Abstract
Objective
Pituitary apoplexy (PA) is described as a clinical syndrome characterized by sudden headache, vomiting, visual impairment, and meningismus caused by rapid enlargement of a pituitary adenoma. We retrospectively analyzed the clinical presentation and surgical outcome in PA presenting with cranial neuropathy.
Methods
Twelve cases (3.3%) of PA were retrospectively reviewed among 359 patients diagnosed with pituitary adenoma. The study included 6 males and 6 females. Mean age of patients was 49.0 years, with a range of 16 to 74 years. Follow-up duration ranged from 3 to 20 months, with an average of 12 months. All patients were submitted to surgery, using the transsphenoidal approach (TSA).
Results
Symptoms included abrupt headache (11/12), decreased visual acuity (12/12), visual field defect (11/12), and cranial nerve palsy of the third (5/12) and sixth (2/12). Mean height of the mass was 29.0 mm (range 15-46). Duration between the ictus and operation ranged from 1 to 15 days (mean 7.0). The symptom duration before operation and the recovery period of cranial neuropathy correlated significantly (p = 0.0286). TSA resulted in improvement of decreased visual acuity in 91.6%, visual field defect in 54.5%, and cranial neuropathy in 100% at 3 months after surgery.
Conclusion
PA is a rare event, complicating 3.3% in our series. Even in blindness following pituitary apoplexy cases, improvement of cranial neuropathy is possible if adequate management is initiated in time. Surgical decompression must be considered as soon as possible in cases with severe visual impairment or cranial neuropathy.
Keywords: Pituitary apoplexy, Pituitary adenoma, Cranial neuropathy, Transsphenoidal approach
INTRODUCTION
Pituitary apoplexy (PA) is an uncommon event heralded by abrupt onset of severe headache, deterioration of visual acuity, restriction of visual fields, disorders of ocular motility, and altered sensorium caused by rapid enlargement of a pituitary adenoma, usually due to hemorrhagic infarction of the tumor5,9,26). Decreased visual acuity and field defect following PA are attributed to rapid expansion of an infarcted and/or hemorrhagic pituitary adenoma that extends laterally into the cavernous sinus or reaches superiorly to compress the cranial nerve III, IV, V, VI and optic chiasm15,16). Initial management of patients presenting with PA includes close observation, hormonal replacement such as administration of corticosteroids and, if necessary, surgical decompression2). However, there is disagreement with regard to the role of early surgery; some advocate urgent decompression of the pituitary fossa, particularly when vision is severely affected, others assert that early neurosurgical intervention is not required10,14,19).
The aim of this study was to analyze clinical presentation and surgical outcome in PA presented with cranial neuropathy. We also analyzed the relationship between clinical outcome and timing of surgery for consideration of the necessity for early surgery.
MATERIALS AND METHODS
Twelve cases (3.3%) of PA were retrospectively reviewed among 359 patients diagnosed with pituitary adenoma from 1996 to 2009. Data collected included the age and sex of the patient, past medical history, clinical presentation, preoperative neurological and ophthalmologic status, and postoperative outcome. Inclusion criteria for pituitary apoplexy consisted of a history of sudden onset of neurological symptoms and signs : 1) sudden onset of severe headache with or without nausea, vomiting, 2) sudden deterioration of vision, including complete blindness. Blindness was defined as the absence of perception of light, and 3) sudden oculomotor paresis such as third and sixth cranial nerve palsy.
Neuroradiological studies included sellar turcica X-ray in the antero-posterior and lateral planes. Computerized tomography (CT) was performed in 8 patients. All patients were studied by magnetic resonance imaging (MRI). All patients were submitted to surgery, using the transsphenoidal approach (TSA). Operative findings were collected from the operative note.
Follow-up duration ranged from 3 to 20 months, with an average follow-up of 12.0 months. Relief of preoperative symptoms and occurrence of postoperative complications were noted from clinical records. All patients underwent preand postoperative ophthalmological examination, including visual acuity (VA) and visual field (VF).
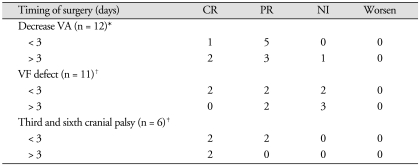
We classified recovery of cranial neuropathy after PA surgery : complete resolution (CR), partial resolution (PR), and no improvement (NI). CR of cranial neuropathy was defined as full recovery within 3 months to the state before development of symptoms, and PR as improvement of symptoms, indicating no recovery to the previous state.
