amino acids by liver and portal-drained viscera of fed sheep. Am J Physiol. 1972 Aug;223(2):438–446. doi: 10.1152/ajplegacy.1972.223.2.438. [DOI] [PubMed] [Google Scholar]
- Wolpe S. D., Davatelis G., Sherry B., Beutler B., Hesse D. G., Nguyen H. T., Moldawer L. L., Nathan C. F., Lowry S. F., Cerami A. Macrophages secrete a novel heparin-binding protein with inflammatory and neutrophil chemokinetic properties. J Exp Med. 1988 Feb 1;167(2):570–581. doi: 10.1084/jem.167.2.570. [DOI] [PMC free article] [PubMed] [Google Scholar]


