Abstract
Cicatricial (scarring) alopecia results from irreversible damage to epithelial stem cells located in the bulge region of the hair follicle, generally as a result of inflammatory mechanisms (eg, in the context of autoimmune disease). In primary cicactricial alopecia (PCA), the hair follicle itself is the key target of autoaggressive immunity. This group of permanent hair loss disorders can be classified into distinct subgroups, characterized by the predominant peri-follicular inflammatory cell type. In none of these PCA forms do we know exactly why hair follicles begin to attract such an infiltrate. Thus, it is not surprising that halting or even reversing this inflammation in PCA is often extremely difficult. However, increasing evidence suggests that healthy hair follicle epithelial stem cells enjoy relative protection from inflammatory assault by being located in an immunologically “privileged” niche. Because this protection may collapse in PCA, one key challenge in PCA research is to identify the specific signaling pathways that endanger, or restore, the relative immunoprotection of these stem cells. After a summary of pathobiological principles that underlie the development and clinical phenotype of PCA, we close by defining key open questions that need to be answered if more effective treatment modalities for this therapeutically very frustrating, but biologically fascinating, group of diseases are to be developed.
Cicatricial alopecias (syn. scarring alopecias; permanent alopecias) are an uncommon and clinically diverse set of disorders that result in permanent and irreversible (scalp) hair loss, often leading to major disfigurement, discomfort, and psychological distress. Clinically, this form of irreversible hair loss is characterized by the disappearance of visible follicular ostia within an area of alopecia. Histologically, this corresponds to hair follicle (HF) destruction and subsequent replacement with fibrous tissue. These disorders may be primary (PCA), with the follicle itself being the target of the disease process, or secondary, where HFs are destroyed coincidentally as part of a more generalized tissue-damaging event (eg, deep skin infection, thermal burn, trauma, or ionizing radiation).1,2
This review will focus on PCA and will discuss current concepts in PCA pathogenesis and important areas for future study.
Why Are Primary Cicatricial Alopecias of Major Interest Beyond the Field of HF Biology?
Given the multiple distinct stem cell populations of the HF,3 its capacity to induce angiogenesis4 and nerve fiber sprouting,5 its major role in wound healing,6,7 and its notoriously underestimated impact on general skin architecture and physiology,8,9 the permanent loss of pilosebaceous units is indeed a catastrophic event for the entire skin, its normal function, and its regenerative potential. Yet, PCA is also of major interest well beyond the “hair and skin horizon.” PCA results from the irreversible destruction of key epithelial stem cells of the HF so that this constantly remodeled, cycling, miniorgan loses its capacity to maintain and regenerate itself, resulting from an inflammatory attack on the HF. Therefore, PCA offers an excellent, highly accessible model for studying how various inflammatory events and immunoregulatory mechanisms—in the adult human system—conspire to assault epithelial stem cell populations. Moreover, PCA provides a model system in which to identify the (currently unknown) signals that incite such an attack—as a key step toward stem cell disease prevention and termination of disease progression.
Furthermore, in both human PCA and mouse models that exhibit PCA phenotypes (see below), one can study and experimentally manipulate how epithelial stem cells are normally protected within their specialized niche10,11 from autoaggressive inflammation, what damage response and repair strategies they use, and what exactly defines the point at which epithelial stem cell damage becomes irreparable and results in stem cell apoptosis or necrosis. Thus, PCA in man and mice provides an instructive yet still under-investigated and under-exploited in situ model for the immunopathology of epithelial stem cells.
Morphological Principles and Variants of PCA
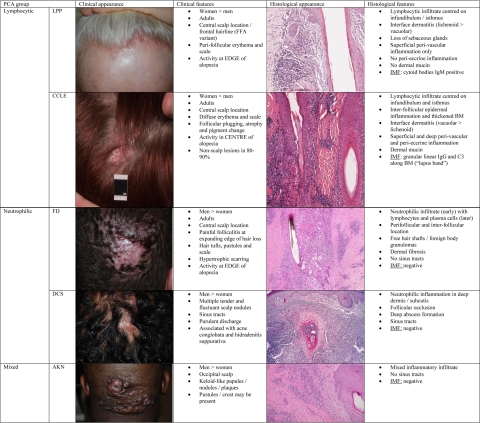
PCA should be suspected when follicular ostia in areas of alopecia are no longer visible. This sign is often accompanied by evidence of cutaneous inflammation (eg, peri-follicular erythema, increased scaling) and may be associated with hair tufting, pustules, skin atrophy, or hypertrophic scarring. On histological examination, a variably dense inflammatory infiltrate is typically seen in the immediate vicinity of PCA-affected HFs. Its composition is suggestive of the specific PCA type present (Figure 1).1 In fact, the current classification of PCAs heavily relies on the predominant inflammatory infiltrate seen. Disorders are grouped as lymphocytic, neutrophilic, mixed, or nonspecific, with further subclassification into a recognized disease state based on the clinical features expressed.12 Additional diagnostic clues may be gained from immunofluorescence, particularly if cutaneous lupus erythematosus or bullous dermatoses is suspected.1 Lesioned HFs in PCA are eventually destroyed and replaced with “scar-like” fibrous tissue. Verhoff Van Gieson elastin stains are helpful in end-stage disease when inflammation is no longer present.13 At this point, the original trigger for the hair loss is often no longer identifiable.
Figure 1.
Key clinical and histological features of selected PCA entities (adapted from Harries et al1). LPP, lichen planopilaris; CCLE, chronic cutaneous lupus erythematosus; FD, folliculitis decalvans; DCS, dissecting cellulitis of the scalp (syn. Perifolliculitis abcedens et suffodiens); AKN, acne keloidalis nuchae; Ig, immunoglobulin; IMF, immunofluorescence; BM, basement membrane).
Unmet Diagnostic and Therapeutic Challenges
Unfortunately, our understanding of PCA etiology and pathogenesis is still in its infancy.12,14 Significant progress has been hampered by our poor understanding of disease progression and natural history, inconsistent and inaccurate disease definition in the literature, the lack of definitive molecular markers for any of the recognized PCA entities, and the unpredictable and incomplete response to therapy that is commonly seen.15,16 Thus, effectively counseling patients about their likely disease prognosis and the expected effectiveness of any chosen therapy is, at present, extremely challenging.
A more in-depth understanding of PCA pathobiology appears to be the essential prerequisite for achieving major progress in identifying definitive diagnostic and prognostic markers for specific PCA entities, for elucidating promising therapeutic targets, and thus for developing more effective strategies to manage these under-estimated, commonly painful, and both cosmetically and psychosocially disruptive disorders.
Epithelial HF Stem Cells
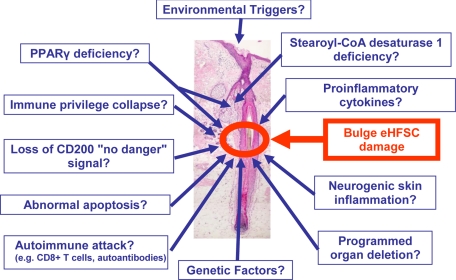
Given that epithelial HF stem cells (eHFSCs) lie at the heart of PCA pathobiology (Figure 2), a brief excursion into the biology of these cells and their importance in HF function is in order (for details, see Refs. 3, 17, 18).
Figure 2.
Possible pathogenic factors in PCA.
As one of the defining features of mammals, HFs show an astounding regenerative capacity, while continuously undergoing serial complex organ transformations, termed the hair cycle. Here, phases of very active growth (anagen) alternate with massive, apoptosis-driven HF regression (catagen) and periods of relative quiescence (telogen).19 This constant and lifelong remodeling activity of the HF absolutely depends on the presence of functional eHFSCs. These cells reside at the insertion point of the arrector pili muscle, in the outermost layer of the outer root sheath—the so-called “bulge” region. This area is also the lowermost portion of the “permanent” HF during hair cycling.20 While usually very difficult to see in human skin, characteristic protrusions (“follicular trochanter”) can provide a useful histological demarcation of the human bulge.21
Further evidence in support of the importance of eHFSCs to HF survival has been elegantly demonstrated by Ito et al.22 In this study, a transgenic mouse model used a “suicide gene,” encoding herpes simplex virus thymidine kinase (HSV-TK) with a keratin 15 promoter, to induce selective bulge cell destruction after gene activation.22 When skin from these mice was grafted onto immunodeficient hosts, there was a rapid and permanent loss of HFs within the grafted skin, but apparently without the presence of inflammation, thereby demonstrating the key role that these bulge cells play in ongoing HF survival.
Special Immunological Features of the HF
As PCA results from an abnormal, autoaggressive immune response that destroys eHFSCs, it is important to recall that, within the skin immune system, the HF immune system stands out in several respects, even under physiological conditions.23 This is likely to be of major relevance to understanding the immunopathobiology of PCA and therefore needs to be briefly summarized.
