Abstract
Highly pathogenic avian influenza viruses (HPAIV) of the H5 and H7 subtypes primarily infect poultry but are occasionally transmitted to humans and other mammalian species, often causing severe disease. Previously we have shown that HPAIV H5N1 causes severe systemic disease in cats. In this study, we investigated whether HPAIV H7N7 isolated from a fatal human case is also able to cause disease in cats. Additionally, we compared the cell tropism of both viruses by immunohistochemistry and virus histochemistry. Three domestic cats were inoculated intratracheally with HPAIV H7N7. Virus excretion was restricted to the pharynx. At necropsy, 7 days post inoculation, lesions were restricted to the respiratory tract in all cats. Lesions consisted of diffuse alveolar damage and colocalized with virus antigen expression in type II pneumocytes and nonciliated bronchiolar cells. The attachment patterns of HPAIV H7N7 and H5N1 were similar: both viruses attached to nonciliated bronchiolar epithelial cells, type II pneumocytes, as well as alveolar macrophages. These data show for the first time that a non-H5 HPAIV is able to infect and cause respiratory disease in cats. The failure of HPAIV H7N7 to spread beyond the respiratory tract was not explained by differences in cell tropism compared to HPAIV H5N1. These findings suggest that HPAIV H5N1 possesses other characteristics that allow it to cause systemic disease in both humans and cats.
Although the natural reservoir for influenza A viruses are free-ranging waterbirds, influenza virus lineages can be found in many other species, including humans, swine, horses, and dogs. Three pandemics in humans have been recorded in the previous century, as the result of introduction of a new subtype of influenza A virus that was efficiently transmissible among humans. Of these three pandemics, the H1N1 virus outbreak in 1918 was the worst, claiming more than 40 million lives worldwide.1 The first influenza pandemic of this century, which started in 2009, was also caused by an H1N1 virus (http://www.who.int/csr/don/2009_05_29/en/index.html).
Highly pathogenic avian influenza viruses (HPAIV) primarily infect poultry, but occasionally also humans. Fatal disease in humans has been reported for HPAIV infections of the H5N1 and H7N7 subtypes. During an outbreak of HPAIV of the H7N7 subtype in the Netherlands in 2003, virus was transmitted to 89 people, of whom one died.2 During the ongoing outbreak of HPAIV of the H5N1 subtype, virus has been transmitted to 492 people, resulting in 291 deaths (http://www.who.int/csr/disease/avian_influenza/country/cases_table_2010_03_30/en/index.html, last accessed May 29, 2009). Since 2003, HPAIV H5N1 has also been transmitted to tigers,3,4 leopards,3 domestic cats,5,6,7,8 a stone marten,9 Owston’s palm civets,10 domestic dogs,11 an American mink,12 and swine.13 These repeated transmissions to mammals, including humans, might facilitate viral adaptations to the mammalian host, which could potentially lead to a pandemic.
Of particular interest are the frequent transmissions of HPAIV H5N1 to felids, which were previously considered to be resistant to influenza A virus disease.6,14 Early in the outbreak of HPAIV H5N1, two tigers and two leopards at a zoo in Thailand became fatally infected with H5N1 virus.3 Experimental studies subsequently proved that domestic cats developed severe disease after H5N1 virus infection, with demonstration of felid-to-felid transmission.15 Interestingly, although influenza viruses are normally restricted to the respiratory tract in mammals, cats infected with HPAIV H5N1 had extensive extra-respiratory spread of the virus.16 This is relevant to the disease in humans, where extra-respiratory disease from HPAIV H5N1 has also been demonstrated.17,18,19,20,21
The pathogenesis of HPAIV infections in humans is not completely understood, partly because data on human HPAIV infections are often from a late stage of disease, which is not representative for events that happen early after infection. Attachment studies have shown that the attachment pattern of avian influenza viruses in cats closely resembles that of humans.22,23 Therefore, studies on influenza A virus infections in cats can provide insight into the pathogenesis of influenza A virus infections in humans.