Statistical comparison between groups, in terms of timing of surgery and improvement in visual outcome, was performed using the Mann-Whitney U-test and Chi-square test. A two tailed p-value of less than 0.05 was considered significant.
RESULTS
Clinical presentation
The study included 6 males and 6 females. Mean age of patients was 49.0 years, with a range of 16 to 74 years. Clinical data are summarized in Table 1. The most common symptoms included sudden headache (n = 11) and decreased visual acuity (n = 12). The visual symptoms included 11 patients with visual field defects, 7 cranial nerve palsy (5 3rd nerve and 2 6th nerve), and 2 unilateral blindness. One patient simultaneously experienced palsy of both the third and sixth cranial nerves (case 4). A meningismus was noted in one patient.
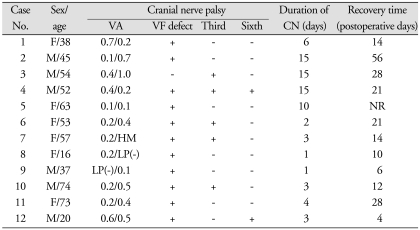
Table 1.
Clinical features of patients with pituitary apoplexy
VA : visual acuity, VF : visual field, CN : cranial neuropathy, HM : hand movement, LP: light perception, NR : no recovery
Radiologic finding
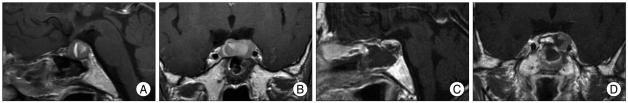
On skull X-rays, the sellar turcica was enlarged in all patients, with erosion of the sellar floor and dorsum sella in two cases. All patients were evaluated by CT scan and/or MRI scan that revealed a sellar lesion. A mass that compressed the optic chiasm or optic tracts was revealed in ten cases. A sella lesion accompanied by hemorrhage and/or hemorrhagic infarction shadow was seen in nine cases (Fig. 1). The largest diameter of the lesions ranged between 15 and 46 mm (mean 29.0 mm).
Fig. 1.
T1-weighted magnetic resonance imaging scans of the brain of patient (case 7). Sagittal (A) and coronal (B) sections reveal a hemorrhagic pituitary adenoma. No evidence of remaining enhancing tumor seen in postoperative first day follow up sellar MRI-sagittal (C) and coronal (D) sections.
Management
Tumor resection was performed using a TSA in all patients. Duration between the ictus and decompression ranged from 1 to 15 days (mean 7.0). In six patients, early surgical decompression, within 72 hr from attack time, was performed due to severe neurological deficit, including visual impairment. Two other patients underwent surgery within 3 to 7 days of presentation. In the other 4 cases presenting, surgical intervention was undertaken within 7 to 15 days of presentation due to late arrival at hospital. During surgery, evidence of hemorrhage within the tumor was detected in 4 patients, and necrotic adenomatous tissue was identified in 5 patients. We also detected direct invasion of the cavernous sinus wall in one patient. Pathologic examination revealed pituitary adenoma with hemorrhage or hemorrhagic infarction in 9 cases.
Postoperative evolution and outcome
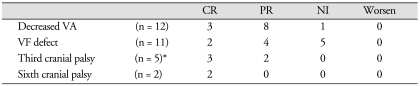
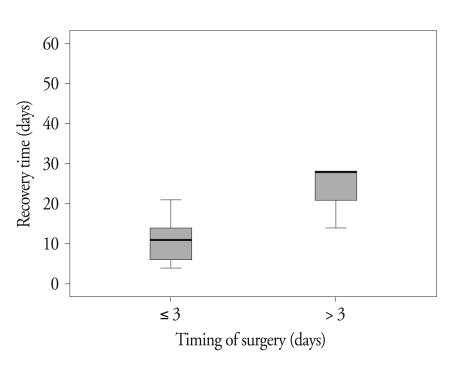
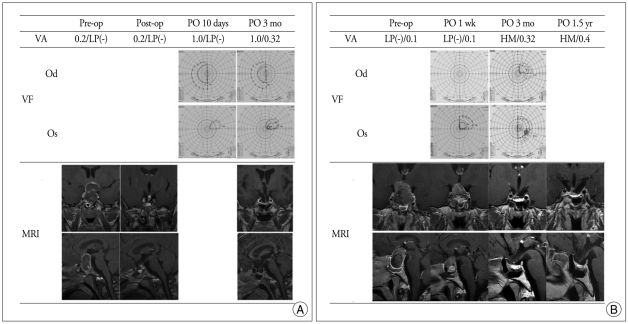
Postoperative MRI at 3-month follow-up showed that tumors were removed gross totally in 8 cases and subtotally in 4 cases. Overall, TSA resulted in improvement of decreased visual acuity in 91.6% (11/12), visual field defect in 54.5% (6/11), and third and sixth cranial nerve palsy in 100% (6/6) at 3 months after surgery (Table 2). As shown in Fig. 2, the symptom duration before operation and the recovery period of cranial neuropathy correlated significantly (p = 0.0286). In two cases of complete blindness following pituitary apoplexy, preoperative visual acuity was 0.2/light perception (LP) (-) in case 8 and LP(-)/0.1 in case 9. Tumors were removed from both patients via a TSA with a delay of 18 hours and 24 hours each. Postoperative MR showed complete removal of the pituitary mass lesion. Both patients showed improved vision : 1.0/0.32 in case 8 and hand movement (HM)/0.32 in case 9 at 3 months after surgery (Fig. 3).