On the one hand, HFs provide the major portal for microbial invasion into mammalian skin. Yet, in immunocompetent individuals, the clinical consequence of successful microbial invasion (ie, bacterial or fungal folliculitis) is an exceptionally rare event—given that the human integument supports about 5 million HFs. This observation alone already suggests that the HF must have established an exquisitely effective anti-infection defense system. Indeed, the distal HF epithelium is richly endowed with intraepithelial T cells and Langerhans cells,10,24,25 is densely surrounded by the key cellular protagonists of innate immunity (ie, peri-follicular mast cells and macrophages), and expresses a battery of antimicrobial peptides, such as human β2-defensin, psoriasin, cathelicidin, and RNAse7.26 The latter not only directly kills potential invaders but also serves as a “danger” signal that, together with chemokines and proinflammatory cytokines released by the HF epithelium, can rapidly attract a massive immunocyte accumulation, up to the point where neutrophils, macrophages, and T cells invade (and potentially destroy) the HF epithelium.
On the other hand, the HF immune system has established a complex system of checks and balances that controls and suppresses excessive HF inflammation. Specifically, large stretches of the HF epithelium express potent endogenous immunosuppressants, such as transforming growth factor (TGF)-β1/2 and α-melanocyte-stimulating hormone. Under physiological conditions, even peri-follicular mast cells may exert primarily immune-inhibitory effects.27 Defined compartments of the HF epithelium, most prominently the anagen hair bulb, go beyond this and appear to have established an area of relative “immune privilege” (IP). This is characterized by low or absent expression of major histocompatability complex (MHC) class I and class II molecules and the local production of immunosuppressants,28,29 including the intrafollicular synthesis of cortisol.30 All this may serve not only to ward off damaging HF inflammation after minor trauma, skin irritation, or microbial invasion, but also to sequester potentially immunogenic follicular autoantigens from immune recognition.23 Consequently, collapse of the IP of the anagen hair bulb is thought to result in one of the most common autoimmune diseases of man, alopecia areata.31
In the context of PCA pathobiology, the most important feature of the normal HF immune system is that the HF IP also extends to the human bulge, the seat of eHFSCs.10 Here, MHC class I and β2-microglobulin as well as MHC class II expression levels are sharply reduced, while the immunoreactivity for locally generated immunosuppressants such as TGF-β2 and macrophage migration inhibitory factor is increased. T cell functions may be inhibited by the expression of indoleamine-2,3-dioxygenase and by natural killer (NK) and cytotoxic T cells by human leukocyte antigen E expression. On stimulation with interferon (IFN)-γ, the bulge IP phenotype collapses,10 just as has been shown for the anagen hair bulb IP.28
In addition, the type-1 trans-membrane glycoprotein, CD200, is predominantly expressed in the human HF bulge.10,32 When CD200 interacts with the CD200 receptor (CD200R), anti-inflammatory and tolerance-promoting signals are generated, while the deletion of CD200 in mice attracts massive peri-follicular inflammation, resulting in murine PCA.33,34 Secretion of proinflammatory cytokines by activated T cells can be down-regulated by CD200 + dendritic cells in vitro,33 and antigen-presenting cell activity is significantly reduced after CD200R binding.35 Therefore, through these mechanisms, CD200 is thought to act as a “no-danger” signal to the local immune system.34
However, the functional significance of these “immune protective” mechanisms in the HF remains a matter of speculation. They may have evolved to reduce the risk of autoimmune hair loss developing in mammals where the process could threaten survival (eg, hair loss in a polar bear).36 Another explanation is that IP protects the eHFSCs that reside in the bulge from immune-mediated attack so as to preserve this critical regenerative cell pool.10 Teleologically, this explanation makes sense considering the importance of eHFSCs to HF regeneration, cycling, and wound healing.17 Further, it should be recognized that the failure of self-recognition (or, to be more precise, failure of central and/or peripheral tolerance of autoantigens) is an independent phenomenon of IP. For actual loss of tolerance against autoantigens to occur leading to manifest autoimmune disease, additional mechanisms are required that go beyond the collapse of IP. Therefore, HF IP collapse will only exceptionally lead to failure of tolerance and frank autoimmune disease.23
Key Concepts in PCA Pathogenesis
Key to understanding the pathogenesis in PCA is the location of the peri-follicular inflammatory infiltrate relative to two key structures of the HF, the hair bulb (where the hair shaft is generated), and the hair bulge (the home of eHFSCs). In the prototypic reversible autoimmune hair loss disorder alopecia areata, the inflammatory infiltrate is centered round the hair bulb; whereas in PCA, the infiltrate is located around the bulge region and distal (noncycling) follicle.17,19 This marked difference in the intracutaneous location of the inflammatory infiltrate has major clinical consequences (ie, reversible versus permanent hair loss) and underscores the topobiological importance of location in immunopathology. Further, HFs can only respond to inflammatory damage in a limited number of ways, with premature catagen induction, “dystrophic anagen,” programmed organ deletion (POD—see below), and scarring all being possible responses seen, depending on the type, intensity, and duration of immune-mediated attack.19
eHFSC Damage
Destruction of eHFSCs appears to be a key element in PCA pathogenesis. Supporting this is the observation that the inflammatory cell infiltrate in PCA is predominantly located round the bulge region and distal follicle, with relative sparing of the proximal bulb. Further, immunostaining of keratin 15, a recognized bulge marker in humans (but not specific for eHFSCs),11 has been shown to be diminished or absent from the bulge region in lichen planopilaris (LPP)37 and chronic cutaneous lupus erythematosus (CCLE),38 predominantly in samples with the pushing densest peri-follicular inflammatory infiltrates, suggesting that the inflammatory process is either destroying the eHFSC pool or pushing these cells towards differentiation as part of a HF repair response. Interestingly, permanent alopecia can also occur in long-standing cases of androgenetic alopecia39 and acute graft-versus-host disease,40 two conditions where inflammatory cell infiltrates around the bulge are frequently seen. However, whether the destructive inflammation is specifically directed against stem cell-specific autoantigens or is, in fact, targeting the more differentiated epithelium in which they reside, is still to be determined. Thus, even if the eHFSCs are not directly destroyed by the inflammatory process (ie, they are not the primary target of immune attack), if a sufficient insult occurs, the regenerative potential of the eHFSC must become overwhelmed (“stem cell exhaust”) for permanent HF loss to occur.
These findings, together with the K15 transgenic mouse model22 (see above), support a key role of eHFSC damage/exhaust in PCA; although, as highlighted in this mouse model, eHFSC destruction alone does not explain the full phenotypic profile (eg, atrophy, erythema, follicular plugging) seen in different PCA entities. Therefore, other pathogenic processes must account for these changes, in addition to the eHFSC damage that is clearly occurring. However, why the follicle is attacked in the first place (ie, what is the intrafollicular target antigen?) and what prevents inflammatory damage to eHFSCs in normal follicles are yet to be determined. Are the normal immunoprotective mechanisms so severely disrupted in PCA that the eHFSCs are exposed to a damaging immune-mediated attack? Whatever the unknown trigger factor may be, the peri-follicular inflammation in PCA appears to be sufficient to destroy eHFSCs and, in so doing, abrogates the astounding capacity of the HF for regeneration and self-repair—while simultaneously inducing all of the other clinical and histopathological hallmarks of the specific causative disease.
Collapse of HF Immune Privilege
HF bulge IP collapse is an attractive theory to explain the exposure of eHFSCs to immune-mediated attack in PCA. Preliminary data would support this, with MHC class I, β2-microblobulin, and MHC class II immune reactivity all being up-regulated in the bulge region of lesioned skin, compared with uninvolved skin, in different PCA entities.41 Unfortunately, it is still unclear whether this phenomenon develops at disease onset, predisposing the follicle to inflammatory attack, or as a secondary response induced by the developing proinflammatory cytokine milieu, and what is the intrafollicular antigenic trigger that precipitates such an autoimmune response.
Loss of CD200 Expression
Along the same lines, it is possible that loss of the “no danger” signal CD200 is important in the pathogenesis of PCA.34 Observations from the CD200 knock-out mouse model support this suggestion.33 Here, CD200-deficient (CD200−/−) mice skin was grafted onto sex-matched wild-type hosts (CD200+/+), producing inflammation, follicle loss, and scarring in the grafted skin. Of note was that the skin grafts survived long-term and the epidermis remained normal throughout. Histologically, a peri- and intrafollicular mononuclear inflammatory cell infiltrate was seen with intrafollicular edema and increased levels of apoptosis. Inflammation eventually resolved after all hair was lost and replaced with tracks of scar tissue.