All data on HPAIV infections in cats are from the HPAIV H5N1 virus outbreak. It is unknown whether disease from HPAIV infections in cats, including its systemic spread, is unique for HPAIV H5N1. To gain more insight into the pathogenesis of HPAIV infection in cats, domestic cats were infected with a non-H5 HPAIV, a H7N7 subtype. This virus was isolated from a fatal human case during the HPAIV outbreak in 2003 in the Netherlands and is known to be highly pathogenic in mouse and ferret models.24,25 Furthermore, pathological investigations on chickens infected during this outbreak showed a similar pathogenesis to that from other HPAIV infections, including HPAIV H5N1.25,26 Our main objective was to determine whether cats could become infected and develop disease from this HPAIV of the H7N7 subtype. Furthermore, we wanted to determine whether the infection was restricted to the respiratory tract or spread systemically. To investigate observed differences between HPAIV H5N1 and HPAIV H7N7 infections, we subsequently compared the attachment pattern of this HPAIV H7N7 isolate with that of HPAIV H5N1 in cats.
Materials and Methods
Virus Preparation
A virus stock was prepared from the influenza A virus A/NL/219/03 (H7N7), which was isolated from a fatal human case during the 2003 outbreak in the Netherlands.2 The virus was propagated in Madin-Darby canine kidney cells. The stock was titrated according to standard methods.27
Experimental Protocol
Four- to six-month-old specific pathogen-free European shorthair cats were purchased from a commercial breeder (Harlan, Indianapolis, IN). Throughout the experiment the animals were housed in negatively pressurized isolator units. Three cats (cat 1–3) were anesthetized with ketamine and infected intratracheally using a catheter containing 2.5 × 104 TCID50 of H7N7 virus. In parallel, two cats were inoculated intratracheally with PBS. Before and at 1, 3, 5, and 7 days post infection (dpi), pharyngeal, nasal and rectal swabs were collected from cats under anesthesia (ketamine) and submersed in 3 ml of virus transport medium (minimal essential medium with antibiotics). Body temperature was measured daily using subcutaneous probes. All cats were euthanized 7 dpi by exsanguination under ketamine anesthesia. Experimental procedures were approved by an independent Animal Care and Use Committee.
Pathological Examination and Immunohistochemistry
Necropsies and tissue sampling were performed according to a standard protocol. The following tissues were collected: conjunctiva, trachea, lung (nine specimens), tongue, esophagus, stomach, duodenum, jejunum, ileum, cecum, colon, tonsil, tracheo-bronchial lymph node, mesenteric lymph node, spleen, thymus, heart, liver, pancreas, kidney, adrenal gland, urinary bladder, olfactory bulb, cerebrum (at the level of the hippocampus), cerebellum, and brain stem. After fixation in 10% neutral-buffered formalin and embedding in paraffin, tissue sections were stained with hematoxylin and eosin for histological evaluation.
For the detection of influenza A virus antigen, all tissues were stained with a primary antibody against the influenza A virus nucleoprotein (HB-65, ATCC Wesel, Germany) as described previously,28 with the following modifications: Tissue sections were pre-incubated with 0.1% protease for 10 minutes at 37°C (Sigma, St. Louis, MO). Binding of the primary antibody was detected using a peroxidase labeled goat-anti-mouse IgG2a (Southern Biotech, Birmingham, AL). Peroxidase was revealed using 3-Amino-9-ethyl-carbazole (AEC, Sigma) resulting in a bright red precipitate. In each staining procedure an isotype control was included as a negative control and influenza A virus-positive tissue as positive control.
Virus Titrations
The following tissues were sampled for virus titration: stomach, liver, kidney, heart, cerebrum, cerebellum, brain stem, olfactory bulb, nasal concha, trachea, jejunum, tonsil, eyelid, tracheo-bronchial lymph node, mesenteric lymph node, spleen, and lung. Tissues were weighed and homogenized in 3 ml of transport medium by use of a homogenizer (Kinematica Polytron, Lucerne, Switzerland). Tenfold serial dilutions of the tissue suspensions and swabs were inoculated in Madin-Darby canine kidney cells in quadruplicate as described previously.27 All experiments were performed under biosafety level 3 conditions.
Virus Histochemistry
The attachment of HPAIV H7N7 (A/NL/219/03) and HPAIV H5N1 (A/Vietnam/1194/2004) was determined on lung, liver, and cerebrum tissues from 3 healthy noninfected domestic cats. Virus histochemistry was performed as described previously.22,23,29
Results
Clinical Signs
None of the three cats showed any severe clinical signs. Two of the cats appeared less active from day 1 onwards, and one cat appeared lethargic at day 7. All three cats showed a body temperature rise of 1 to 1.5°C at 2 dpi that lasted until 3–6 dpi.
