Abstract
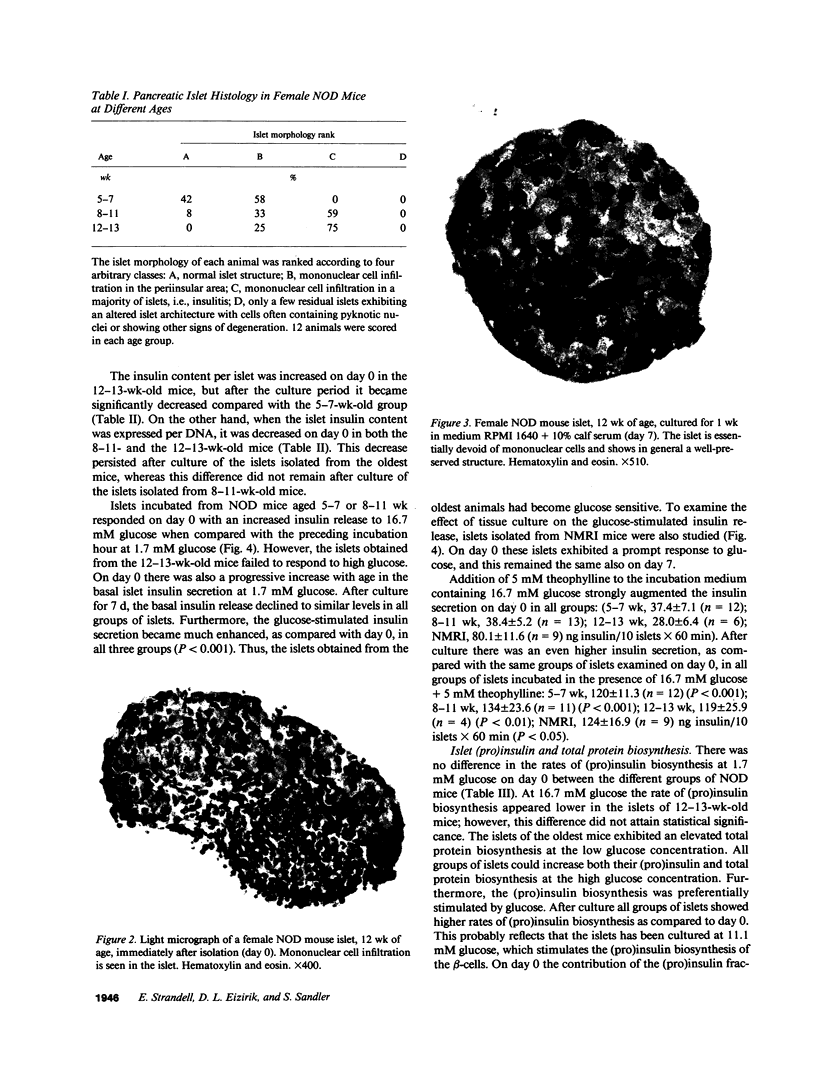
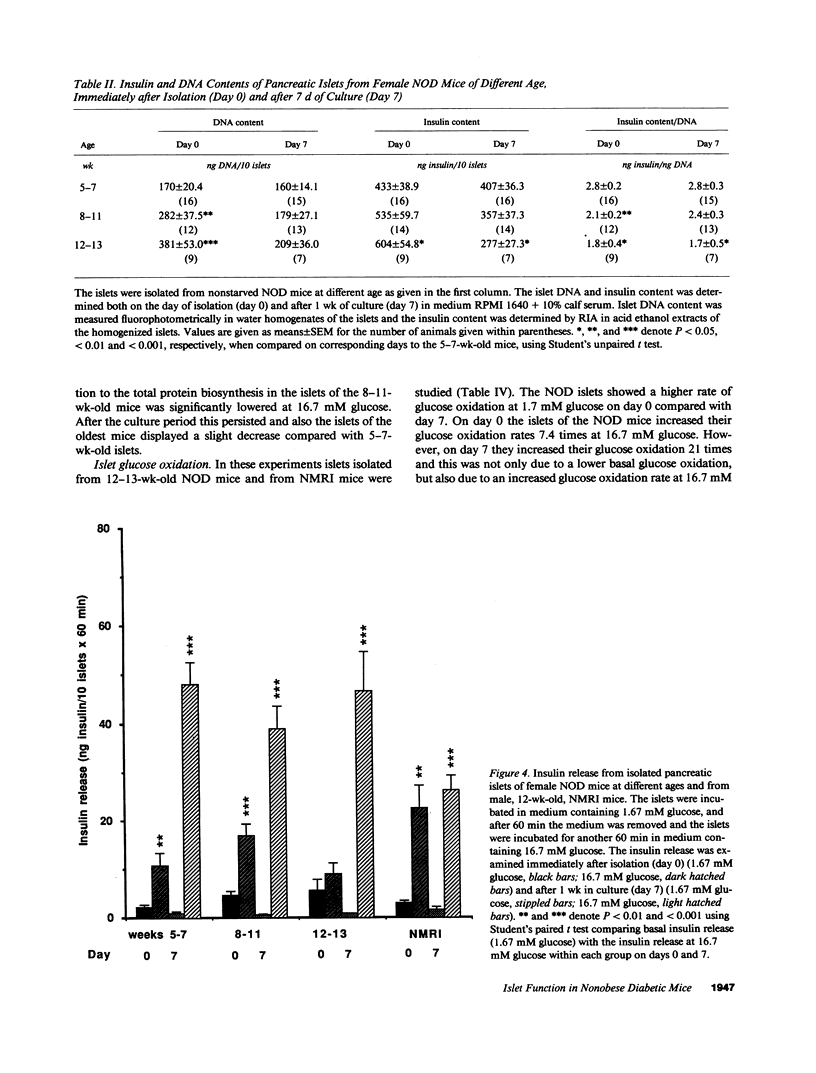
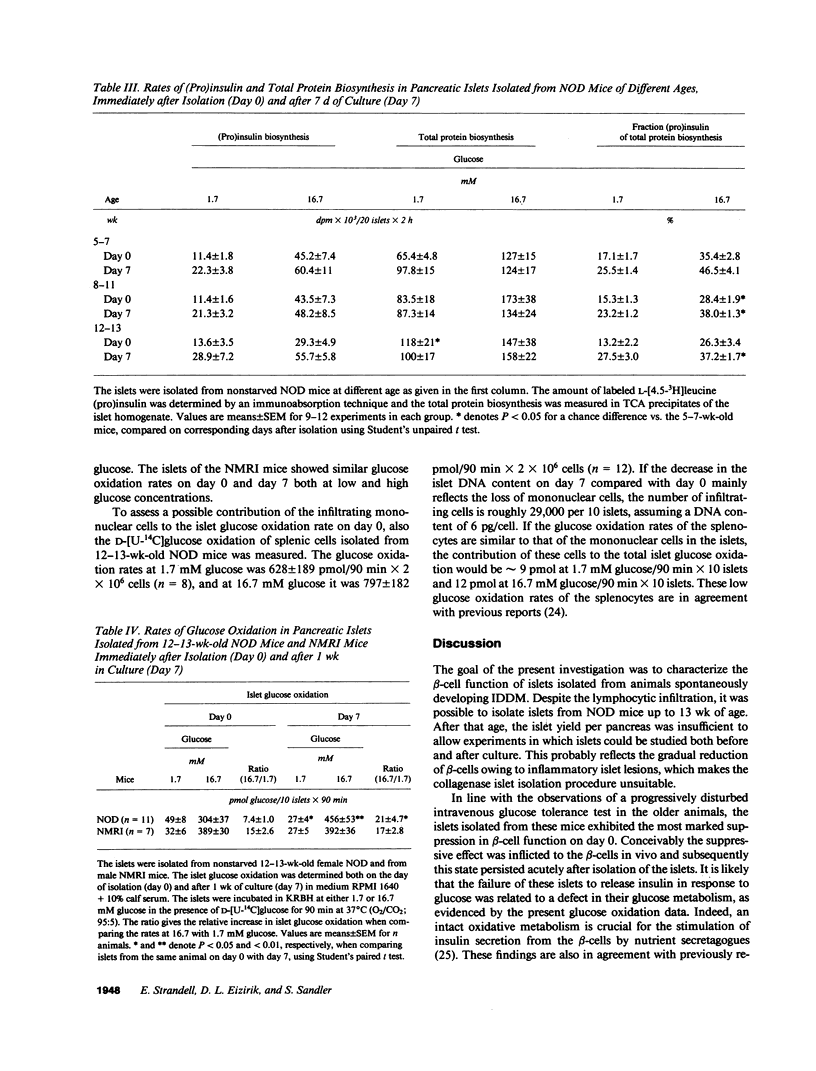
Insulin-dependent diabetes mellitus (IDDM) is characterized by a progressive autoimmune destruction of the pancreatic beta-cells. One of the best-suited animal models for IDDM is the nonobese diabetic (NOD) mouse. In this investigation pancreatic islets were isolated from female NOD mice aged 5-7, 8-11, and 12-13 wk and examined immediately (day 0) or after 7 d of culture (day 7). The mice showed a progressive disturbance in glucose tolerance with age, and a correspondingly increased frequency of pancreatic insulitis. Islets isolated from the oldest mice often contained inflammatory cells on day 0, which resulted in an elevated islet DNA content. During culture these islets became depleted of infiltrating cells and the DNA content of the islets decreased on day 7. Islets of the eldest mice failed to respond with insulin secretion to high glucose, whereas a response was observed in the other groups. After culture all groups of islets showed a markedly improved insulin secretion. Islets from the 12-13-wk-old mice displayed a lower glucose oxidation rate at 16.7 mM glucose on day 0 compared with day 7. Islet (pro)insulin and total protein biosynthesis was essentially unaffected. In conclusion, islets obtained from 12-13-wk-old NOD mice exhibit an impaired glucose metabolism, which may explain the suppressed insulin secretion observed immediately after isolation. This inhibition of beta-cell function can be reversed in vitro. Thus, there may be a stage during development of IDDM when beta-cell destruction can be counteracted and beta-cell function restored, provided the immune aggression is arrested.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson A., Sandler S. Viability tests of cryopreserved endocrine pancreatic cells. Cryobiology. 1983 Apr;20(2):161–168. doi: 10.1016/0011-2240(83)90005-6. [DOI] [PubMed] [Google Scholar]
- Appels B., Burkart V., Kantwerk-Funke G., Funda J., Kolb-Bachofen V., Kolb H. Spontaneous cytotoxicity of macrophages against pancreatic islet cells. J Immunol. 1989 Jun 1;142(11):3803–3808. [PubMed] [Google Scholar]
- Bendtzen K. Immune hormones (cytokines); pathogenic role in autoimmune rheumatic and endocrine diseases. Autoimmunity. 1989;2(2):177–189. doi: 10.3109/08916938909019954. [DOI] [PubMed] [Google Scholar]