Cytotoxic Cell-Mediated HF Damage in PCA
Peri-follicular inflammation is almost universally seen at some point in all PCA entities, leading many authors to examine the potential role of cell-mediated cytotoxicity in disease pathogenesis. In CCLE, the inflammatory infiltrate is composed predominantly of activated T lymphocytes,42 with T cells expressing chemokine receptor-4 frequently found in scarring lesions, suggesting a direct tissue-damaging role of these cells through epithelial invasion and induction of apoptosis.43 Further, colocalization of the skin-homing marker CLA (cutaneous lymphocyte antigen) with the cytotoxic marker granzyme B is significantly higher in CCLE lesions that scar, compared with nonscarring lesions, again, supporting their role in direct cytotoxic tissue damage.44 Laser capture microdissection and subsequent microarray analysis of CCLE tissue subpopulations suggest that inappropriate activation of innate immune pathways underlies this cytotoxic response, leading to tissue damage and scarring.45
Other cellular changes seen in CCLE are increased numbers of γδ-T cells46 and plasmacytoid dendritic cells,47 along with increased expression of various cell surface and adhesion molecules (eg, intercellular adhesion molecule-1, vascular cell adhesion molecule, and E-selectin) that are likely to be important in promoting cell trafficking into lesional skin.48
Proinflammatory Response in PCA
As in multiple other inflammatory diseases, including alopecia areata,31 INF is a key cytokine in the proinflammatory response. In CCLE, local production of type 1 INF induces a Th1 pattern of inflammation through induction of various chemokines (eg, CXCL9, 10, and 11) with subsequent recruitment of CXCR3+ T cells into affected skin.49,50 Interestingly, CXCL10 is expressed predominantly in the basal layer of the epidermis in areas of cytotoxic T-cell basal layer invasion.51 The proinflammatory cytokines tumor necrosis factor (TNF)-α, interleukin 2, and INF-γ are also up-regulated in CCLE.52 Similarly, in LPP, proinflammatory cytokines, including INF-γ, are expressed in the inflammatory infiltrate with levels of the profibrotic cytokine TGF-β also being greatly raised.53 In early lesion of folliculitis decalvans, activated T-helper cells are readily seen, with intercellular adhesion molecule-1 and interleukin 8 being up-regulated and inducing neutrophil migration into the tissues. In older lesions, fibroblast growth factor and TGF-β are up-regulated and thought to mediate the fibrotic reaction seen.54
Wenzel et al proposed the following mechanism for the initiation and propagation of the inflammatory reaction in CCLE: type 1 INF expression is triggered by an (yet) unknown stimulus, which induces chemokine and antiviral protein production and, in turn, attracts CXCR3+ T cells and plasmacytoid dendritic cells into the skin. Further release of proinflammatory cytokine from plasmacytoid dendritic cells and accumulated apoptotic cells, together with direct chemokine release from T cells, perpetuate the ongoing inflammatory response.55 It is conceivable that the pathogenesis of lymphocytic PCA follows a similar scenario.
Increased Apoptosis in PCA
Apoptotic keratinocytes are commonly observed during the histological assessment of PCA, suggesting a potential role in these disorders. In CCLE, apoptosis is increased in the HF, epidermis, and inflammatory infiltrate56,57,58,59; with the epithelial proliferation marker Ki-67 and the tumor suppressor gene p53 also being up-regulated. Here, p53 acts as the “guardian of the genome” by either facilitating DNA repair or by directing irreconcilably damaged cells into apoptosis.57,58,59 In LPP, gene expression profiling identified 206 up-regulated genes in affected scalp tissue, of which many were functionally involved in macrophage activation, apoptosis, cell migration, and tissue remodeling. About 30% of these up-regulated genes were regulated by p53, suggesting a central role in LPP pathogenesis.60
The cell surface receptor Fas (CD95) triggers apoptosis on binding to its receptor, Fas ligand (FasL; CD95L). In CCLE, Fas expression is increased in both the inflammatory infiltrate and on epidermal keratinocytes,56,61 and FasL+ cells (mainly infiltrating macrophages) are also found to accumulate around HFs. This peri-follicular FasL+ infiltrate is thought to play a key role in the follicular destruction commonly seen in CCLE through inappropriate apoptosis induction.56,61 Interestingly, expression of the constitutive apoptosis inhibitor Bcl-2 is also reduced in CCLE, predisposing to apoptosis-related tissue injury.56
Sebaceous Gland Dysfunction and PCA
The hypothesis that sebaceous gland abnormalities may play a role in PCA pathogenesis was originally derived from observations of the spontaneous mouse mutant, asebia (Scd1ab), which exhibits a scarring alopecia phenotype (Table 1).22,32,62,63,64,65,66,67,68 In this model, hairs are scant or absent and follicles are replaced by fibrous tracts. A defect in stearoyl-CoA desaturase 1, important in sebaceous gland fatty acid composition, results in sebaceous glands atrophy and abnormal gland secretions, in turn leading to delayed inner root sheath disintegration, retrograde hair shaft growth, and penetration of the bulb, with resulting foreign body reaction and eventual destruction of the HF. However, although sebaceous glands are atrophic or absent early in the disease course in humans, desquamation of the inner root sheath is not delayed, and retrograde follicle growth has not been described. So although this (and similar) models (Table 1)22,33,62,63,64,65,66,67,68 hold interesting lessons for general pathological mechanisms that may lead to PCA, promoting the hypothesis that sebaceous gland rather than HF dysfunction is the primary defect, there is as yet insufficient evidence that these mouse models faithfully represent the principles that drive specific PCA entities. It remains to be seen how much relevance these models actually bear to human disease.
Table 1.
Selected Mouse Mutants with a Cicatricial Alopecia Phenotype
| Mouse model (symbol) | Gene defect [mouse chromosome] | Type | Phenotype | Histology | Compared to human disease | Reference |
|---|---|---|---|---|---|---|
| Asebia (Scd1ab) Asebia Jackson (Scd1ab-J) Asebia Jackson-2 (Scd1ab-2J) | Stearoyl-CoA desaturase 1 enzyme [Ch 19] | S | Recessive inheritance; Scd1ab = reduced hair growth apparent by day 7; sparse coat in adults; scaly skin; reduced growth; reduced eye lubrication; Scd1ab-J; Scd1ab-2J = progressive scarring alopecia; scaly skin; runted growth; photophobia | Scarring alopecia; hypoplasia of sebaceous glands; abnormally long anagen follicle; retained IRS and hair follicle plugging; short shaft at surface; retrograde movement of the hair shaft with shaft penetration of the bulb and resultant; foreign body reaction; increased mast cell numbers | n.s. | 62, 63 |
| Alopecia 1 (Alo-1) | Mapped to Bsk locus 11 Mb region [Ch 11] | ENU | Dominant inheritance; early onset progressive alopecia; complete alopecia by 20 weeks; occasional excoriation/erosions in older mice | Thickened epidermis; early sebaceous gland loss; early infiltrate of mononuclear cells in dermis only; follicles deep into sc fat; destruction of follicles with necrosis; MHC I- and ICAM-1-positive in proximal follicle ( = IP collapse) | n.s. | 64 |
| Alopecia 2 (Alo-2) | Mapped to Bsk locus 24 Mb region [Ch 11] | ENU | Identical to Alo-1 | See Alo-1. Infiltrate and ICAM-1 expression down to sc fat; infiltrate deeper than that seen in Alo-1 | n.s. | 64 |
| Male NZ black/KN (NZB/KN) | Unknown | Tg | Normal onset hair cycle; initial lesions around tail; indurated skin with alopecia; progressive hair loss on back; severity and incidence of alopecia increases with age (77% developed alopecia lesions by 6 months) | Reduced follicle density; atrophic epidermis; intra- and peri-follicular mononuclear cell infiltrate; CD3+ and CD4+ increased around bulge; peri-vascular infiltrate; increased total number (and degranulated) mast cells; positive direct immunofluorescence for Ig M in 75% | Auto-immune-induced permanent alopecia | 65 |
| PPARγ conditional null (Pparg) | Peroxisome proliferator activated receptor-γ [Ch 6] | Tg | Progressive alopecia with spare and matted hair; marked pruritus and scratching behaviour; scaly skin; smaller size to uninvolved littermates | Hyperkeratosis; plugged follicles; follicular destruction; peri-follicular fibrosis; inflammatory infiltrate comprising T cells, macrophages, plasma cells and mast cells; abnormal sebaceous glands; abnormal lipid accumulation within follicle | LPP | 66 |
| Keratin 15 transgenic (Krtl-15-HSV-TK ROSA26) | Keratin 15 [Ch = 11] (NB. mice died within 7 days due to gastrointestinal toxicity) | Tg | Krtl-15-HSV-TK ROSA26 mice skin grafted onto immune-deficient mice; bulge suicide gene activated by ganciclovir at 3 months; complete hair loss within 8 days; no visible inflammation; normal looking epidermis; graft survives long-term. | Complete loss of follicles; no inflammation | n.s. | 22 |
| CD200 null (CD200−/−) | CD200 (OX-2) [Ch = 16] | Tg | CD200−/− skin grafted onto sex-matched WT hosts; hair lost in grafts after 40 days; epidermis normal; long-term graft survival | Peri- and intra-follicular mononuclear inflammatory infiltrate; intra-follicular edema and apoptosis; inflammation resolved after hair loss; residual scarring | n.s. | 33, 67 |
| Lympho-proliferation (Faspr) | Fas antigen [Ch 19] | S | Patchy hair loss; CCLE-like skin lesions in 80% by 5 months; photo-sensitive; SLE-like symptoms | Progressive dermal T-lymphocyte infiltration; similar histology to CCLE; direct immunofluorescence shows IgG at the dermo-epidermal junction | CCLE/SLE | 68 |
Ch, chromosome; ENU, N-ethyl-N-nitrosurea mutagenesis; Bsk, bareskin; IRS, inner root sheath; n.s., not specified; S, spontaneous; Tg, transgenic; N.M., not mapped; MHC 1, major histocompatability class 1; ICAM-1, intercellular adhesion molecule 1; LPP, lichen planopilaris; CCLE, chronic cutaneous lupus erythematosus; SLE, systemic lupus erythematosus; CA, cicatricial alopecia.
