SYNOPSIS
Objectives
In 2007, a localized outbreak of tularemia occurred among visitors to a lodge on the western side of Utah Lake, Utah. We assessed risk factors for disease and attempted to identify undiagnosed clinically compatible illnesses.
Methods
We conducted a retrospective cohort study by recruiting all people who had visited the lodge on the western side of Utah Lake from June 3 to July 28, 2007. A self-administered questionnaire was distributed to a sub-cohort of people who were part of an organized group that had at least one tularemia patient. Questions assessed risk and protective factors and disease symptoms.
Results
During the outbreak period, 14 cases of tularemia were reported from five of Utah's 12 health districts. The weekly attack rate ranged from 0 to 2.1/100 lodge visitors from June 3 to July 28. Illness onset dates ranged from June 15 to July 8. The median delay between onset of symptoms and laboratory test for tularemia was 14 days (range: 7–34 days). Cohort study respondents who reported deer-fly bites while at the lodge (adjusted risk ratio [ARR] = 7.2, 95% confidence interval [CI] 2.4, 22.0) and who reported having worn a hat (ARR=5.6, 95% CI 1.3, 24.6) were more likely to become ill.
Conclusions
This was Utah's second documented deer-fly-associated human tularemia outbreak. People participating in outdoor activities in endemic areas should be aware of disease risks and take precautions. Educational campaigns can aid in earlier disease recognition, reporting, and, consequently, outbreak detection.
Tularemia is an uncommon zoonotic disease caused by Francisella tularensis (F. tularensis). Transmission to humans usually occurs through tick or deer-fly bites, ingestion of contaminated food or water, inhalation of contaminated aerosols, or handling of infected animals, especially rabbits, hares, rodents, and domestic cats.1 In the United States, a total of 1,368 cases of tularemia were reported from 44 states during 1990–2000, for a national average of 124 cases/year.2 The majority of cases occur singly and appear to be sporadic; however, focal outbreaks have been reported in association with tick bites,3–5 deer-fly bites,6 muskrat trapping,7 and aerosol-related exposures.8 Because F. tularensis has been developed as a potential bioweapon,9 rapid characterization of the source and route of transmission is important whenever outbreaks occur.
During June and July 2007, a total of 14 cases of tularemia were reported to the Utah Department of Health, compared with a mean of 2.5 cases/year (range: 0–5) between 1992 and 2006. A preliminary public health investigation determined that all patients had visited a lodge located on the western side of Utah Lake shortly before becoming ill. The lodge provides camping and outdoor recreation opportunities for local church-affiliated groups. To determine the scope of the outbreak and to identify risk factors for infection, we initiated active case finding and conducted a cohort study of visitors to the lodge from June 3 to July 28, 2007. This article describes the characteristics of the outbreak, presents results of the cohort study, and offers recommendations to improve tularemia diagnosis and prevent future outbreaks.
METHODS
Case ascertainment and clinical features
To identify additional possible cases among lodge visitors, we reviewed the guest registry and recorded the identity and size of all groups who had visited from June 3 to July 28, 2007. These dates were chosen because the lodge is used for youth group activities and family reunions from June to August, and the cohort study recruitment began at the end of July. Group leaders were contacted and asked to conduct among their group members an assessment of illness with fever within two weeks of visiting the lodge. All four lodge workers were questioned about exposures and illness. In addition, health-care providers and staff at health clinics and emergency departments were notified by blast faxes and listserv e-mails and asked to immediately report any suspect cases. A press release was issued to raise awareness among the public.
Information on clinical and demographic features was collected for all reported cases by interviewing patients or a family member. For purposes of this investigation, we defined a suspect case as fever (temperature [T] >100°F) associated with other clinical features consistent with ulceroglandular, glandular, oculoglandular, oropharyngeal, intestinal (pain, vomiting, and diarrhea), pneumonic, or typhoidal (septicemic) tularemia in a person who had visited the lodge from June 3 to July 28, 2007. We defined a confirmed human case as F. tularensis cultured from a clinical specimen or with a fourfold or greater increase in serum antibody titer to F. tularensis antigen. A probable human case was defined as F. tularensis antigens detected by a fluorescent assay, F. tularensis deoxyribonucleic acid (DNA) detected by a validated polymerase chain reaction (PCR) test, or a single elevated serum antibody titer in a person without history of tularemia vaccination.