Table 2.
Recovery of cranial neuropathy in pituitary apoplexy
*A patient had both third and sixth cranial nerve palsy. CR : complete resolution, PR : partial resolution, NI : no improvement, VA : visual acuity, VF : visual field
Fig. 2.
The relationship between the timing of surgery and recovery time of cranial neuropathy. The symptom duration before operation and the recovery period of cranial neuropathy correlated significantly (p = 0.0286).
Fig. 3.
In case 8 and 9 of complete blindness following pituitary apoplexy, the tumors of both patients were removed via a TSA with the delay of 18 hours and 24 hours each. A : Pre-operative unilateral blindness showed partial recovery in both visual acuity and visual field in case 8. B : In case 9, pre-operative decreased visual acuity was partially recovered after operation. The improvement of the left monocular blindness occurred, not immediately, but several days after surgery. VA : visual acuity, VF : visual field, LP : light perception, HM : hand movement, MRI : magnetic resonance imaging.
Eleven patients except a patient with no improvement experienced improved cranial neuropathy, with the earliest beginning of recovery at 4 days, and the latest at 56 days. Recovery of third nerve palsy was slower than that of sixth cranial nerve palsies. There was no patient who showed further improvement of cranial neuropathy after 3 months.
In terms of the relationship between visual outcome and timing of TSA, as shown in Table 3, CR and PR of decreased visual acuity were seen in all patients who underwent surgery within the first 3 days of presentation, as compared to 83.3% (5/6) in whom surgery was delayed beyond 3 days. Similarly, CR and PR of visual field defects were observed in 66.6% (4/6) associated with patients who had undergone surgery within the first 3 days of presentation, as compared to 40.0% (2/5) in whom surgery was delayed beyond 3 days. CR and PR of cranial nerve palsy were seen in all patients who underwent surgery regardless of timing. However, there were no significant differences in visual outcome between early and delayed surgery group (*p = 0.5050; †p = 0.1213; ‡p = 1.000).
Table 3.
Timing of surgery and visual outcome after transsphenoidal surgery
*p = 0.5050, †p = 0.1213, ‡p = 1.000. CR : complete resolution, PR : partial resolution, NI : no improvement, VA : visual acuity, VF : visual field
DISCUSSION
In 1896, Percival Bailey was the first to describe a clinical case of pituitary apoplexy, resulting from catastrophic hemorrhage of a pituitary adenoma2). Incidence of pituitary apoplexy varies from 0.6% to 22%, depending on the definition used. However, the majority of authors agree that PA is a clinical syndrome that results from hemorrhage, infarction, or hemorrhagic infarction of a pituitary tumor that results in its sudden and fulminant expansion3,25). Incidence of symptomatic PA varies from 0.6% to 9%3,4,25).
Most of PA develop in a either known or unknown pituitary adenoma. PA rarely occurs in a non-adenomatous pituitary lesion, such as an abscess, a metastatic tumor, or a lymphocytic hypophysitis7,8,12). All of our patients presented a pituitary adenoma.
Cranial nerve dysfunction due to pituitary tumor was thought to occur in 5-17% of pituitary tumor patients, development of third cranial nerve palsy has been reported most frequently, followed by sixth, then fourth or fifth cranial nerve palsies, in that order20).