Virology
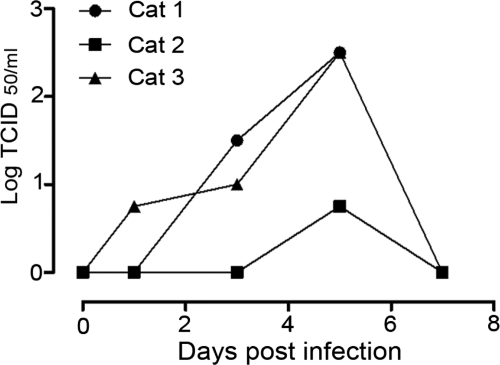
In all infected cats, virus was isolated from the pharyngeal swabs. No virus was isolated from any of the nasal or rectal swabs. The maximum titer in the pharyngeal swabs (100.75–102.5 TCID50/ml) reached its peak at 5 dpi in all three cats (Figure 1). At necropsy on 7 dpi, virus was isolated from the lung (103.1–107.7 TCID50/g) and the trachea (104.0–104.4 TCID50/g) in all three cats (Table 1). In two of the cats (cat 2 and 3), virus was isolated from the tonsil (102.7–104.5 TCID50/g). In cat 1 virus was isolated from the heart (102.3 TCID50/g), and in cat 3 virus was isolated from the tracheo-bronchial lymph node (101.1 TCID50/g) (Table 1). No virus could be isolated from any of the swabs from the sham-infected cats.
Figure 1.
Virus titers in pharyngeal swabs from individual cats infected with HPAIV H7N7 virus.
Table 1.
Virus Titers (Log10 TCID50/gram tissue) from Tissues Collected 7 Days Postinfection
| Tissue | Cat 1 | Cat 2 | Cat 3 |
|---|---|---|---|
| Heart | 2.3 | <1 | <1 |
| Lung | 5.1 | 7.7 | 3.1 |
| Trachea | 4.0 | 4.0 | 4.4 |
| Tracheo-bronchial lymph node | <1 | <1 | 1.1 |
| Tonsil | <1 | 2.7 | 4.5 |
Brain, eye, jejunum, kidney, liver, mesenteric lymph node, nasal concha, spleen, and stomach all tested negative.
Gross Pathology
All three cats had multifocal or coalescing pulmonary lesions, which were red, slightly raised, and firmer than normal. The estimated lung volume affected ranged from 25 to 80%. All cats had enlarged tracheo-bronchial lymph nodes, and one cat (cat 2) had enlarged tonsils. The stomachs of cat 1 and 2 were half-filled, while the stomach of cat 3 was empty. In cat 2, the pelvis of the left kidney was distended and filled with urine and the upper one-third of the left ureter was distended.
Histopathology
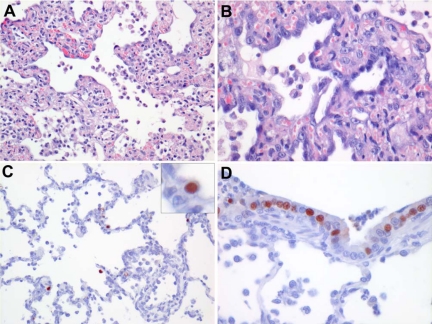
In the lungs of all three cats, there were multiple foci of subacute to chronic necrosis and inflammation in the lung parenchyma centered on the bronchioles. The alveolar and bronchiolar lumina were filled with variable numbers of large mononuclear cells, neutrophils, and lymphocytes, mixed with edema fluid, fibrin, erythrocytes, and cell debris. The alveolar and bronchiolar walls had lost their epithelium focally. In the alveoli, there was partial to complete re-epithelialization with low to high cuboidal epithelial cells (type II pneumocyte hyperplasia). The alveolar and bronchiolar walls were mildly thickened with edema fluid, macrophages, neutrophils, and lymphocytes. The connective tissue around the pulmonary arteries and veins was widened and contained few macrophages, neutrophils, and lymphocytes (Figure 2, A and B).
Figure 2.