- Bolaffi J. L., Nowlain R. E., Cruz L., Grodsky G. M. Progressive damage of cultured pancreatic islets after single early exposure to streptozocin. Diabetes. 1986 Sep;35(9):1027–1033. doi: 10.2337/diab.35.9.1027. [DOI] [PubMed] [Google Scholar]
- Comens P. G., Wolf B. A., Unanue E. R., Lacy P. E., McDaniel M. L. Interleukin 1 is potent modulator of insulin secretion from isolated rat islets of Langerhans. Diabetes. 1987 Aug;36(8):963–970. doi: 10.2337/diab.36.8.963. [DOI] [PubMed] [Google Scholar]
- Eisenbarth G. S., Connelly J., Soeldner J. S. The "natural" history of type I diabetes. Diabetes Metab Rev. 1987 Oct;3(4):873–891. doi: 10.1002/dmr.5610030404. [DOI] [PubMed] [Google Scholar]
- Eizirik D. L., Sandler S. Function and metabolism of pancreatic beta-cells maintained in culture following experimentally induced damage. Pharmacol Toxicol. 1989 Sep;65(3):163–168. doi: 10.1111/j.1600-0773.1989.tb01149.x. [DOI] [PubMed] [Google Scholar]
- Eizirik D. L., Sandler S. Functional restoration of cultured mouse pancreatic islets after in vitro exposure to alloxan. Pharmacol Toxicol. 1988 Nov;63(5):396–399. doi: 10.1111/j.1600-0773.1988.tb00975.x. [DOI] [PubMed] [Google Scholar]
- Eizirik D. L., Strandell E., Bendtzen K., Sandler S. Functional characteristics of rat pancreatic islets maintained in culture after exposure to human interleukin 1. Diabetes. 1988 Jul;37(7):916–919. doi: 10.2337/diab.37.7.916. [DOI] [PubMed] [Google Scholar]
- Formby B., Miller N., Garret R., Peterson C. M. Effects of low-dose cyclosporine prophylaxis in nonobese diabetic mice. J Pharmacol Exp Ther. 1987 Jun;241(3):1106–1111. [PubMed] [Google Scholar]
- Ganda O. P., Srikanta S., Brink S. J., Morris M. A., Gleason R. E., Soeldner J. S., Eisenbarth G. S. Differential sensitivity to beta-cell secretagogues in "early," type I diabetes mellitus. Diabetes. 1984 Jun;33(6):516–521. doi: 10.2337/diab.33.6.516. [DOI] [PubMed] [Google Scholar]
- Gillis S., Watson J. Interleukin-2 dependent culture of cytolytic T cell lines. Immunol Rev. 1981;54:81–109. doi: 10.1111/j.1600-065x.1981.tb00435.x. [DOI] [PubMed] [Google Scholar]