Causes of cranial nerve palsy in pituitary tumor patients have been discussed by various investigators5,17,22-24). Causes included direct compression of the cranial nerve by the tumor or transmission of pressure on the cavernous sinus wall from tumor expansion, edematous expansion due to hemorrhage in the tumor, ischemic infarction of the tumor, and direct infiltration by the tumor. PA can provoke or aggravate cranial nerve palsy, either alone or via interaction with other predisposing factors, such as irradiation, alteration of pressure gradients (i.e., changes in blood pressure, or lumbar punctures), trauma, estrogens, bromocriptine therapy, and anti-coagulants.
Because the third cranial nerve is located horizontally in the same plane as the pituitary gland, pressure from lateral growth of a pituitary tumor to compress the cavernous sinus is relatively easily transmitted to the third cranial nerve. This leads to compression of the third cranial nerve between the tumor and the interclinoid ligament, commonly resulting in development of third cranial nerve palsy, which tends to bring about slow-onset nerve palsy6,21,22,23,24). Sudden-onset of third cranial nerve palsy has been attributed to compromise of the vascular supply to the nerve due to compression of the vasa nervosum originating in the internal carotid artery24).
Because the compliance of the third nerve with compression or stretching is relatively lower than that of other cranial nerves, the recovery of the third nerve is slow. Hence, recovery or improvement of third cranial nerve palsy may be the most important indicator of complete recovery after surgery in PA11). In the present study, only one among 5 cases of third cranial nerve palsy was accompanied by sixth cranial nerve palsy. During recovery of third cranial nerve palsy, sixth cranial nerve palsy had already recovered.
Although much less frequent, sixth cranial nerve palsy has also been reported. A proposed mechanism by which isolated sixth nerve palsy, or sixth nerve palsy along with third nerve palsy may occur, is extension of the tumor backwards, along Dorello's canal, which contains the sixth cranial nerve along with the inferior petrosal sinus. Sudden lateral expansion of the adenoma into the cavernous sinus more commonly provokes third cranial palsy than sixth (or fourth) cranial nerve palsy11,20,27).
Dubuisson et al.8) studied 24 patients who presented with PA. Postoperative follow-up of patients with a mean 5.5 year follow-up period showed restoration of normal vision in 92% of preoperatively affected patients, while 85% of preoperative oculomotor pareses were improved or resolved. In this study, TSA resulted in an improvement of decreased visual acuity in 91.6%, visual field defect in 54.5%, and cranial neuropathy in 100% at 3 months after surgery.
Historically, early decompression of the pituitary fossa has been advocated, the rationale being the possibility of better visual outcome. Onesti et al.18) noted improved visual field defect in all 16 patients who were surgically treated. Six of these patients regained full fields with surgery within 2 to 72 days after the deficit. Bills et al.3) note that a significant better outcome of visual deficits and pituitary dysfunction was reported in those patients decompressed within the first week. Randeva et al.21) noted that surgical management led to improvement in ocular paresis and in the deficits of VA and VF in most patients, no patient suffered deterioration of vision. Although improvements in ocular paresis with respect to the timing of surgery were not statistically significant, possibly due to the small numbers, complete resolution of ocular paresis was more frequently seen in those who underwent surgery within 8 days compared to those who had surgery after 8 days. Agrawal and Mahapatra1) noted that no improvement of vision occurred in patients with blindness following pituitary apoplexy if surgical intervention was delayed beyond a week. This finding is in concordance with previous reports showing good visual outcome if optic nerves are decompressed within one week. Kim et al.11) noted that time to recovery from cranial nerve palsies accompanying pituitary tumors after surgery and the interval between development of symptoms and surgery were positively and significantly correlated. The interval between the appearance of symptoms and surgery may be regarded as an important factor for recovery after surgery.
Several previous obsevational studies have also favored a more conservative approach in the management of pituitary apopexy. In a small prospective study of 12 patients with pituitary apoplexy, Maccagnan et al.13) reported complete resolution of ophthalmoplegia in six of seven patients managed conservatively, with improvement in the remainaing one. In all five patients managed surgically, TSA resulted in prompt neurological and visual improvement, but with complete recovery in only one. Gruber et al.10) studied a clinical outcomes in patients presenting with pituitary apoplexy and the results of conservative and surgical management. Ten patients proceeded to early pituitary surgery and twenty were managed conservatively. There was no evidence that early operative decompression is associated with an improved outcome. In both studies, however, patients presenting with no or mild visual field deficit and cranioneuropathy were managed conservatively with corticosteroids and monitoring of visual acuity and field. Patients who showed deterioration in visual function should be considered for decompressive surgery. In our analysis, we found 6 patients who underwent surgery within 3 days. Although there were no significant differences, result for visual outcome in patients who underwent surgery within 3 days tended to show better outcome compared to those who underwent surgery beyond 3 days. Although there were small number of patients in present study, the symptoms duration before operation and the recovery period correlated significantly (p = 0.0286), indicating that recovery from cranial nerve palsy was much more rapid in patients who underwent surgery soon after development of symptoms. Our findings would support early decompression surgery at the time of presentation with apoplexy, which may be important to improvement of visual outcome and cranial neuropathy.