PPARγ Deficiency in PCA
An exciting development that promises to bridge the divide between supporters of the eHFSC destruction theory of PCA and those who feel sebaceous gland dysfunction is a central factor in PCA pathogenesis has recently emerged. Gene expression analysis from humans with LPP has identified a deficiency in peroxisome proliferator-activated receptor (PPAR)-γ–mediated signaling, which suggested that defective lipid metabolism and peroxisome processing may be important in LPP pathogenesis. Indeed, deletion of PPARγ in K15+ bulge cells in mutant mice results in an LPP-like skin and hair phenotype. Thus, PPAR-γ–mediated signaling via appropriate endogenous ligands may be vital for maintaining normal eHFSC function.66 Consequently, PPAR-γ stimulation by clinically available agonists such as pioglitazone would seem to be a promising therapeutic strategy for the future therapy of at least those PCA variants that show features of LPP. A first case report supports this important new concept in PCA management.69
Programmed Organ Deletion
Programmed cell death is a key tool for sculpting tissues and organs during development and physiological tissue remodeling, such as during HF morphogenesis and HF involution in the catagen stage of HF cycling.9 Interestingly, in the noninflamed back skin of clinically normal mice, dense peri-follicular infiltrates of inflammatory cells (primarily activated macrophages) are found centered round the bulge of isolated HFs and appear responsible for removal of these structures.70 Histological evidence for mild peri-follicular inflammation is actually much more common in clinically noninflamed human skin than is often appreciated (eg, in androgenetic alopecia),39 with POD potentially explaining why the total number of scalp HFs per unit area actually declines slightly with advancing age. Therefore, it has been proposed that immunologically mediated POD is a physiological mechanism for the removal of damaged/malfunctioning HF and that PCA may represent an exaggerated, uncontrolled variant of this otherwise normal response pattern to major HF damage.70
Neurogenic Skin Inflammation
In mice, psycho-emotional stressors have been shown to profoundly influence hair growth and cycling through the induction of nerve growth factor (NGF) and increased release of the prototypic stress-associated neuropeptide, substance P (SP), which conspire to induce mast cell–dependent neurogenic skin inflammation.71 This results in dense peri-follicular inflammatory cell infiltrates (especially around the bulge), accumulation and degranulation of peri-follicular mast cells, increased HF keratinocyte apoptosis, and reduced hair matrix keratinocyte proliferation, followed by premature HF entry into catagen.71,72,73,74
Of potential significance to PCA pathogenesis is the observation that SP+ nerve fibers are particularly dense around the insertion point of the arrector pili muscle and the bulge.72 Peri-follicular inflammation is often centered round this area, and stress-induced apoptosis is particularly prominent in the bulge.73 Further evidence from human HF organ cultures has shown that the addition of SP induces catagen, increases mast cell degranulation, and, in anagen follicles, up-regulates MHC class I and β2-microglobulin, indicating IP collapse.74 In addition, SP stimulation up-regulates the expression of (catagen-promoting) neurotrophins and their receptor (p75NTR) in human scalp HF.74 Because SP is also a known fibroblast growth factor,75 in theory, even scar formation may be promoted by SP-driven, peri-follicular neurogenic inflammation. Taken together, these findings suggest a possible role of stress-induced, SP-dependent, peri-follicular neurogenic inflammation in PCA, for example, by precipitating IP collapse and/or promoting excessive POD of HFs.
Mouse Models for Cicatricial Alopecia
Selected mouse models with a PCA phenotype are presented (Table 1).22,33,62,63,64,65,66,67,68 However, further investigation and correlation with human disease is required before these models can be accepted as true representations of human PCA.
Environmental Triggers for PCA
Various environmental triggers (eg, infection, trauma, medication) have been proposed as potential triggers for PCA.
Infection appears to be a major factor in the pathogenesis of folliculitis decalvans, based on the observation that Staphylococcus aureus is almost universally isolated from affected skin,76,77,78 and that the disease often responds to antistaphylococcal antibiotic therapy.16,77 However, considering how common Staphylococcal carriage is in the general population (20 to 30%), it is likely that an abnormal host response to this bacteria plays a key role in disease development.79 One theory is that S. aureus produces toxins (termed “superantigens”), which can form abnormal complexes with MHC class II receptors and stimulate the proliferation of T-cells80 and, in turn, propagates the excessive inflammatory response seen.81 Interestingly, it is also possible that these bacteria can survive phagocytosis, which may explain the frequent observation of disease recrudesce, even after an apparently successful course of therapy.81,82 Further, new lesions of acne necrotica varioliformis can be experimentally reproduced by injecting Staphylococci and Streptococci, isolated from active lesions from the same patient back into their normal skin. This may also represent an abnormal host response to these common bacteria.83
Trauma has been implicated in the pathogenesis of various PCA entities. In central centrifugal cicatricial alopecia (syn. follicular degeneration syndrome),84 a history of traumatic hair-care practices is often, though not always, identified.13,85 Interestingly, premature desquamation of the inner root sheath (highlighted by keratin 75 expression86), initially proposed as a useful diagnostic marker for the condition,87 is also frequently found in other scarring and nonscaring alopecias, and so is not unique to this condition.88 Acne keloidalis nuchae,89,90 folliculitis decalvans,91,92,93 and erosive pustular dermatosis of the scalp (EPD)94 have also been linked with scalp trauma. Grattan et al have proposed that EPD represents a “non-specific inflammatory response to injury of aging and sun-damaged scalps.”94 However, atrophic skin at sun-protected body sites have also been reported to develop EPD-like changes, suggesting that skin atrophy, and not sun damage, may be more important in pathogenesis.95
Drug therapy has also been implicated in PCA development; Graham Little syndrome has been reported following hepatitis B vaccination,96 acne keloidalis nuchae has been associated with anticonvulsant and cyclosporin therapy,97,98,99 and drug-induced causes of alopecia mucinosa100,101,102,103 and CCLE have been described.104
Genetic Factors in PCA
Genetic factors are likely to underpin the pathogenesis of all PCA entities in some form, either by predisposing an individual to a certain disorder or through a direct genetic effect, causing the condition. The latter is highlighted by keratosis follicularis spinulosa decalvans, where both x-linked and autosomal-dominant modes of inheritance have been demonstrated in different pedigrees. For x-linked keratosis follicularis spinulosa decalvans, linkage analysis has narrowed down the locus to Xp22.13-p22.2105,106,107,108; and a UK pedigree shows similar genetic features.109
The influence of genetic and environmental factors on other PCA may be further explored by reviewing familial cases of different disorders, for example, folliculitis decalvans in identical twins,110 dissecting cellulitis in two brothers,111 and familial cases of pseudopelade of Brocq.112,113,114 Interestingly, both a mother and daughter, each with Graham Little syndrome, were found to have human leukocyte antigen DR1 on human leukocyte antigen typing.115 Disappointingly, these limited genetic analyses have as yet contributed little to furthering our understanding of PC pathogenesis.
Conclusion and Perspectives
Here, we have portrayed PCA as a group of chronic inflammatory diseases characterized by irreversible damage to eHFSCs. No matter whether lymphocytes predominate in the peri-follicular infiltrate (eg, LPP and CCLE) or neurotrophils (eg, folliculitis decalvans), the critical factor appears to be whether these stem cells located in the HF bulge pass a “point-of-no-return,” beyond which no repair is possible. We have also discussed that healthy eHFSCs appear to enjoy relative protection from inflammatory assaults by being located in an immunologically “privileged” niche and that increasing evidence suggests that this protection may collapse, at least in the most prevalent forms of PCA, such as LPP and CCLE. Further, we have delineated specific PCA-promoting pathways along which inflammatory stem cell damage and “stem cell exhaust” may occur. Once these pathways have become better defined on the molecular level, is should be possible to discard the often confusing traditional PCA nomenclature and to replace it with a new diagnostic system that classifies PCAs on the basis of the predominant damage pathway rather than according to the observed histopathological pattern phenomena.