Histological changes (A and B) and virus antigen distribution (C and D) in lung tissue of cat no. 1 at 7 days after experimental infection with HPAIV H7N7. A: Alveoli with a thickened wall, covered by hyperplastic type II pneumocytes. Alveolar lumina are filled with mononuclear cells, neutrophils, and lymphocytes, mixed with edema fluid, fibrin, erythrocytes, and cell debris (original magnification, ×200). B: Alveolus covered by hyperplastic type II pneumocytes (original magnification, ×400). C: Influenza virus antigen expression in a few type II pneumocytes in alveoli at the edge of a lesion (original magnification, ×200). Inset: Influenza virus antigen expression in a type II pneumocyte. D: Nonciliated bronchiolar cells expressing influenza A virus antigen (original magnification, ×400).
In cat 3, there was evidence of moderate lymphocyte hyperplasia in lymph nodes. In the conjunctiva of cat 1, there was diffuse loss of histological architecture, intra- and intercellular edema, and infiltration with moderate numbers of neutrophils. Lesions considered unrelated to the H7N7 virus infection were a hydronephrosis in the kidney and a focal chronic hematoma in the spleen in cat 2 and a small lingual ulcer on the tongue in cat 3. Other tissues examined had no significant lesions.
Immunohistochemistry
Influenza virus antigen was visible as diffuse to granular red staining, which was most prominent in the nucleus of the cell. In all three cats, influenza virus antigen was only present in the lungs. Lesions in the lung were more widespread than the distribution of virus antigen. Virus antigen was present predominantly in type II pneumocytes in the alveoli (Figure 2C) and occasionally in nonciliated epithelial cells in the bronchioles (Figure 2D). The bronchiolar epithelium in domestic cats consists of more than 95% of nonciliated cells and less than 5% of ciliated cells.30,31
Virus Histochemistry
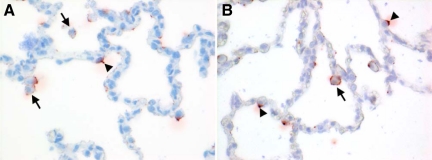
To determine whether the lack of systemic dissemination of HPAIV H7N7—which we had observed in HPAIV H5N1 infected cats—could be due to differences in cell tropism we compared the pattern of virus attachment of the two viruses in respiratory and extra-respiratory tissues of non-infected healthy cats. In the lung, both viruses attached predominantly to nonciliated cuboidal cells in the bronchioles and to type II pneumocytes and alveolar macrophages in the alveoli (Figure 3, A and B). Neither of the viruses attached to cells in liver or cerebrum.
Figure 3.
Virus attachment is visible as red precipitate of HPAIV H7N7 (A) and H5N1 (B) in alveoli of uninfected cats. Both viruses attach to type II pneumocytes (arrowheads) and alveolar macrophages (arrows).
Discussion
This study shows for the first time that cats are susceptible to disease after infection with a HPAIV of another subtype than H5N1. Cats developed respiratory disease after intratracheal infection with a HPAIV H7N7, isolated from a fatal human case during the 2003 outbreak in the Netherlands. Interestingly, H7N7 virus replication and associated lesions were limited to the respiratory tract. This is in contrast with HPAIV H5N1 infection in cats, which resulted in systemic virus replication associated with severe necrosis and inflammation.5,6,7,15,16
The fact that both HPAIV H5N1 and HPAIV H7N7 are able to infect cats implies that cats might be susceptible to infection by multiple subtypes of HPAIV. (Recent demonstration of pandemic H1N1 virus infection in cats indicates that cats might be susceptible to infection by human influenza viruses as well.32) The role of cats should therefore be considered during outbreaks of any HPAIV subtype because they may transmit virus from one poultry farm to another poultry farm, or to other species (including humans).33 Furthermore, the ability of HPAIV to infect cats could facilitate adaptations to the mammalian host and thereby increase the pandemic potential of the virus.34
The difference in histological pulmonary lesions between HPAIV H5N1 and H7N7 virus-infected cats seems to be associated with the stage of infection. HPAIV H7N7 Infection was resolving at 7 dpi, as indicated by the lack of virus excretion and the few cells that contained virus antigen in the pulmonary lesions. In contrast, HPAIV H5N1 infection seemed to be ongoing at 7 dpi, with still increasing virus titers from the pharynx and many influenza virus antigen containing cells in the pulmonary lesions.15,16
The differences in virus distribution between HPAIV H7N7- and H5N1-infected cats could not be explained by differences in genome, pattern of virus attachment, or pattern of virus replication. Regarding genome, there were no obvious differences between the two viruses. For example, both viruses have a lysine at position 627 of PB2, which is associated with increased pathogenicity of influenza virus infection in mammals.24 Regarding pattern of virus attachment, both viruses attached to nonciliated epithelial cells in the bronchioles and to type II pneumocytes and alveolar macrophages in the alveoli. Regarding pattern of virus replication, both viruses replicated in the same cell types, nonciliated bronchiolar epithelial cells and type II pneumocytes, as detected by immunohistochemistry at 7 dpi. The only difference was infection of alveolar macrophages: these were not observed in HPAIV H7N7-infected cats and were occasionally observed in HPAIV H5N1-infected cats. It cannot be excluded that this difference contributes to the difference in pathogenicity between these two viruses.