Laboratory methods
Clinical specimens (e.g., whole blood, lesion swabs, or lymph node aspirates) and suspect F. tularensis isolates identified at hospital laboratories were submitted to the Utah Public Health Laboratories for further evaluation. Clinical specimens were inoculated onto chocolate agar plates and incubated in a 5% carbon dioxide atmosphere at 37°C for ≤3 days. Colonies with growth characteristic for F. tularensis were confirmed by direct fluorescent antibody (DFA) assay by using fluorescent isothiocyanate-labeled rabbit anti-F. tularensis subspecies tularensis antibodies (Centers for Disease Control and Prevention [CDC], Fort Collins, Colorado). Additionally, DNA was extracted from whole blood, lesion swabs, lymph node aspirates, or culture isolates by using the QIAamp® DNA Blood Mini Kit (Qiagen Inc., Valencia, California). Real-time PCR specific for F. tularensis DNA was performed by using the Laboratory Response Network standardized protocol and reagents (CDC, Atlanta, Georgia). Confirmed isolates and materials positive by PCR were forwarded to CDC, Fort Collins, as previously described for further characterization and molecular subtyping.10
A microagglutination assay was used to test serum or plasma for antibodies to F. tularensis.11 Serial dilutions of serum or plasma were incubated at room temperature overnight with safranin-O-stained, formalin-killed antigen (CDC, Fort Collins). A titer was assigned to the highest dilution of serum or plasma, producing full agglutination. Samples with a titer of 1:128 or greater were reported as positive. To demonstrate a fourfold change in antibody titer, acute and convalescent samples were tested concurrently.
Cohort study
Participants in the cohort study were recruited from groups that had visited the lodge from June 3 to July 28, 2007, and had at least one member with confirmed, probable, or suspect tularemia. Members of these groups were assumed to have the potential for an infectious exposure on the basis of time of visit to the lodge; they were retrospectively assessed for exposures and symptoms, and then case status was determined. Youth groups were from different counties throughout Utah, and one family group included people living both within and outside of Utah. Local health department personnel contacted group leaders who resided within that jurisdiction and asked them to provide contact information for group members and to help with study recruitment efforts. A self-administered questionnaire was distributed to group members by postal mail, e-mail, or at a church meeting. The questionnaire assessed exposures, risk factors, protective factors, and disease symptoms. Parental consent was required for participants aged <18 years. A parent completed the questionnaire for participants younger than 12 years of age.
Recruited groups were included in the cohort study analysis if the group response rate was ≥40%. Analysis of variance and Chi-square tests were used to compare ill and well participants with regard to age and sex, respectively. Univariate and multivariate risk ratios (RRs) and 95% confidence intervals (CIs) were determined by Poisson regression with robust error variance. The study protocol was not submitted for Institutional Review Board approval because the study was conducted as part of the public health response to an outbreak.
RESULTS
Outbreak description
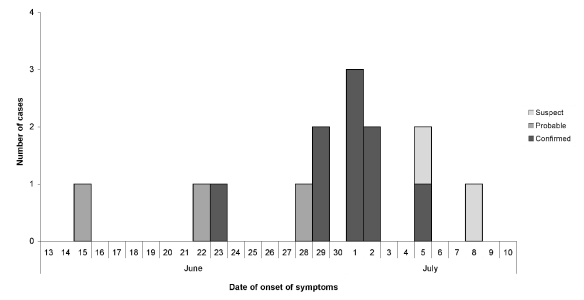
Fourteen tularemia patients (nine confirmed, three probable, and two suspect) were identified with illness onset dates from June 15 to July 8 (Figure). Of the nine patients with confirmed illness, five had F. tularensis cultured from clinical specimens, and four had a fourfold or greater change in serum antibody titer to F. tularensis antigen (three of these four patients also had F. tularensis DNA detected by PCR). The three probable patients had F. tularensis DNA detected by PCR (two of these three patients also had F. tularensis antigens detected by DFA). Two suspect patients had fever and swollen lymph nodes with or without skin ulcers.
Figure.