CONCLUSION
PA is a rare event, complicating 3.3% in our series. However, cranial neuropathy following PA was relatively common. Even in cases of blindness following pituitary apoplexy, improvement of cranial neuropathy was possible with rapid diagnosis and initiation of adequate and timely management. It suggests that cranial nerve palsy due to PA can be treated with a high rate of recovery if surgery is performed as early as possible after diagnosis. Thus, early surgical decompression must be considered in cases with severe visual impairment or cranial neuropathy.
References
- 1.Agrawal D, Mahapatra AK. Visual outcome of blind eyes in pituitary apoplexy after transsphenoidal surgery : a series of 14 eyes. Surg Neurol. 2005;63:42–46. doi: 10.1016/j.surneu.2004.03.014. discussion 46. [DOI] [PubMed] [Google Scholar]
- 2.Arafah BM, Harrington JF, Madhoun ZT, Selman WR. Improvement of pituitary function after surgical decompression for pituitary tumor apoplexy. J Clin Endocrinol Metab. 1990;71:323–328. doi: 10.1210/jcem-71-2-323. [DOI] [PubMed] [Google Scholar]
- 3.Bills DC, Meyer FB, Laws ER, Jr, Davis DH, Ebersold MJ, Scheithauer BW, et al. A retrospective analysis of pituitary apoplexy. Neurosurgery. 1993;33:602–608. doi: 10.1227/00006123-199310000-00007. discussion 608-609. [DOI] [PubMed] [Google Scholar]
- 4.Brisman MH, Katz G, Post KD. Symptoms of pituitary apoplexy rapidly reversed with bromocriptine. case report. J Neurosurg. 1996;85:1153–1155. doi: 10.3171/jns.1996.85.6.1153. [DOI] [PubMed] [Google Scholar]
- 5.Cardoso ER, Peterson EW. Pituitary apoplexy : a review. Neurosurgery. 1984;14:363–373. doi: 10.1227/00006123-198403000-00021. [DOI] [PubMed] [Google Scholar]
- 6.Cho WJ, Joo SP, Kim TS, Seo BR. Pituitary apoplexy presenting as lsolated third cranial nerve palsy with ptosis : two case reports. J Korean Neurosurg Soc. 2009;45:118–121. doi: 10.3340/jkns.2009.45.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dan NG, Feiner RI, Houang MT, Turner JJ. Pituitary apoplexy in association with lymphocytic hypophysitis. J Clin Neurosci. 2002;9:577–580. doi: 10.1054/jocn.2001.0975. [DOI] [PubMed] [Google Scholar]
- 8.Dubuisson AS, Beckers A, Stevenaert A. Classical pituitary tumor apoplexy : clinical features, management and outcomes in a series of 24 patients. Clin Neurol Neurosurg. 2007;109:63–70. doi: 10.1016/j.clineuro.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Ebersold MJ, Laws ER, Jr, Scheithaure BW, Randall RV. Pituitary apoplexy treated by trans-sphenoidal surgery. A clinicopathological and immunocytochemical study. J Neurosurg. 1983;58:315–320. doi: 10.3171/jns.1983.58.3.0315. [DOI] [PubMed] [Google Scholar]
- 10.Gruber A, Clayton J, Kumar S, Robertson I, Howlett TA, Mansell P. Pituitary apoplexy : retrospective review of 30 patients--is surgical intervention always necessary? Br J Neurosrug. 2006;20:379–385. doi: 10.1080/02688690601046678. [DOI] [PubMed] [Google Scholar]
- 11.Kim SH, Lee KC, Kim SH. Cranial nerve palsies accompanying pituitary tumor. J Clin Neurosci. 2007;14:1158–1162. doi: 10.1016/j.jocn.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 12.Kingdon CC, Sidhu PS, Cohen J. Pituitary apoplexy secondary to an underlying abscess. J Infect. 1996;33:53–55. doi: 10.1016/s0163-4453(96)92814-5. [DOI] [PubMed] [Google Scholar]