Among the multiple possibilities that need to be considered in terms of damage pathways leading to bulge stem cell destruction (Figure 2), we have singled out the maintenance and restoration of eHFSC immune privilege and of normal PPAR-γ receptor–mediated signaling as two particularly important parameters to better understand the pathobiology of the predominant PCAs, that is, lymphocytic PCAs, and for the development of more effective forms of PCA treatment.
This clinically important group of chronic inflammatory dermatoses is diagnostically difficult, remains therapeutically very frustrating, and continues to be largely ignored by professional immuno- and stem cell pathologists. We do hope, however, that these reflections have shed light on what makes PCAs both biologically and clinically fascinating: PCAs offer superbly accessible model systems for studying how and why epithelial stem cells are normally protected from immunological damage, what happens when these defenses crumble, and what can be done to restore them. Moreover, they invite one to study how epithelial cells respond to defined types of inflammatory damage and what physicians may do to boost both their immunoprotective and damage repair strategies.
Acknowledgments
We are grateful to Drs. David Pattwell and Katja Meyer for helpful editorial suggestions and to Prof. Christopher Griffiths for continued advice and support.
Footnotes
Address reprint requests to Ralf Paus, M.D., Department of Dermatology, University of Lübeck, Ratzeburger Allee 160, D-23538 Lübeck, Germany. E-mail: Ralf.Paus@uk-sh.de.
Supported by the Geoffrey-Dowling Fellowship from the British Association of Dermatologists (M.J.H.), a grant from the University of Lübeck Research Focus Programme on Autoimmunity and the Manchester NIHR Biomedical Research Centre (R.P.), as well as research grants from the Cicatricial Alopecia Research Foundation (CARF) and British Skin Foundation (BSF) (M.J.H. and R.P.).
CME Disclosure: None of the authors disclosed any relevant financial relationships.
References
- Harries MJ, Trueb RM, Tosti A, Messenger AG, Chaudhry I, Whiting DA, Sinclair R, Griffiths CE, Paus R. How not to get scar(r) ed: pointers to the correct diagnosis in patients with suspected primary cicatricial alopecia. Br J Dermatol. 2009;160:482–501. doi: 10.1111/j.1365-2133.2008.09008.x. [DOI] [PubMed] [Google Scholar]
- Whiting DA. Cicatricial alopecia: clinico-pathological findings and treatment. Clin Dermatol. 2001;19:211–225. doi: 10.1016/s0738-081x(00)00132-2. [DOI] [PubMed] [Google Scholar]
- Tiede S, Kloepper JE, Bodo E, Tiwari S, Kruse C, Paus R. Hair follicle stem cells: walking the maze. Eur J Cell Biol. 2007;86:355–376. doi: 10.1016/j.ejcb.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Mecklenburg L, Tobin DJ, Muller-Rover S, Handjiski B, Wendt G, Peters EM, Pohl S, Moll I, Paus R. Active hair growth (anagen) is associated with angiogenesis. J Invest Dermatol. 2000;114:909–916. doi: 10.1046/j.1523-1747.2000.00954.x. [DOI] [PubMed] [Google Scholar]
- Botchkarev VA, Botchkareva NV, Peters EM, Paus R. Epithelial growth control by neurotrophins: leads and lessons from the hair follicle. Prog Brain Res. 2004;146:493–513. doi: 10.1016/S0079-6123(03)46031-7. [DOI] [PubMed] [Google Scholar]
- Gharzi A, Reynolds AJ, Jahoda CA. Plasticity of hair follicle dermal cells in wound healing and induction. Exp Dermatol. 2003;12:126–136. doi: 10.1034/j.1600-0625.2003.00106.x. [DOI] [PubMed] [Google Scholar]
- Lau K, Paus R, Tiede S, Day P, Bayat A. Exploring the role of stem cells in cutaneous wound healing. Exp Dermatol. 2009;18:921–933. doi: 10.1111/j.1600-0625.2009.00942.x. [DOI] [PubMed] [Google Scholar]
- Hansen LS, Coggle JE, Wells J, Charles MW. The influence of the hair cycle on the thickness of mouse skin. Anat Rec. 1984;210:569–573. doi: 10.1002/ar.1092100404. [DOI] [PubMed] [Google Scholar]
- Stenn KS, Paus R. Controls of hair follicle cycling. Physiol Rev. 2001;81:449–494. doi: 10.1152/physrev.2001.81.1.449. [DOI] [PubMed] [Google Scholar]
- Meyer KC, Klatte JE, Dinh HV, Harries MJ, Reithmayer K, Meyer W, Sinclair R, Paus R. Evidence that the bulge region is a site of relative immune privilege in human hair follicles. Br J Dermatol. 2008;159:1077–1085. doi: 10.1111/j.1365-2133.2008.08818.x. [DOI] [PubMed] [Google Scholar]
- Kloepper JE, Tiede S, Brinckmann J, Reinhardt DP, Meyer W, Faessler R, Paus R. Immunophenotyping of the human bulge region: the quest to define useful in situ markers for human epithelial hair follicle stem cells and their niche. Exp Dermatol. 2008;17:592–609. doi: 10.1111/j.1600-0625.2008.00720.x. [DOI] [PubMed] [Google Scholar]
- Olsen EA, Bergfeld WF, Cotsarelis G, Price VH, Shapiro J, Sinclair R, Solomon A, Sperling L, Stenn K, Whiting DA, Bernardo O, Bettencourt M, Bolduc C, Callendar V, Elston D, Hickman J, Ioffreda M, King L, Linzon C, McMichael A, Miller J, Mulinari F, Trancik R. Summary of North American Hair Research Society (NAHRS)-sponsored Workshop on Cicatricial Alopecia. Duke University Medical Center, February 10 and 11, 2001. J Am Acad Dermatol. 2003;48:103–110. doi: 10.1067/mjd.2003.68. [DOI] [PubMed] [Google Scholar]
- Elston DM, McCollough ML, Warschaw KE, Bergfeld WF. Elastic tissue in scars and alopecia. J Cutan Pathol. 2000;27:147–152. doi: 10.1034/j.1600-0560.2000.027003147.x. [DOI] [PubMed] [Google Scholar]
- Stenn KS, Cotsarelis G, Price VH. Report from the cicatricial alopecia colloquium. J Invest Dermatol. 2006;126:539–541. doi: 10.1038/sj.jid.5700148. [DOI] [PubMed] [Google Scholar]
- Harries MJ, Meyer KC, Paus R. Hair loss as a result of cutaneous autoimmunity: frontiers in the immunopathogenesis of primary cicatricial alopecia. Autoimmun Rev. 2009;8:478–483. doi: 10.1016/j.autrev.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Harries MJ, Sinclair RD, Macdonald-Hull S, Whiting DA, Griffiths CE, Paus R. Management of primary cicatricial alopecias: options for treatment. Br J Dermatol. 2008;159:1–22. doi: 10.1111/j.1365-2133.2008.08591.x. [DOI] [PubMed] [Google Scholar]
- Cotsarelis G. Epithelial stem cells: a folliculocentric view. J Invest Dermatol. 2006;126:1459–1468. doi: 10.1038/sj.jid.5700376. [DOI] [PubMed] [Google Scholar]
- Schneider MR, Schmidt-Ullrich R, Paus R. The hair follicle as a dynamic miniorgan. Curr Biol. 2009;19:R132–142. doi: 10.1016/j.cub.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Paus R, Cotsarelis G. The biology of hair follicles. N Engl J Med. 1999;341:491–497. doi: 10.1056/NEJM199908123410706. [DOI] [PubMed] [Google Scholar]
- Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329–1337. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- Tiede S, Kloepper JE, Whiting DA, Paus R. The ‘follicular trochanter’: an epithelial compartment of the human hair follicle bulge region in need of further characterization. Br J Dermatol. 2007;157:1013–1016. doi: 10.1111/j.1365-2133.2007.08138.x. [DOI] [PubMed] [Google Scholar]
- Ito M, Liu Y, Yang Z, Nguyen J, Liang F, Morris RJ, Cotsarelis G. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med. 2005;11:1351–1354. doi: 10.1038/nm1328. [DOI] [PubMed] [Google Scholar]