Epidemic curve of laboratory-confirmed, probable, and suspected tularemia cases reported in Utah (n=14) by date of onset of symptoms, June–July 2007
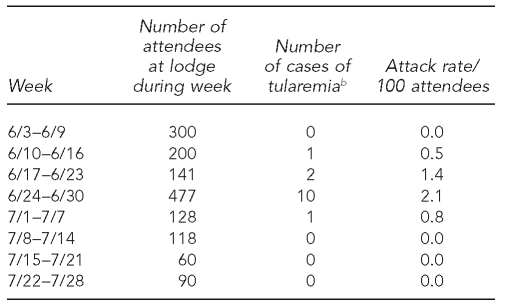
Case reports and active surveillance identified all 14 patients; no additional patients were identified by the cohort study. Attendance records at the lodge indicated that 1,514 people had visited the lodge from June 3 to July 28; the attack rate was 0.9%. The attack rate by week of lodge attendance peaked at 2% during the week beginning June 24 (Table 1). This week also had the greatest number of attendees and the greatest absolute number of cases among attendees. Patients were exposed during a four-week period, June 10–July 7. The peak number of cases by onset occurred during the subsequent week (Figure), consistent with the usual incubation period for tularemia (3–5 days; range: 1–14 days).12 None of the four lodge workers reported any symptoms, and they were not included in the cohort study. No other tularemia cases were reported in Utah during this period.
Table 1.
Lodge attendance, number of tularemia cases, and attack rate (patients/100 attendees) by week, June 3–July 28, 2007, Utah Lake, Utaha
aTularemia patients were included in the week during which they attended the lodge and not in the week of reported illness onset. Weekly attack rate was calculated as number of patients among lodge attendees who had visited the lodge during a week, divided by the number of lodge attendees during that week.
bNumber of attendees during a given week who would become patients with confirmed, probable, or suspected tularemia
Patients resided in five of Utah's 29 counties and were aged 1–77 years (median = 19 years). All patients were white and eight patients (57%) were male. All patients presented with fever, fatigue, and loss of appetite. The majority of patients also presented with chills (93%), headache (93%), swollen lymph nodes (86%), or skin ulcers (86%). Other symptoms included sore throat (64%), cough (57%), abdominal pain (50%), diarrhea (43%), chest pain (43%), red eyes (36%), mouth ulcer (21%), and vomiting (7%). The clinical presentation was ulceroglandular tularemia for 12 patients (86%) and glandular tularemia for two patients (14%). Six patients (43%) were hospitalized; no deaths occurred.
The median delay between onset of symptoms and the date the laboratory test for tularemia was conducted was 14 days (range: 7–34 days). Tularemia diagnosis was delayed because of other diagnoses or treatments for different conditions. Initial diagnoses (before diagnosis of tularemia) included, but were not limited to, cellulitis, methicillin-resistant Staphylococcus aureus infection, and insect bite with concomitant gastroenteritis. Case reporting to public health officials began on July 13, when physicians at two different hospitals notified the local health department that they had treated patients with skin ulcers and fever. This was 28 days after the onset of illness for the first confirmed case.
Cohort study
Six of seven groups that visited the lodge between June 3 and July 28 and were recruited for the cohort study were included in the analysis. The excluded group had a response rate of 5%. Questionnaires were completed for 441 (73%) of 604 eligible participants in the six included groups, including 13 of 14 confirmed, probable, or suspect tularemia patients. No information was available for people who did not respond. Respondents' ages ranged from <1 to 80 years (median = 17 years); 226 respondents (51%) were male. Ill respondents did not differ from well respondents with regard to age or sex (p=0.341 and p=0.451, respectively).
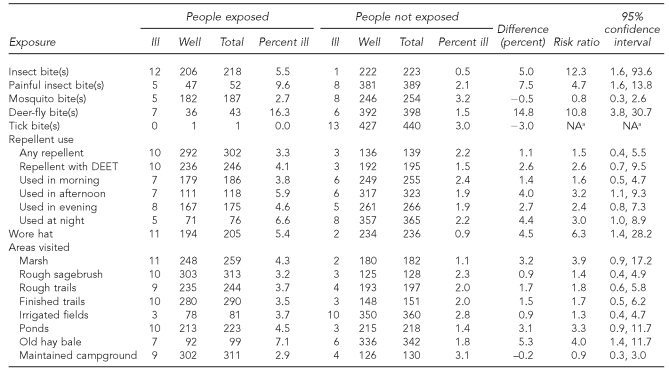
Respondents who reported any type of insect bite while at the lodge were more likely to have become ill than those who did not (RR=12.3, 95% CI 1.6, 93.6) (Table 2). The same was true for respondents who reported especially painful insect bites (RR=4.7, 95% CI 1.6, 13.8) and deer-fly bites (RR=10.8, 95% CI 3.8, 30.7). Mosquito bites and tick bites were not significant risk factors for illness (p>0.05). No statistically significant risks were associated with a bite or sting of a gnat, horsefly, other biting fly, wasp, ant, or spider (p>0.05; data not shown).