- Halban P. A., Wollheim C. B., Blondel B., Renold A. E. Long-term exposure of isolated pancreatic islets to mannoheptulose: evidence for insulin degradation in the beta cell. Biochem Pharmacol. 1980 Oct 1;29(19):2625–2633. doi: 10.1016/0006-2952(80)90077-5. [DOI] [PubMed] [Google Scholar]
- Haskins K., Portas M., Bergman B., Lafferty K., Bradley B. Pancreatic islet-specific T-cell clones from nonobese diabetic mice. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8000–8004. doi: 10.1073/pnas.86.20.8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heding L. G. Determination of total serum insulin (IRI) in insulin-treated diabetic patients. Diabetologia. 1972 Aug;8(4):260–266. doi: 10.1007/BF01225569. [DOI] [PubMed] [Google Scholar]
- Hinegardner R. T. An improved fluorometric assay for DNA. Anal Biochem. 1971 Jan;39(1):197–201. doi: 10.1016/0003-2697(71)90476-3. [DOI] [PubMed] [Google Scholar]
- Howell S. L., Taylor K. W. Potassium ions and the secretion of insulin by islets of Langerhans incubated in vitro. Biochem J. 1968 Jun;108(1):17–24. doi: 10.1042/bj1080017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson L., Sandler S. The influence of cyclosporin A on the vascular permeability of the pancreatic islets and on diabetes induced by multiple low doses of streptozotocin in the mouse. Virchows Arch A Pathol Anat Histopathol. 1988;412(3):225–230. doi: 10.1007/BF00737146. [DOI] [PubMed] [Google Scholar]
- KEEN H., FIELD J. B., PASTAN I. H. A simple method for in vitro metabolic studies using small volumes of tissue and medium. Metabolism. 1963 Feb;12:143–147. [PubMed] [Google Scholar]
- KISSANE J. M., ROBINS E. The fluorometric measurement of deoxyribonucleic acid in animal tissues with special reference to the central nervous system. J Biol Chem. 1958 Jul;233(1):184–188. [PubMed] [Google Scholar]
- Kanatsuna T., Baekkeskov S., Lernmark A., Ludvigsson J. Immunoglobulin from insulin-dependent diabetic children inhibits glucose-induced insulin release. Diabetes. 1983 Jun;32(6):520–524. doi: 10.2337/diab.32.6.520. [DOI] [PubMed] [Google Scholar]
- Kano Y., Kanatsuna T., Nakamura N., Kitagawa Y., Mori H., Kajiyama S., Nakano K., Kondo M. Defect of the first-phase insulin secretion to glucose stimulation in the perfused pancreas of the nonobese diabetic (NOD) mouse. Diabetes. 1986 Apr;35(4):486–490. doi: 10.2337/diab.35.4.486. [DOI] [PubMed] [Google Scholar]