- Paus R, Nickoloff BJ, Ito T. A ‘hairy’ privilege. Trends Immunol. 2005;26:32–40. doi: 10.1016/j.it.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Paus R, van der Veen C, Eichmuller S, Kopp T, Hagen E, Muller-Rover S, Hofmann U. Generation and cyclic remodeling of the hair follicle immune system in mice. J Invest Dermatol. 1998;111:7–18. doi: 10.1046/j.1523-1747.1998.00243.x. [DOI] [PubMed] [Google Scholar]
- Christoph T, Muller-Rover S, Audring H, Tobin DJ, Hermes B, Cotsarelis G, Ruckert R, Paus R. The human hair follicle immune system: cellular composition and immune privilege. Br J Dermatol. 2000;142:862–873. doi: 10.1046/j.1365-2133.2000.03464.x. [DOI] [PubMed] [Google Scholar]
- Reithmayer K, Meyer KC, Kleditzsch P, Tiede S, Uppalapati SK, Glaser R, Harder J, Schroder JM, Paus R. Human hair follicle epithelium has an antimicrobial defence system that includes the inducible antimicrobial peptide psoriasin (S100A7) and RNase 7. Br J Dermatol. 2009;161:78–89. doi: 10.1111/j.1365-2133.2009.09154.x. [DOI] [PubMed] [Google Scholar]
- Waldmann H. Immunology: protection and privilege. Nature. 2006;442:987–988. doi: 10.1038/nature05165. [DOI] [PubMed] [Google Scholar]
- Ito T, Ito N, Bettermann A, Tokura Y, Takigawa M, Paus R. Collapse and restoration of MHC class-I-dependent immune privilege: exploiting the human hair follicle as a model. Am J Pathol. 2004;164:623–634. doi: 10.1016/S0002-9440(10)63151-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Ito N, Saatoff M, Hashizume H, Fukamizu H, Nickoloff BJ, Takigawa M, Paus R. Maintenance of hair follicle immune privilege is linked to prevention of NK cell attack. J Invest Dermatol. 2008;128:1196–1206. doi: 10.1038/sj.jid.5701183. [DOI] [PubMed] [Google Scholar]
- Ito N, Ito T, Kromminga A, Bettermann A, Takigawa M, Kees F, Straub RH, Paus R. Human hair follicles display a functional equivalent of the hypothalamic-pituitary-adrenal axis and synthesize cortisol. FASEB J. 2005;19:1332–1334. doi: 10.1096/fj.04-1968fje. [DOI] [PubMed] [Google Scholar]
- Gilhar A, Paus R, Kalish RS. Lymphocytes, neuropeptides, and genes involved in alopecia areata. J Clin Invest. 2007;117:2019–2027. doi: 10.1172/JCI31942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama M, Terunuma A, Tock CL, Radonovich MF, Pise-Masison CA, Hopping SB, Brady JN, Udey MC, Vogel JC. Characterization and isolation of stem cell-enriched human hair follicle bulge cells. J Clin Invest. 2006;116:249–260. doi: 10.1172/JCI26043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblum MD, Olasz EB, Yancey KB, Woodliff JE, Lazarova Z, Gerber KA, Truitt RL. Expression of CD200 on epithelial cells of the murine hair follicle: a role in tissue-specific immune tolerance? J Invest Dermatol. 2004;123:880–887. doi: 10.1111/j.0022-202X.2004.23461.x. [DOI] [PubMed] [Google Scholar]
- Rosenblum MD, Yancey KB, Olasz EB, Truitt RL. CD200, a “no danger” signal for hair follicles. J Dermatol Sci. 2006;41:165–174. doi: 10.1016/j.jdermsci.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Barclay AN, Wright GJ, Brooke G, Brown MH. CD200 and membrane protein interactions in the control of myeloid cells. Trends Immunol. 2002;23:285–290. doi: 10.1016/s1471-4906(02)02223-8. [DOI] [PubMed] [Google Scholar]
- Paus R, Ito N, Takigawa M, Ito T. The hair follicle and immune privilege. J Investig Dermatol Symp Proc. 2003;8:188–194. doi: 10.1046/j.1087-0024.2003.00807.x. [DOI] [PubMed] [Google Scholar]
- Pozdnyakova O, Mahalingam M. Involvement of the bulge region in primary scarring alopecia. J Cutan Pathol. 2008;35:922–925. doi: 10.1111/j.1600-0560.2007.00937.x. [DOI] [PubMed] [Google Scholar]
- Al-Refu K, Edward S, Ingham E, Goodfield M. Expression of hair follicle stem cells detected by cytokeratin 15 stain: implications for pathogenesis of the scarring process in cutaneous lupus erythematosus. Br J Dermatol. 2009;160:1188–1196. doi: 10.1111/j.1365-2133.2009.09074.x. [DOI] [PubMed] [Google Scholar]
- Jaworsky C, Kligman AM, Murphy GF. Characterization of inflammatory infiltrates in male pattern alopecia: implications for pathogenesis. Br J Dermatol. 1992;127:239–246. doi: 10.1111/j.1365-2133.1992.tb00121.x. [DOI] [PubMed] [Google Scholar]
- Murphy GF, Lavker RM, Whitaker D, Korngold R. Cytotoxic folliculitis in GvHD. Evidence of follicular stem cell injury and recovery. J Cutan Pathol. 1991;18:309–314. doi: 10.1111/j.1600-0560.1991.tb01541.x. [DOI] [PubMed] [Google Scholar]
- Harries MJ, Meyer KC, Chaudhry IH, Griffiths CE, Paus R. Does collapse of immune privilege in the hair-follicle bulge play a role in the pathogenesis of primary cicatricial alopecia? Clin Exp Dermatol. 2010;35:637–644. doi: 10.1111/j.1365-2230.2009.03692.x. [DOI] [PubMed] [Google Scholar]
- Tebbe B, Mazur L, Stadler R, Orfanos CE. Immunohistochemical analysis of chronic discoid and subacute cutaneous lupus erythematosus–relation to immunopathological mechanisms. Br J Dermatol. 1995;132:25–31. doi: 10.1111/j.1365-2133.1995.tb08620.x. [DOI] [PubMed] [Google Scholar]
- Wenzel J, Henze S, Brahler S, Bieber T, Tuting T. The expression of human leukocyte antigen-DR and CD25 on circulating T cells in cutaneous lupus erythematosus and correlation with disease activity. Exp Dermatol. 2005;14:454–459. doi: 10.1111/j.0906-6705.2005.00301.x. [DOI] [PubMed] [Google Scholar]
- Wenzel J, Uerlich M, Worrenkamper E, Freutel S, Bieber T, Tuting T. Scarring skin lesions of discoid lupus erythematosus are characterized by high numbers of skin-homing cytotoxic lymphocytes associated with strong expression of the type I interferon-induced protein MxA. Br J Dermatol. 2005;153:1011–1015. doi: 10.1111/j.1365-2133.2005.06784.x. [DOI] [PubMed] [Google Scholar]
- Wenzel J, Zahn S, Bieber T, Tuting T. Type I interferon-associated cytotoxic inflammation in cutaneous lupus erythematosus. Arch Dermatol Res. 2009;301:83–86. doi: 10.1007/s00403-008-0892-8. [DOI] [PubMed] [Google Scholar]
- Volc-Platzer B, Anegg B, Milota S, Pickl W, Fischer G. Accumulation of gamma delta T cells in chronic cutaneous lupus erythematosus. J Invest Dermatol. 1993;100:84S–91S. doi: 10.1111/1523-1747.ep12356084. [DOI] [PubMed] [Google Scholar]
- Farkas L, Beiske K, Lund-Johansen F, Brandtzaeg P, Jahnsen FL. Plasmacytoid dendritic cells (natural interferon- alpha/beta-producing cells) accumulate in cutaneous lupus erythematosus lesions. Am J Pathol. 2001;159:237–243. doi: 10.1016/s0002-9440(10)61689-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg F, Hasan T, Skoglund C, Stephansson E. Early events in ultraviolet light-induced skin lesions in lupus erythematosus: expression patterns of adhesion molecules ICAM-1. VCAM-1 and E-selectin. Acta Derm Venereol. 1999;79:431–436. doi: 10.1080/000155599750009852. [DOI] [PubMed] [Google Scholar]
- Wenzel J, Worenkamper E, Freutel S, Henze S, Haller O, Bieber T, Tuting T. Enhanced type I interferon signalling promotes Th1-biased inflammation in cutaneous lupus erythematosus. J Pathol. 2005;205:435–442. doi: 10.1002/path.1721. [DOI] [PubMed] [Google Scholar]
- Flier J, Boorsma DM, van Beek PJ, Nieboer C, Stoof TJ, Willemze R, Tensen CP. Differential expression of CXCR3 targeting chemokines CXCL10. CXCL9, and CXCL11 in different types of skin inflammation. J Pathol. 2001;194:398–405. doi: 10.1002/1096-9896(200108)194:4<397::aid-path899>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Wenzel J, Zahn S, Mikus S, Wiechert A, Bieber T, Tuting T. The expression pattern of interferon-inducible proteins reflects the characteristic histological distribution of infiltrating immune cells in different cutaneous lupus erythematosus subsets. Br J Dermatol. 2007;157:752–757. doi: 10.1111/j.1365-2133.2007.08137.x. [DOI] [PubMed] [Google Scholar]