Table 2.
Exposure-specific risk ratios from the 2007 Utah Lake, Utah, tularemia outbreak cohort study (n=441)
aRisk ratio and 95% confidence interval could not be calculated because no ill people were exposed.
NA = not applicable
Overall, use of insect repellent was not protective (RR=1.5, 95% CI 0.4, 5.5), and neither was use of DEET-containing repellent specifically (RR=2.6, 95% CI 0.7, 9.5). Significant risks for illness included use of insect repellent in the afternoon (RR=3.2, 95% CI 1.1, 9.3) and at night (RR=3.0, 95% CI 1.0, 8.9) and wearing a hat (RR=6.3, 95% CI 1.4, 28.2). A limited number of respondents reported use of permethrin (n=9, 2.0%) or picaridin (n=16, 3.6%).
Specific areas of the grounds at the lodge were investigated as potential high-risk exposure areas. Respondents who reported having spent time on or near a stack of old hay bales were more likely to have become ill (RR=4.0, 95% CI 1.4, 11.7). No statistically significant associations were identified for other areas. Ill respondents did not differ from well respondents with regard to number of days spent at the lodge, where they had slept while at the lodge, activities (e.g., volleyball, horseshoes, exploring trails, square dancing, or visiting the dairy), type of clothing worn (long- or short-sleeve shirts, long or short pants, or light- or dark-colored shirts and pants), contact with animals, or having seen live or dead rabbits (p>0.05; data not shown). A total of 109 respondents (24.7%) reported having seen live rabbits, and 112 respondents (25.4%) reported having seen dead rabbits.
Risk factors that were significantly associated with illness in univariate analysis were included in a multivariate model. Respondents who reported deer-fly bites while at the lodge (adjusted RR=7.2, 95% CI 2.4, 22.0) and who reported having worn a hat (adjusted RR=5.6, 95% CI 1.3, 24.6) were more likely to have become ill even after adjustment for other factors (data not shown).
Of the seven patients who did not report deer-fly bites, six patients (86%) recalled some type of insect bite (other/unspecified biting fly [n=1], mosquito [n=1], and unknown insect[s] [n=4]), and one patient (14%) denied having had any insect bites. All patients with any report of an insect bite also reported having spent time on or near a stack of old hay bales. None of the patients reported having touched wild animals or domestic dogs or horses.
DISCUSSION
This was a naturally occuring outbreak of tularemia involving 14 patients with exposures along the western side of Utah Lake. Results of the cohort study were consistent with transmission by deer flies, a known vector for tularemia in Utah. This finding was supported by local reports and other cohort study results that both deer-fly and rabbit populations were high throughout the outbreak area during June and July 2007. Evidence of a tularemia epizootic among rabbits and a consequent rabbit die-off was gathered from an environmental study, as previously reported.10 Eleven of 12 (92%) collected rabbit carcasses tested positive for F. tularensis, including two F. tularensis subsp. holarctica (type B) and nine F. tularensis subsp. tularensis (type A; four type A1 and one type A2). Subtyping of isolates and PCR-positive materials from 11 patients revealed that all were infected with F. tularensis type A (six type A1 and five type A2).13 This is the first known documentation of multiple subspecies and clades in a localized outbreak, contrasting with previous findings of the geographic characteristics and associated vectors of F. tularensis subspecies and clades.1,10 The deer flies, which were collected three weeks after the outbreak, did not test positive for F. tularensis, but this was not a surprising finding because long-term maintenance of F. tularensis has not been shown to occur.
Tularemia is endemic in Utah, and an outbreak in Utah linked to an epizootic among rabbits has been previously reported.6 During a three-month period in 1971, a total of 39 tularemia cases were reported in Utah. As in the 2007 outbreak, the majority of patients (72%) were presumably infected through deer-fly bites. A major difference between the 1971 and 2007 Utah outbreaks was the location of the infectious exposure. The 1971 outbreak involved exposures within 11 counties in Utah. The 2007 outbreak was localized to a particular area on the western side of Utah Lake.