- 13.Maccagnan P, Macedo CL, Kayath MJ, Nogueira RG, Abucham J. Conservative management of pituitary apoplexy : a prospective study. J Clin Endocrinol Metab. 1995;80:2190–2197. doi: 10.1210/jcem.80.7.7608278. [DOI] [PubMed] [Google Scholar]
- 14.McFadzean RM, Doyle D, Rampling R, Teasdale E, Teasdale G. Pituitary apoplexy and its effect on vision. Neurosurgery. 1991;29:669–675. doi: 10.1097/00006123-199111000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Molitch ME. Gonadotroph-cell pituitary adenomas. N Engl J Med. 1991;324:626–627. doi: 10.1056/NEJM199102283240909. [DOI] [PubMed] [Google Scholar]
- 16.Muthukumar N, Rossette D, Soundaram M, Senthilbabu S, Badrinarayanan T. Blindness following pituitary apoplexy : timing of surgery and neuro-ophthalmic outcome. J Clin Neurosci. 2008;15:873–879. doi: 10.1016/j.jocn.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 17.Nawar RN, AbdelMannan D, Selman WR, Arafah BM. Pituitary tumor apoplexy : a review. J Intensive Care Med. 2008;23:75–90. doi: 10.1177/0885066607312992. [DOI] [PubMed] [Google Scholar]
- 18.Onesti ST, Wisniewski T, Post KD. Clinical versus subclinical pituitary apoplexy : presentation, surgical management, and outcome in 21 patients. Neurosurgery. 1990;26:980–986. [PubMed] [Google Scholar]
- 19.Peter M, De Tribolet N. Visual outcome after transsphenoidal surgery for pituitary adenomas. Br J Neurosrug. 1995;9:151–157. doi: 10.1080/02688699550041485. [DOI] [PubMed] [Google Scholar]
- 20.Petermann SH, Newman NJ. Pituitary macroadenoma manifesting as an isolated fourth nerve palsy. Am J Ophthalmol. 1999;127:235–236. doi: 10.1016/s0002-9394(98)00407-3. [DOI] [PubMed] [Google Scholar]
- 21.Randeva HS, Schoebel J, Byrne J, Esiri M, Adams CB, Wass JA. Classical pituitary apoplexy : clinical features, management and outcome. Clin Endocrinol (Oxf) 1999;51:181–188. doi: 10.1046/j.1365-2265.1999.00754.x. [DOI] [PubMed] [Google Scholar]
- 22.Riedl M, Clodi M, Kotzmann H, Hainfellner JA, Schima W, Reitner A, et al. Apoplexy of a pituitary macroadenoma with reversible third, fourth and sixth cranial nerve palsies following administration of hypothalamic releasing hormones : MR features. Eur J Radiol. 2000;36:1–4. doi: 10.1016/s0720-048x(00)00148-0. [DOI] [PubMed] [Google Scholar]
- 23.Rossitch E, Jr, Carrazana EJ, Black PM. Isolated oculomotor nerve palsy following apoplexy of a pituitary adenoma. J Neurosurg Sci. 1992;36:103–105. [PubMed] [Google Scholar]
- 24.Saul RF, Hilliker JK. Third nerve palsy : the presenting sign of a pituitary adenoma in five patients and the only neurological sign in four patients. J Clin Neuroophthalmol. 1985;5:185–193. [PubMed] [Google Scholar]
- 25.Semple PL, Webb MK, de Villiers JC, Laws ER., Jr Pituitary apoplexy. Neurosurgery. 2005;56:65–72. doi: 10.1227/01.neu.0000144840.55247.38. discussion 72-73. [DOI] [PubMed] [Google Scholar]
- 26.Verrees M, Arafah BM, Selman WR. Pituitary tumor apoplexy : characteristics, treatment, and outcomes. Neurosurg Focus. 2004;16:E6. doi: 10.3171/foc.2004.16.4.7. [DOI] [PubMed] [Google Scholar]
- 27.Warwar RE, Bhullar SS, Pelstring RJ, Fadell RJ. Sudden death from pituitary apoplexy in a patient presenting with an isolated sixth cranial nerve palsy. J Neuroophthalmol. 2006;26:95–97. doi: 10.1097/01.wno.0000223270.01813.57. [DOI] [PubMed] [Google Scholar]