Other factors, such as replication efficiency and mechanisms to escape innate immune response, might contribute to the observed difference in virus distribution between HPAIV H5N1 and H7N7. Virus isolation from the heart of one cat indicated there might be some spillover of HPAIV H7N7 from the lung into the bloodstream. Because there was no virus antigen detected by immunohistochemistry in the heart or any other extra-respiratory tissues, there is no evidence for active virus replication at these sites.
The differences in tissue tropism between HPAIV H7N7 and HPAIV H5N1 in other experimental animal species corresponds with our findings in cats. In both mice and ferrets, HPAIV H7N7 was detected in extra-respiratory tract tissues by virus isolation. However, virus replication in these tissues could not be confirmed by immunohistochemistry in mice, while immunohistochemistry was not performed in ferrets.26 In comparison, HPAIV H5N1 did show virus replication in extra-respiratory tissues by immunohistochemistry in both mice and ferrets.35,36
The lesions found in the fatal human case, from which the HPAIV H7N7 was isolated, are similar to the lesions observed in these experimentally infected cats. In the human fatal case, who died 15 days after infection, the main histological lesion observed was diffuse alveolar damage, without any significant lesions in any other organs by gross or histological examination.2,21 Although virus was isolated from the lungs, virus antigen could not be detected in the lungs or any other organs. In the cats, which were euthanized at 7 dpi, the main histological lesion was also diffuse alveolar damage, without evidence for systemic dissemination of the HPAIV H7N7. Virus antigen—albeit scarce—was associated with the histological lesions. The difference in the presence of virus antigen may be because the infection in the fatal human case was about 1 week more chronic than that in cats.24
The extra-respiratory spread of HPAIV H5N1 in cats, which was not observed in HPAIV H7N7-infected cats, fits with the extra-respiratory spread of HPAIV H5N1 in some of the confirmed human cases. In HPAIV H5N1 virus-infected humans, virus has been detected in blood by virus isolation17,37 and in brain, intestine, liver, and placenta by immunohistochemistry and in situ hybridization.19,20 Because influenza A viruses are normally restricted to the respiratory tract in humans and other mammals, HPAIV H5N1 seems to have a unique pathogenesis, even when compared to other HPAIV.
Acknowledgments
We thank Theo Bestebroer, Lonneke Leijten, Frank van der Panne, and Robert Dias d’Ullois for excellent technical assistance, and Emmie de Wit and Ron Fouchier for helpful comments.
Footnotes
Address reprint requests to Thijs Kuiken, Ph.D., Department of Virology, Erasmus MC, PO Box 2040, 3000 CA, Rotterdam, The Netherlands. E-mail: t.kuiken@erasmusmc.nl.
Supported by the VIRGO Consortium, an innovative cluster approved by the Netherlands Genomics Initiative, and partially funded by the Dutch government (grant number BSIK03012) and the Novaflu EU grant QLRT 2001-01034.