Two risk factors for disease in this outbreak were having been bitten by a deer fly and having worn a hat. In a univariate analysis, having worn insect repellent, including DEET, was a risk factor. Neither wearing a hat nor wearing insect repellent was a protective factor, as might be expected. Approximately half of all cohort study respondents reported having worn a hat, and more than half reported use of DEET. People who wore DEET were possibly protected against tularemia transmitted by tick bites. If used correctly, DEET can be effective against certain arthropods that transmit tularemia (i.e., mosquitoes and ticks), but it has not been reported to be effective against deer flies. This likely explains, at least in part, why wearing insect repellent was not a protective factor. Another potential explanation of these risk factors might be differential recall (i.e., recall bias); ill respondents might have been more likely to recall having used insect repellent or wearing a hat, which are both considered optimal behaviors. Finally, these factors might simply be markers of exposure, reflecting greater repellent use and hat wearing among people who spent more time outdoors where deer flies were active.
This outbreak highlighted the importance of collaboration among public health agencies with knowledge both of tularemia and of the occurrence of other cases, physicians examining patients with compatible symptoms, and the Utah Public Health Laboratories, which, as a Laboratory Reference Network laboratory, possessed the expertise to confirm diagnoses. Expertise in entomology and wildlife ecology provided by the Utah Department of Natural Resources/Division of Wildlife Resources and CDC were also needed to characterize the outbreak.
Tularemia is a disease of low prevalence, which might mask risk and contribute to delayed diagnosis and misdiagnosis. Four weeks passed from the first patient's illness onset to public health notification. After two physicians recognized unusual clinical manifestations among patients and reported the cases to public health, personnel at state and local health departments were able to recognize the outbreak and, consequently, respond with public health messaging to find additional cases and help prevent future cases. Thus, the importance of public health agency involvement was further illustrated. Increased awareness among health-care providers of the endemic nature of tularemia in Utah might aid in earlier recognition, reporting, and, consequently, outbreak detection. Establishing ongoing communication between wildlife resources workers with knowledge of animal die-offs and vector density and public health workers likely to learn of cases can also improve ability to recognize a tularemia outbreak and allow earlier implementation of prevention measures. Effective collaboration between health-care providers and public health agencies is essential.
Prevention is the key to stopping the spread of infectious diseases. The general public should be educated to avoid bites of ticks, mosquitoes, and flies, including correct use of insect repellent (including the fact that DEET might not protect against deer-fly bites), wearing light-colored long-sleeved shirts and long pants while outdoors, and use of mosquito netting while sleeping outdoors or in an unscreened structure. Education for leaders of groups planning to spend time in areas endemic to tularemia is also appropriate. Educational materials should be distributed to leaders of outdoor groups (e.g., scouting groups, summer camps, wilderness survival and recreational groups, and church groups) and to outdoor retailers. Group events are teachable moments, and group leaders should inform group members of disease risks and how to minimize those risks and recognize disease.
Limitations
Limitations of the cohort study included missing information and potential recall bias associated with the long period that elapsed between respondents' visits to the lodge and questionnaire completion. This time delay was caused, in part, by delayed diagnosis of certain patients and, consequently, delayed outbreak detection. Another limitation was a low response rate among one group, which was not included in the cohort study analysis. Finally, we only recruited participants from groups that had at least one confirmed, probable, or suspect case. If exposure also occurred during the visits of those groups not included, their exclusion might have limited the ability to detect factors contributing to the absence of tularemia patients among those groups. However, we do not anticipate that members of groups who were not recruited would have differed significantly from cohort participants with regard to demographic variables or risk factors, because they were all members of groups affiliated with the same church and engaged in similar activities (i.e., hiking and camping). Cohort study exclusions did not affect calculation of attack rates because total attendance numbers by week were available.
CONCLUSIONS
Rapid public health response to confirmed or suspect tularemia cases and identification of the mode of disease transmission are important because the exposure can be ongoing. However, tularemia diagnosis is often delayed because of nonspecific symptoms; public health officials are often notified of unusual disease before recognition of the diagnosis of tularemia because of severe symptoms. Increased awareness of areas in which tularemia is endemic and tularemia signs, symptoms, and treatment should be promoted through an educational campaign among the medical community. A high index of suspicion for tularemia should be maintained if a patient is clinically compatible (e.g., febrile illness and skin ulcers or swollen lymph nodes) and has had outdoor exposure in Utah or other endemic areas.