- Toro JR, Finlay D, Dou X, Zheng SC, LeBoit PE, Connolly MK. Detection of type 1 cytokines in discoid lupus erythematosus. Arch Dermatol. 2000;136:1497–1501. doi: 10.1001/archderm.136.12.1497. [DOI] [PubMed] [Google Scholar]
- Moretti S, Amato L, Massi D, Bianchi B, Gallerani I, Fabbri P. Evaluation of inflammatory infiltrate and fibrogenic cytokines in pseudopelade of Brocq suggests the involvement of T-helper 2 and 3 cytokines. Br J Dermatol. 2004;151:84–90. doi: 10.1111/j.1365-2133.2004.05976.x. [DOI] [PubMed] [Google Scholar]
- Chiarini C, Torchia D, Bianchi B, Volpi W, Caproni M, Fabbri P. Immunopathogenesis of folliculitis decalvans: clues in early lesions. Am J Clin Pathol. 2008;130:526–534. doi: 10.1309/NG60Y7V0WNUFH4LA. [DOI] [PubMed] [Google Scholar]
- Wenzel J, Tuting T. Identification of type I interferon-associated inflammation in the pathogenesis of cutaneous lupus erythematosus opens up options for novel therapeutic approaches. Exp Dermatol. 2007;16:454–463. doi: 10.1111/j.1600-0625.2007.00556.x. [DOI] [PubMed] [Google Scholar]
- Baima B, Sticherling M. Apoptosis in different cutaneous manifestations of lupus erythematosus. Br J Dermatol. 2001;144:958–966. doi: 10.1046/j.1365-2133.2001.04182.x. [DOI] [PubMed] [Google Scholar]
- Chung JH, Kwon OS, Eun HC, Youn JI, Song YW, Kim JG, Cho KH. Apoptosis in the pathogenesis of cutaneous lupus erythematosus. Am J Dermatopathol. 1998;20:233–241. doi: 10.1097/00000372-199806000-00002. [DOI] [PubMed] [Google Scholar]
- Pablos JL, Santiago B, Galindo M, Carreira PE, Ballestin C, Gomez-Reino JJ. Keratinocyte apoptosis and p53 expression in cutaneous lupus and dermatomyositis. J Pathol. 1999;188:63–68. doi: 10.1002/(SICI)1096-9896(199905)188:1<63::AID-PATH303>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Zamolo G, Coklo M, Santini Dusevic D, Kastelan M, Batinac T, Materljan E, Brumini G. Expression of p53 and apoptosis in discoid lupus erythematosus. Croat Med J. 2005;46:678–684. [PubMed] [Google Scholar]
- Karnik P, Smetanick M, McKormick TS, Mirmirani P. Microarray-based gene expression profiling in primary cicatricial alopecia implicates p53-dependent abnormal cytokine production, concurrent apoptosis and tissue remodelling in disease pathogenesis. J Invest Dermatol. 2005;124:A79. [Google Scholar]
- Nakajima M, Nakajima A, Kayagaki N, Honda M, Yagita H, Okumura K. Expression of Fas ligand and its receptor in cutaneous lupus: implication in tissue injury. Clin Immunol Immunopathol. 1997;83:223–229. doi: 10.1006/clin.1997.4352. [DOI] [PubMed] [Google Scholar]
- Stenn KS. Insights from the asebia mouse: a molecular sebaceous gland defect leading to cicatricial alopecia. J Cutan Pathol. 2001;28:445–447. doi: 10.1034/j.1600-0560.2001.028009445.x. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Eilertsen KJ, Ge L, Zhang L, Sundberg JP, Prouty SM, Stenn KS, Parimoo S. Scd1 is expressed in sebaceous glands and is disrupted in the asebia mouse. Nat Genet. 1999;23:268–270. doi: 10.1038/15446. [DOI] [PubMed] [Google Scholar]
- Wood GA, Flenniken A, Osborne L, Fleming C, Vukobradovic I, Morikawa L, Xu Q, Porter R, Adamson SL, Rossant J, McKerlie C. Two mouse mutations mapped to chromosome 11 with differing morphologies but similar progressive inflammatory alopecia. Exp Dermatol. 2005;14:373–379. doi: 10.1111/j.0906-6705.2005.00291.x. [DOI] [PubMed] [Google Scholar]
- Hiroi A, Ito T, Seo N, Uede K, Yoshimasu T, Ito M, Nakamura K, Ito N, Paus R, Furukawa F. Male New Zealand Black/KN mice: a novel model for autoimmune-induced permanent alopecia? Br J Dermatol. 2006;155:437–445. doi: 10.1111/j.1365-2133.2006.07204.x. [DOI] [PubMed] [Google Scholar]
- Karnik P, Tekeste Z, McCormick TS, Gilliam AC, Price VH, Cooper KD, Mirmirani P. Hair follicle stem cell-specific PPARgamma deletion causes scarring alopecia. J Invest Dermatol. 2009;129:1243–1257. doi: 10.1038/jid.2008.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblum MD, Woodliff JE, Madsen NA, McOlash LJ, Keller MR, Truitt RL. Characterization of CD 200-receptor expression in the murine epidermis. J Invest Dermatol. 2005;125:1130–1138. doi: 10.1111/j.0022-202X.2005.23948.x. [DOI] [PubMed] [Google Scholar]
- Furukawa F, Yoshimasu T. Animal models of spontaneous and drug-induced cutaneous lupus erythematosus. Autoimmun Rev. 2005;4:345–350. doi: 10.1016/j.autrev.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Mirmirani P, Karnik P. Lichen planopilaris treated with a peroxisome proliferator-activated receptor gamma agonist. Arch Dermatol. 2009;145:1363–1366. doi: 10.1001/archdermatol.2009.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichmuller S, van der Veen C, Moll I, Hermes B, Hofmann U, Muller-Rover S, Paus R. Clusters of perifollicular macrophages in normal murine skin: physiological degeneration of selected hair follicles by programmed organ deletion. J Histochem Cytochem. 1998;46:361–370. doi: 10.1177/002215549804600310. [DOI] [PubMed] [Google Scholar]
- Peters EM, Arck PC, Paus R. Hair growth inhibition by psychoemotional stress: a mouse model for neural mechanisms in hair growth control. Exp Dermatol. 2006;15:1–13. doi: 10.1111/j.0906-6705.2005.00372.x. [DOI] [PubMed] [Google Scholar]
- Peters EM, Botchkarev VA, Botchkareva NV, Tobin DJ, Paus R. Hair-cycle-associated remodeling of the peptidergic innervation of murine skin, and hair growth modulation by neuropeptides. J Invest Dermatol. 2001;116:236–245. doi: 10.1046/j.1523-1747.2001.01232.x. [DOI] [PubMed] [Google Scholar]
- Arck PC, Handjiski B, Hagen E, Joachim R, Klapp BF, Paus R. Indications for a ’brain-hair follicle axis (BHA)’: inhibition of keratinocyte proliferation and up-regulation of keratinocyte apoptosis in telogen hair follicles by stress and substance P. FASEB J. 2001;15:2536–2538. doi: 10.1096/fj.00-0699fje. [DOI] [PubMed] [Google Scholar]
- Peters EM, Liotiri S, Bodo E, Hagen E, Biro T, Arck PC, Paus R. Probing the effects of stress mediators on the human hair follicle: substance P holds central position. Am J Pathol. 2007;171:1872–1886. doi: 10.2353/ajpath.2007.061206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson J, von Euler AM, Dalsgaard CJ. Stimulation of connective tissue cell growth by substance P and substance K. Nature. 1985;315:61–63. doi: 10.1038/315061a0. [DOI] [PubMed] [Google Scholar]
- Powell J, Dawber RP. Folliculitis decalvans and tufted folliculitis are specific infective diseases that may lead to scarring, but are not a subset of central centrifugal scarring alopecia. Arch Dermatol. 2001;137:373–374. [PubMed] [Google Scholar]
- Powell JJ, Dawber RP, Gatter K. Folliculitis decalvans including tufted folliculitis: clinical, histological and therapeutic findings. Br J Dermatol. 1999;140:328–333. doi: 10.1046/j.1365-2133.1999.02675.x. [DOI] [PubMed] [Google Scholar]
- Bogg A. Folliculitis decalvans. Acta Derm Venereol. 1963;43:14–24. [Google Scholar]
- Vandenbergh MF, Verbrugh HA. Carriage of Staphylococcus aureus: epidemiology and clinical relevance. J Lab Clin Med. 1999;133:525–534. doi: 10.1016/s0022-2143(99)90181-6. [DOI] [PubMed] [Google Scholar]
- Marrack P, Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990;248:1066. [PubMed] [Google Scholar]