References
- Oxford JS. Influenza A pandemics of the 20th century with special reference to 1918: virology, pathology and epidemiology. Rev Med Virol. 2000;10:119–133. doi: 10.1002/(sici)1099-1654(200003/04)10:2<119::aid-rmv272>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Fouchier RA, Schneeberger PM, Rozendaal FW, Broekman JM, Kemink SA, Munster V, Kuiken T, Rimmelzwaan GF, Schutten M, Van Doornum GJ, Koch G, Bosman A, Koopmans M, Osterhaus AD. Avian influenza A virus (H7N7) associated with human conjunctivitis and a fatal case of acute respiratory distress syndrome. Proc Natl Acad Sci USA. 2004;101:1356–1361. doi: 10.1073/pnas.0308352100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keawcharoen J, Oraveerakul K, Kuiken T, Fouchier RA, Amonsin A, Payungporn S, Noppornpanth S, Wattanodorn S, Theambooniers A, Tantilertcharoen R, Pattanarangsan R, Arya N, Ratanakorn P, Osterhaus DM, Poovorawan Y. Avian influenza H5N1 in tigers and leopards. Emerg Infect Dis. 2004;10:2189–2191. doi: 10.3201/eid1012.040759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanawongnuwech R, Amonsin A, Tantilertcharoen R, Damrongwatanapokin S, Theamboonlers A, Payungporn S, Nanthapornphiphat K, Ratanamungklanon S, Tunak E, Songserm T, Vivatthanavanich V, Lekdumrongsak T, Kesdangsakonwut S, Tunhikorn S, Poovorawan Y. Probable tiger-to-tiger transmission of avian influenza H5N1. Emerg Infect Dis. 2005;11:699–701. doi: 10.3201/eid1105.050007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopfleisch R, Wolf PU, Uhl W, Gerst S, Harder T, Starick E, Vahlenkamp TW, Mettenleiter TC, Teifke JP. Distribution of lesions and antigen of highly pathogenic avian influenza virus A/Swan/Germany/R65/06 (H5N1) in domestic cats after presumptive infection by wild birds. Vet Pathol. 2007;44:261–268. doi: 10.1354/vp.44-3-261. [DOI] [PubMed] [Google Scholar]
- Yingst SL, Saad MD, Felt SA. Qinghai-like H5N1 from domestic cats, northern Iraq. Emerg Infect Dis. 2006;12:1295–1297. doi: 10.3201/eid1208.060264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songserm T, Amonsin A, Jam-on R, Sae-Heng N, Meemak N, Pariyothorn N, Payungporn S, Theamboonlers A, Poovorawan Y. Avian influenza H5N1 in naturally infected domestic cat. Emerg Infect Dis. 2006;12:681–683. doi: 10.3201/eid1204.051396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leschnik M, Weikel J, Mostl K, Revilla-Fernandez S, Wodak E, Bago Z, Vanek E, Benetka V, Hess M, Thalhammer JG. Subclinical infection with avian influenza A (H5N1) virus in cats. Emerg Infect Dis. 2007;13:243–247. doi: 10.3201/eid1302.060608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopfleisch R, Wolf PU, Wolf C, Harder T, Starick E, Niebuhr M, Mettenleiter TC, Teifke JP. Encephalitis in a stone marten (Martes foina) after natural infection with highly pathogenic avian influenza virus subtype H5N1. J Comp Pathol. 2007;137:155–159. doi: 10.1016/j.jcpa.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Roberton SI, Bell DJ, Smith GJ, Nicholls JM, Chan KH, Nguyen DT, Tran PQ, Streicher U, Poon LL, Chen H, Horby P, Guardo M, Guan Y, Peiris JS. Avian influenza H5N1 in viverrids: implications for wildlife health and conservation. Proc Biol Sci. 2006;273:1729–1732. doi: 10.1098/rspb.2006.3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songserm T, Amonsin A, Jam-on R, Sae-Heng N, Pariyothorn N, Payungporn S, Theamboonlers A, Chutinimitkul S, Thanawongnuwech R, Poovorawan Y. Fatal avian influenza A H5N1 in a dog. Emerg Infect Dis. 2006;12:1744–1747. doi: 10.3201/eid1211.060542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reperant LA, Rimmelzwaan GF, Kuiken T. Avian influenza viruses in mammals. Rev Sci Tech. 2009;28:137–159. doi: 10.20506/rst.28.1.1876. [DOI] [PubMed] [Google Scholar]