- Klandorf H., Chirra A. R., DeGruccio A., Girman D. J. Dimethyl sulfoxide modulation of diabetes onset in NOD mice. Diabetes. 1989 Feb;38(2):194–197. doi: 10.2337/diab.38.2.194. [DOI] [PubMed] [Google Scholar]
- Kolb H. Mouse models of insulin dependent diabetes: low-dose streptozocin-induced diabetes and nonobese diabetic (NOD) mice. Diabetes Metab Rev. 1987 Jul;3(3):751–778. doi: 10.1002/dmr.5610030308. [DOI] [PubMed] [Google Scholar]
- Leiter E. H. Murine macrophages and pancreatic beta cells. Chemotactic properties of insulin and beta-cytostatic action of interleukin 1. J Exp Med. 1987 Oct 1;166(4):1174–1179. doi: 10.1084/jem.166.4.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiter E. H., Prochazka M., Coleman D. L. The non-obese diabetic (NOD) mouse. Am J Pathol. 1987 Aug;128(2):380–383. [PMC free article] [PubMed] [Google Scholar]
- MacKay P., Boulton A., Rabinovitch A. Lymphoid cells of BB/W diabetic rats are cytotoxic to islet beta cells in vitro. Diabetes. 1985 Jul;34(7):706–709. doi: 10.2337/diab.34.7.706. [DOI] [PubMed] [Google Scholar]
- MacKay P., Jacobson J., Rabinovitch A. Spontaneous diabetes mellitus in the Bio-Breeding/Worcester rat. Evidence in vitro for natural killer cell lysis of islet cells. J Clin Invest. 1986 Mar;77(3):916–924. doi: 10.1172/JCI112390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaisse W. J. Insulin release : the fuel concept. Diabete Metab. 1983 Dec;9(4):313–320. [PubMed] [Google Scholar]
- McEvoy R. C., Andersson J., Sandler S., Hellerström C. Multiple low-dose streptozotocin-induced diabetes in the mouse. Evidence for stimulation of a cytotoxic cellular immune response against an insulin-producing beta cell line. J Clin Invest. 1984 Sep;74(3):715–722. doi: 10.1172/JCI111487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy M. V., Chan E. K., Teruya M., Forrest L. E., Likhite V., Charles M. A. Macrophage-mediated islet cell cytotoxicity in BB rats. Diabetes. 1989 Oct;38(10):1329–1331. doi: 10.2337/diab.38.10.1329. [DOI] [PubMed] [Google Scholar]
- Nerup J., Mandrup-Poulsen T., Mølvig J. The HLA-IDDM association: implications for etiology and pathogenesis of IDDM. Diabetes Metab Rev. 1987 Jul;3(3):779–802. doi: 10.1002/dmr.5610030309. [DOI] [PubMed] [Google Scholar]
- Newsholme E. A., Crabtree B., Ardawi M. S. The role of high rates of glycolysis and glutamine utilization in rapidly dividing cells. Biosci Rep. 1985 May;5(5):393–400. doi: 10.1007/BF01116556. [DOI] [PubMed] [Google Scholar]