- Brooke RC, Griffiths CE. Folliculitis decalvans. Clin Exp Dermatol. 2001;26:120–122. doi: 10.1046/j.1365-2230.2001.00746.x. [DOI] [PubMed] [Google Scholar]
- Rogers DE, Tompsett R. The survival of staphylococci within human leukocytes. J Exp Med. 1952;95:209–230. doi: 10.1084/jem.95.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strumia MM. Experimental acne varioliformis. Arch Derm Syphilol. 1924;10:702. [Google Scholar]
- LoPresti P, Papa CM, Kligman AM. Hot comb alopecia. Arch Dermatol. 1968;98:234–238. [PubMed] [Google Scholar]
- Sperling LC, Sau P. The follicular degeneration syndrome in black patients. ‘Hot comb alopecia’ revisited and revised. Arch Dermatol. 1992;128:68–74. [PubMed] [Google Scholar]
- Sperling LC, Hussey S, Sorrells T, Wang JA, Darling T. Cytokeratin 75 expression in central, centrifugal, cicatricial alopecia–new observations in normal and diseased hair follicles. J Cutan Pathol. 37:243–248. doi: 10.1111/j.1600-0560.2009.01330.x. [DOI] [PubMed] [Google Scholar]
- Sperling LC, Cowper SE. The histopathology of primary cicatricial alopecia. Semin Cutan Med Surg. 2006;25:41–50. doi: 10.1016/j.sder.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Horenstein MG, Simon J. Investigation of the hair follicle inner root sheath in scarring and non-scarring alopecia. J Cutan Pathol. 2007;34:762–768. doi: 10.1111/j.1600-0560.2006.00696.x. [DOI] [PubMed] [Google Scholar]
- Salami T, Omeife H, Samuel S. Prevalence of acne keloidalis nuchae in Nigerians. Int J Dermatol. 2007;46:482–484. doi: 10.1111/j.1365-4632.2007.03069.x. [DOI] [PubMed] [Google Scholar]
- Knable AL, Jr, Hanke CW, Gonin R. Prevalence of acne keloidalis nuchae in football players. J Am Acad Dermatol. 1997;37:570–574. doi: 10.1016/s0190-9622(97)70173-7. [DOI] [PubMed] [Google Scholar]
- Dalziel KL, Telfer NR, Wilson CL, Dawber RP. Tufted folliculitis. A specific bacterial disease? Am J Dermatopathol. 1990;12:37–41. doi: 10.1097/00000372-199002000-00006. [DOI] [PubMed] [Google Scholar]
- Luelmo-Aguilar J, Gonzalez-Castro U, Castells-Rodellas A. Tufted hair folliculitis. A study of four cases. Br J Dermatol. 1993;128:454–457. doi: 10.1111/j.1365-2133.1993.tb00210.x. [DOI] [PubMed] [Google Scholar]
- Pujol RM, Matias-Guiu X, Garcia-Patos V, de Moragas JM. Tufted-hair folliculitis. Clin Exp Dermatol. 1991;16:199–201. doi: 10.1111/j.1365-2230.1991.tb00346.x. [DOI] [PubMed] [Google Scholar]
- Grattan CE, Peachey RD, Boon A. Evidence for a role of local trauma in the pathogenesis of erosive pustular dermatosis of the scalp. Clin Exp Dermatol. 1988;13:7–10. doi: 10.1111/j.1365-2230.1988.tb00639.x. [DOI] [PubMed] [Google Scholar]
- Patton D, Lynch PJ, Fung MA, Fazel N. Chronic atrophic erosive dermatosis of the scalp and extremities: a recharacterization of erosive pustular dermatosis. J Am Acad Dermatol. 2007;57:421–427. doi: 10.1016/j.jaad.2007.04.026. [DOI] [PubMed] [Google Scholar]
- Bardazzi F, Landi C, Orlandi C, Neri I, Varotti C. Graham Little-Piccardi-Lasseur syndrome following HBV vaccination. Acta Derm Venereol. 1999;79:93. doi: 10.1080/000155599750011886. [DOI] [PubMed] [Google Scholar]
- Azurdia RM, Graham RM, Weismann K, Guerin DM, Parslew R. Acne keloidalis in caucasian patients on cyclosporin following organ transplantation. Br J Dermatol. 2000;143:465–467. doi: 10.1046/j.1365-2133.2000.03694.x. [DOI] [PubMed] [Google Scholar]
- Grunwald MH, Ben-Dor D, Livni E, Halevy S. Acne keloidalis-like lesions on the scalp associated with antiepileptic drugs. Int J Dermatol. 1990;29:559–561. doi: 10.1111/j.1365-4362.1990.tb03468.x. [DOI] [PubMed] [Google Scholar]
- Carnero L, Silvestre JF, Guijarro J, Albares MP, Botella R. Nuchal acne keloidalis associated with cyclosporin. Br J Dermatol. 2001;144:429–430. doi: 10.1046/j.1365-2133.2001.04049.x. [DOI] [PubMed] [Google Scholar]
- Crowson AN, Magro CM. Antidepressant therapy: a possible cause of atypical cutaneous lymphoid hyperplasia. Arch Dermatol. 1995;131:925–929. doi: 10.1001/archderm.131.8.925. [DOI] [PubMed] [Google Scholar]
- Magro CM, Crowson AN. Drugs with antihistaminic properties as a cause of atypical cutaneous lymphoid hyperplasia. J Am Acad Dermatol. 1995;32:419–428. doi: 10.1016/0190-9622(95)90063-2. [DOI] [PubMed] [Google Scholar]
- Magro CM, Crowson AN. Drug-induced immune dysregulation as a cause of atypical cutaneous lymphoid infiltrates: a hypothesis. Hum Pathol. 1996;27:125–132. doi: 10.1016/s0046-8177(96)90365-2. [DOI] [PubMed] [Google Scholar]
- Yanagi T, Sawamura D, Shimizu H. Follicular mucinosis associated with imatinib (STI571). Br J Dermatol. 2004;151:1276–1278. doi: 10.1111/j.1365-2133.2004.06295.x. [DOI] [PubMed] [Google Scholar]
- Vedove CD, Del Giglio M, Schena D, Girolomoni G. Drug-induced lupus erythematosus. Arch Dermatol Res. 2009;301:99–105. doi: 10.1007/s00403-008-0895-5. [DOI] [PubMed] [Google Scholar]
- Oosterwijk JC, Nelen M, Van Zandvoort PM, Van Osch LD, Oranje AP, Wittebol-Post D, Van Oost BA. Confirmation of X-linked inheritance and provisional mapping of the keratosis follicularis spinulosa decalvans gene on Xp in a large Dutch family. Ophthalmic Paediatr Genet. 1992;13:27–30. doi: 10.3109/13816819209070050. [DOI] [PubMed] [Google Scholar]
- Porteous ME, Strain L, Logie LJ, Herd RM, Benton EC. Keratosis follicularis spinulosa decalvans: confirmation of linkage to Xp22.13-p22.2. J Med Genet. 1998;35:336–337. doi: 10.1136/jmg.35.4.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosterwijk JC, Nelen M, van Zandvoort PM, van Osch LD, Oranje AP, Wittebol-Post D, van Oost BA. Linkage analysis of keratosis follicularis spinulosa decalvans, and regional assignment to human chromosome Xp21.2-p22.2. Am J Hum Genet. 1992;50:801–807. [PMC free article] [PubMed] [Google Scholar]
- Oosterwijk JC, van der Wielen MJ, van de Vosse E, Voorhoeve E, Bakker E. Refinement of the localisation of the X linked keratosis follicularis spinulosa decalvans (KFSD) gene in Xp22.13-p22.2. J Med Genet. 1995;32:736–739. doi: 10.1136/jmg.32.9.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herd RM, Benton EC. Keratosis follicularis spinulosa decalvans: report of a new pedigree. Br J Dermatol. 1996;134:138–142. [PubMed] [Google Scholar]
- Douwes KE, Landthaler M, Szeimies RM. Simultaneous occurrence of folliculitis decalvans capillitii in identical twins. Br J Dermatol. 2000;143:195–197. doi: 10.1046/j.1365-2133.2000.03616.x. [DOI] [PubMed] [Google Scholar]
- Bjellerup M, Wallengren J. Familial perifolliculitis capitis abscedens et suffodiens in two brothers successfully treated with isotretinoin. J Am Acad Dermatol. 1990;23:752–753. doi: 10.1016/s0190-9622(08)81076-6. [DOI] [PubMed] [Google Scholar]
- Binazzi M. [Apropos of an observation of Brocq’s pseudopelade in a father and daughter.]. Minerva Dermatol. 1964;39:261–266. [PubMed] [Google Scholar]
- Collier PM, James MP. Pseudopelade of Brocq occurring in two brothers in childhood. Clin Exp Dermatol. 1994;19:61–64. doi: 10.1111/j.1365-2230.1994.tb01119.x. [DOI] [PubMed] [Google Scholar]
- Sahl WJ. Pseudopelade: an inherited alopecia. Int J Dermatol. 1996;35:715–719. doi: 10.1111/j.1365-4362.1996.tb00644.x. [DOI] [PubMed] [Google Scholar]
- Viglizzo G, Verrini A, Rongioletti F. Familial Lassueur-Graham-Little-Piccardi syndrome. Dermatology. 2004;208:142–144. doi: 10.1159/000076489. [DOI] [PubMed] [Google Scholar]