- Choi YK, Nguyen TD, Ozaki H, Webby RJ, Puthavathana P, Buranathal C, Chaisingh A, Auewarakul P, Hanh NT, Ma SK, Hui PY, Guan Y, Peiris JS, Webster RG. Studies of H5N1 influenza virus infection of pigs by using viruses isolated in Vietnam and Thailand in 2004. J Virol. 2005;79:10821–10825. doi: 10.1128/JVI.79.16.10821-10825.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinshaw VS, Webster RG, Easterday BC, Bean WJ., Jr Replication of avian influenza A viruses in mammals. Infect Immun. 1981;34:354–361. doi: 10.1128/iai.34.2.354-361.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiken T, Rimmelzwaan G, van Riel D, Van Amerongen G, Baars M, Fouchier R, Osterhaus A. Avian H5N1 influenza in cats. Science. 2004;306:241. doi: 10.1126/science.1102287. [DOI] [PubMed] [Google Scholar]
- Rimmelzwaan GF, van Riel D, Baars M, Bestebroer TM, Van Amerongen G, Fouchier RA, Osterhaus AD, Kuiken T. Influenza A virus (H5N1) infection in cats causes systemic disease with potential novel routes of virus spread within and between hosts. Am J Pathol. 2006;168:176–183. doi: 10.2353/ajpath.2006.050466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong MD, Bach VC, Phan TQ, Vo MH, Tran TT, Nguyen BH, Beld M, Le TP, Truong HK, Nguyen VV, Tran TH, Do QH, Farrar J. Fatal avian influenza A (H5N1) in a child presenting with diarrhea followed by coma. N Engl J Med. 2005;352:686–691. doi: 10.1056/NEJMoa044307. [DOI] [PubMed] [Google Scholar]
- Uiprasertkul M, Puthavathana P, Sangsiriwut K, Pooruk P, Srisook K, Peiris M, Nicholls JM, Chokephaibulkit K, Vanprapar N, Auewarakul P. Influenza A H5N1 replication sites in humans. Emerg Infect Dis. 2005;11:1036–1041. doi: 10.3201/eid1107.041313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korteweg C, Gu J. Pathology, molecular biology, and pathogenesis of avian influenza A (H5N1) infection in humans. Am J Pathol. 2008;172:1155–1170. doi: 10.2353/ajpath.2008.070791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J, Xie Z, Gao Z, Liu J, Korteweg C, Ye J, Lau LT, Lu J, Gao Z, Zhang B, McNutt MA, Lu M, Anderson VM, Gong E, Yu AC, Lipkin WI. H5N1 infection of the respiratory tract and beyond: a molecular pathology study. Lancet. 2007;370:1137–1145. doi: 10.1016/S0140-6736(07)61515-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiken T, Taubenberger JK. Pathology of human influenza revisited. Vaccine. 2008;26 (Suppl 4):D59–D66. doi: 10.1016/j.vaccine.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Riel D, Munster VJ, de Wit E, Rimmelzwaan GF, Fouchier RA, Osterhaus AD, Kuiken T. H5N1 virus attachment to lower respiratory tract. Science. 2006;312:399. doi: 10.1126/science.1125548. [DOI] [PubMed] [Google Scholar]
- van Riel D, Munster VJ, de Wit E, Rimmelzwaan GF, Fouchier RA, Osterhaus AD, Kuiken T. Human and avian influenza viruses target different cells in the lower respiratory tract of humans and other mammals. Am J Pathol. 2007;171:1215–1223. doi: 10.2353/ajpath.2007.070248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munster VJ, de Wit E, van Riel D, Beyer WE, Rimmelzwaan GF, Osterhaus AD, Kuiken T, Fouchier RA. The molecular basis of the pathogenicity of the Dutch highly pathogenic human influenza A H7N7 viruses. J Infect Dis. 2007;196:258–265. doi: 10.1086/518792. [DOI] [PubMed] [Google Scholar]
- van Riel D, van den Brand JM, Munster VJ, Besteboer TM, Fouchier RA, Osterhaus AD, Kuiken T. Pathology and virus distribution in chickens naturally infected with highly pathogenic avian influenza A virus (H7N7) during the 2003 outbreak in The Netherlands. Vet Pathol. 2009;46:971–976. doi: 10.1354/vp.08-VP-0215-K-BC. [DOI] [PubMed] [Google Scholar]