- Nomikos I. N., Prowse S. J., Carotenuto P., Lafferty K. J. Combined treatment with nicotinamide and desferrioxamine prevents islet allograft destruction in NOD mice. Diabetes. 1986 Nov;35(11):1302–1304. doi: 10.2337/diab.35.11.1302. [DOI] [PubMed] [Google Scholar]
- Ohneda A., Kobayashi T., Nihei J., Tochino Y., Kanaya H., Makino S. Insulin and glucagon in spontaneously diabetic non-obese mice. Diabetologia. 1984 Oct;27(4):460–463. doi: 10.1007/BF00273911. [DOI] [PubMed] [Google Scholar]
- Prochazka M., Leiter E. H., Serreze D. V., Coleman D. L. Three recessive loci required for insulin-dependent diabetes in nonobese diabetic mice. Science. 1987 Jul 17;237(4812):286–289. doi: 10.1126/science.2885918. [DOI] [PubMed] [Google Scholar]
- Rabinovitch A., Pukel C., Baquerizo H. Interleukin-1 inhibits glucose-modulated insulin and glucagon secretion in rat islet monolayer cultures. Endocrinology. 1988 Jun;122(6):2393–2398. doi: 10.1210/endo-122-6-2393. [DOI] [PubMed] [Google Scholar]
- Reich E. P., Sherwin R. S., Kanagawa O., Janeway C. A., Jr An explanation for the protective effect of the MHC class II I-E molecule in murine diabetes. Nature. 1989 Sep 28;341(6240):326–328. doi: 10.1038/341326a0. [DOI] [PubMed] [Google Scholar]
- Sandler S., Andersson A., Hellerström C. Inhibitory effects of interleukin 1 on insulin secretion, insulin biosynthesis, and oxidative metabolism of isolated rat pancreatic islets. Endocrinology. 1987 Oct;121(4):1424–1431. doi: 10.1210/endo-121-4-1424. [DOI] [PubMed] [Google Scholar]
- Sandler S., Andersson A. Modulation of streptozotocin-induced insulitis and hyperglycaemia in the mouse. Acta Pathol Microbiol Immunol Scand A. 1985 Mar;93(2):93–98. doi: 10.1111/j.1699-0463.1985.tb03925.x. [DOI] [PubMed] [Google Scholar]
- Schwizer R. W., Leiter E. H., Evans R. Macrophage-mediated cytotoxicity against cultured pancreatic islet cells. Transplantation. 1984 Jun;37(6):539–544. doi: 10.1097/00007890-198406000-00002. [DOI] [PubMed] [Google Scholar]
- Signore A., Pozzilli P., Gale E. A., Andreani D., Beverley P. C. The natural history of lymphocyte subsets infiltrating the pancreas of NOD mice. Diabetologia. 1989 May;32(5):282–289. doi: 10.1007/BF00265543. [DOI] [PubMed] [Google Scholar]