People with outdoor exposures (e.g., camping, hiking, or hunting) are most likely to become infected in the summer and experience tularemia with an ulceroglandular manifestation. Establishing effective communication among the medical community, public health workers, and wildlife resource workers is critical in detecting and responding to tularemia outbreaks. Educational messaging that targets the public and also focuses more specifically on people who participate in outdoor activities (e.g., hiking, camping, and hunting) is important not only for prevention of tularemia, but also for prevention of other diseases (e.g., disease associated with West Nile virus infection).
Footnotes
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
REFERENCES
- 1.Staples JE, Kubota KA, Chalcraft LG, Mead PS, Petersen JM. Epidemiologic and molecular analysis of human tularemia, United States, 1964-2004. Emerg Infect Dis. 2006;12:1113–8. doi: 10.3201/eid1207.051504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tularemia—United States, 1990-2000. MMWR Morb Mortal Wkly Rep. 2002;51(9):181–4. [PubMed] [Google Scholar]
- 3.Saliba GS, Harmston FC, Diamond BE, Zymet CL, Goldenberg MI, Chin TD. An outbreak of human tularemia associated with the American dog tick, Dermacentor variabilis. Am J Trop Med Hyg. 1966;15:531–8. doi: 10.4269/ajtmh.1966.15.531. [DOI] [PubMed] [Google Scholar]
- 4.Schmid GP, Kornblatt AN, Connors CA, Patton C, Carney J, Hobbs J, et al. Clinically mild tularemia associated with tick-borne Francisella tularensis. J Infect Dis. 1983;148:63–7. doi: 10.1093/infdis/148.1.63. [DOI] [PubMed] [Google Scholar]
- 5.Markowitz LE, Hynes NA, de la Cruz P, Campos E, Barbaree JM, Plikaytis BD, et al. Tick-borne tularemia: an outbreak of lymphadenopathy in children. JAMA. 1985;254:2922–5. doi: 10.1001/jama.254.20.2922. [DOI] [PubMed] [Google Scholar]
- 6.Klock LE, Olsen PF, Fukushima T. Tularemia epidemic associated with the deerfly. JAMA. 1973;226:149–52. [PubMed] [Google Scholar]
- 7.Young LS, Bickness DS, Archer BG, Clinton JM, Leavens LJ, Feeley JC, et al. Tularemia epidemic: Vermont, 1968. Forty-seven cases linked to contact with muskrats. N Engl J Med. 1969;280:1253–60. doi: 10.1056/NEJM196906052802301. [DOI] [PubMed] [Google Scholar]
- 8.Feldman KA, Enscore RE, Lathrop SL, Matyas BT, McGuill M, Schriefer ME, et al. An outbreak of primary pneumonic tularemia on Martha's Vineyard. N Engl J Med. 2001;345:1601–6. doi: 10.1056/NEJMoa011374. [DOI] [PubMed] [Google Scholar]
- 9.Dennis DT, Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, et al. Tularemia as a biological weapon: medical and public health management. JAMA. 2001;285:2763–73. doi: 10.1001/jama.285.21.2763. [DOI] [PubMed] [Google Scholar]
- 10.Petersen JM, Carlson JK, Dietrich G, Eisen RJ, Coombs J, Janusz AM, et al. Multiple Francisella tularensis subspecies and clades, tularemia outbreak, Utah. Emerg Infect Dis. 2008;14:1928–30. doi: 10.3201/eid1412.080482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown SL, McKinney FT, Klein GC, Jones WL. Evaluation of a safranin-O-stained antigen microagglutination test for Francisella tularensis antibodies. J Clin Microbiol. 1980;11:146–8. doi: 10.1128/jcm.11.2.146-148.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sjostedt A, Tularemia . In: Control of communicable diseases manual. 18th ed. Heymann DL, editor. Washington: American Public Health Association; 2003. pp. 573–6. [Google Scholar]
- 13.Molins CR, Carlson JK, Coombs J, Petersen JM. Identification of Francisella tularensis subsp. tularensis A1 and A2 infections by real-time polymerase chain reaction. Diagn Microbiol Infect Dis. 2009;64:6–12. doi: 10.1016/j.diagmicrobio.2009.01.006. [DOI] [PubMed] [Google Scholar]