- Belser JA, Lu X, Maines TR, Smith C, Li Y, Donis RO, Katz JM, Tumpey TM. Pathogenesis of avian influenza (H7) virus infection in mice and ferrets: enhanced virulence of Eurasian H7N7 viruses isolated from humans. J Virol. 2007;81:11139–11147. doi: 10.1128/JVI.01235-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimmelzwaan GF, Baars M, Claas EC, Osterhaus AD. Comparison of RNA hybridization, hemagglutination assay, titration of infectious virus and immunofluorescence as methods for monitoring influenza virus replication in vitro. J Virol Methods. 1998;74:57–66. doi: 10.1016/s0166-0934(98)00071-8. [DOI] [PubMed] [Google Scholar]
- Rimmelzwaan GF, Kuiken T, Van Amerongen G, Bestebroer TM, Fouchier RA, Osterhaus AD. Pathogenesis of influenza A (H5N1) virus infection in a primate model. J Virol. 2001;75:6687–6691. doi: 10.1128/JVI.75.14.6687-6691.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Riel D, den Bakker MA, Leijten LM, Chutinimitkul S, Munster VJ, de Wit E, Rimmelzwaan GF, Fouchier RA, Osterhaus AD, Kuiken T. Seasonal and pandemic human influenza viruses attach better to human upper respiratory tract epithelium than avian influenza viruses. Am J Pathol. 2010;176:1614–1618. doi: 10.2353/ajpath.2010.090949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plopper CG, Mariassy AT, Hill LH. Ultrastructure of the nonciliated bronchiolar epithelial (Clara) cell of mammalian lung: iI. A comparison of horse, steer, sheep, dog, and cat. Exp Lung Res. 1980;1:155–169. doi: 10.3109/01902148009069645. [DOI] [PubMed] [Google Scholar]
- Hyde DM, Plopper CG, Weir AJ, Murnane RD, Warren DL, Last JA, Pepelko WE. Peribronchiolar fibrosis in lungs of cats chronically exposed to diesel exhaust. Lab Invest. 1985;52:195–206. [PubMed] [Google Scholar]
- Lohr CV, DeBess EE, Baker RJ, Hiett SL, Hoffman KA, Murdoch VJ, Fischer KA, Mulrooney DM, Selman RL, Hammill-Black WM. Pathology and viral antigen distribution of lethal pneumonia in domestic cats due to pandemic (H1N1) 2009 influenza A virus. Vet Pathol. 2010;47:378–386. doi: 10.1177/0300985810368393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiken T, Fouchier R, Rimmelzwaan G, Osterhaus A, Roeder P. Feline friend or potential foe? Nature. 2006;440:741–742. doi: 10.1038/440741a. [DOI] [PubMed] [Google Scholar]
- Sponseller BA, Strait E, Jergens A, Trujillo J, Harmon K, Koster L, Jenkins-Moore M, Killian M, Swenson S, Bender H, Waller K, Miles K, Pearce T, Yoon KJ, Nara P. Influenza A pandemic (H1N1) 2009 virus infection in domestic cat. Emerg Infect Dis. 2010;16:534–537. doi: 10.3201/eid1603.091737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maines TR, Lu XH, Erb SM, Edwards L, Guarner J, Greer PW, Nguyen DC, Szretter KJ, Chen LM, Thawatsupha P, Chittaganpitch M, Waicharoen S, Nguyen DT, Nguyen T, Nguyen HH, Kim JH, Hoang LT, Kang C, Phuong LS, Lim W, Zaki S, Donis RO, Cox NJ, Katz JM, Tumpey TM. Avian influenza (H5N1) viruses isolated from humans in Asia in 2004 exhibit increased virulence in mammals. J Virol. 2005;79:11788–11800. doi: 10.1128/JVI.79.18.11788-11800.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Tumpey TM, Morken T, Zaki SR, Cox NJ, Katz JM. A mouse model for the evaluation of pathogenesis and immunity to influenza A (H5N1) viruses isolated from humans. J Virol. 1999;73:5903–5911. doi: 10.1128/jvi.73.7.5903-5911.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chutinimitkul S, Bhattarakosol P, Srisuratanon S, Eiamudomkan A, Kongsomboon K, Damrongwatanapokin S, Chaisingh A, Suwannakarn K, Chieochansin T, Theamboonlers A, Poovorawan Y. H5N1 influenza A virus and infected human plasma. Emerg Infect Dis. 2006;12:1041–1043. doi: 10.3201/eid1206.060227. [DOI] [PMC free article] [PubMed] [Google Scholar]