- Spinas G. A., Hansen B. S., Linde S., Kastern W., Mølvig J., Mandrup-Poulsen T., Dinarello C. A., Nielsen J. H., Nerup J. Interleukin 1 dose-dependently affects the biosynthesis of (pro)insulin in isolated rat islets of Langerhans. Diabetologia. 1987 Jul;30(7):474–480. doi: 10.1007/BF00279615. [DOI] [PubMed] [Google Scholar]
- Strandell E., Eizirik D. L., Korsgren O., Sandler S. Functional characteristics of cultured mouse pancreatic islets following exposure to different streptozotocin concentrations. Mol Cell Endocrinol. 1988 Sep;59(1-2):83–91. doi: 10.1016/0303-7207(88)90198-0. [DOI] [PubMed] [Google Scholar]
- Tochino Y. The NOD mouse as a model of type I diabetes. Crit Rev Immunol. 1987;8(1):49–81. [PubMed] [Google Scholar]
- Weir G. C., Leahy J. L., Bonner-Weir S. Experimental reduction of B-cell mass: implications for the pathogenesis of diabetes. Diabetes Metab Rev. 1986;2(1-2):125–161. doi: 10.1002/dmr.5610020108. [DOI] [PubMed] [Google Scholar]
- Welsh M., Eizirik D. L., Strandell E. Heat-shock treatment of mouse pancreatic islets results in a partial loss of islet cells but no remaining functional impairment among the surviving beta cells. J Mol Endocrinol. 1988 Jul;1(1):27–31. doi: 10.1677/jme.0.0010027. [DOI] [PubMed] [Google Scholar]
- Wicker L. S., Miller B. J., Coker L. Z., McNally S. E., Scott S., Mullen Y., Appel M. C. Genetic control of diabetes and insulitis in the nonobese diabetic (NOD) mouse. J Exp Med. 1987 Jun 1;165(6):1639–1654. doi: 10.1084/jem.165.6.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicker L. S., Miller B. J., Fischer P. A., Pressey A., Peterson L. B. Genetic control of diabetes and insulitis in the nonobese diabetic mouse. Pedigree analysis of a diabetic H-2nod/b heterozygote. J Immunol. 1989 Feb 1;142(3):781–784. [PubMed] [Google Scholar]
- Yamada K., Nonaka K., Hanafusa T., Miyazaki A., Toyoshima H., Tarui S. Preventive and therapeutic effects of large-dose nicotinamide injections on diabetes associated with insulitis. An observation in nonobese diabetic (NOD) mice. Diabetes. 1982 Sep;31(9):749–753. doi: 10.2337/diab.31.9.749. [DOI] [PubMed] [Google Scholar]
- Zucker P. F., Archer M. C. Streptozotocin toxicity to cultured pancreatic islets of the Syrian hamster. Cell Biol Toxicol. 1988 Sep;4(3):349–356. doi: 10.1007/BF00058742. [DOI] [PubMed] [Google Scholar]




