Abstract
Epidermal growth factor receptor (EGFR) and MET are molecular targets for lung cancer treatment. The relationships between expression, activation, and gene abnormalities of these two targets are currently unclear. Here, we demonstrate that a panel of 40 lung cancer cell lines could be classified into two groups. Group I was characterized by (1) high phosphorylations of MET and EGFR, (2) frequent mutation or amplification of EGFR, MET, and human epidermal growth factor receptor-2 (HER2), (3) high expressions of bronchial epithelial markers (thyroid transcription factor-1 (TTF-1), MUC1, and Cytokeratin 7 (CK7)); and (4) high expressions of MET, human epidermal growth factor receptor-3, E-cadherin, cyclooxygenase-2, and laminin gamma2. In contrast, Group II exhibited little or no phosphorylation of MET and EGFR; no mutation or amplification of EGFR, MET, and HER2; were triple-negative for TTF-1, MUC1, and CK7; and showed high expressions of vimentin, fibroblast growth factor receptor-1, and transcription factor 8. Importantly, Group I was more sensitive to gefitinib and more resistant to cisplatin and paclitaxel than Group II. The clinical relevance was confirmed in publicly available data on 442 primary lung adenocarcinoma patients; survival benefits by postoperative chemotherapy were seen in only patients with tumors corresponding to Group II. Overall, co-activation of EGFR and MET defines a distinct subgroup of lung carcinoma with characteristic genetic abnormalities, gene expression pattern, and response to chemotherapeutic reagents.
Lung cancer is the leading cause of cancer death in many developed countries, including the United States and Japan.1,2 Patients with lung carcinoma have a poor prognosis: most have disease recurrence after complete surgical resection, and those with stage I disease have a 5-year survival of only 70%.3 Among the four major histological types of lung cancer, adenocarcinoma has been rising in incidence in recent years4 and is a focus of intense investigations.
The discovery of the mutation of the epidermal growth factor receptor (EGFR) gene has been a major breakthrough in lung cancer.5 EGFR mutation is associated with adenocarcinoma histology, nonsmoking history, female sex, and clinical response to EGFR inhibitors.6 The short deletion of exon 19 and the point mutation of exon 21 are the most common of the EGFR mutations, together accounting for about 90% of the mutations overall. KRAS is another oncogene frequently mutated in lung adenocarcinoma.7 Mutations of KRAS and EGFR lead to enhanced activation of similar signaling pathways, such as AKT and ERK, and they occur in a mutually exclusive manner.5 Intriguingly, the EGFR mutation has reported associations with a bronchioloalveolar or papillary histological pattern,8 with the expression of thyroid transcription factor-1,9 and with micropapillary pattern,10,11 an aggressive variant of papillary adenocarcinoma.12,13 The KRAS mutation, on the other hand, is associated with mucinous histology.14 The genetic-histological correlations and mutually exclusive occurrence of EGFR and KRAS mutations suggest that it may be possible to classify lung adenocarcinomas based on integrated information on molecular abnormalities and histological patterns. Yet as of this writing, the associations of the adenocarcinoma subtypes with the various molecular mutations are still not entirely clear. Another recent study has shown high mutation rates of EGFR, KRAS, and BRAF in micropapillary lung adenocarcinomas,15 raising the possibility that the associations of the micropapillary morphology with various genetic mutations may differ between Western and Japanese populations. Further research to elucidate the genetic-histological correlations and molecular classifications of lung adenocarcinomas is clearly warranted.
MET is another molecular target of potential relevance to lung adenocarcinomas. MET is highly expressed in non-small cell lung cancer,16,17,18 and its overexpression is associated with an advanced cancer stage and shorter patient survival.17,18 Our group recently investigated the expression and activation of MET in lung adenocarcinoma tissues and cell lines.19,20 MET overexpression and activation have been found in a significant proportion of lung adenocarcinomas, and they have been confirmed to be associated with papillary histology, a morphological feature linked to tumors with EGFR mutations.10,21 These results suggest that these two tyrosine kinase receptors may be closely related.
In this study we extended our prior investigations by analyzing a larger panel of lung cancer cell lines, mainly of lung adenocarcinomas. Specifically, we investigated the following molecular parameters and the possible relationships among them: (a) activation of EGFR and MET; (b) gene status of EGFR, MET and KRAS; and (c) gene expression profiles. We have found that adenocarcinoma cell lines could be classified into two groups based on integrated information on EGFR and MET abnormalities and the expression patterns of several genes, including thyroid transcription factor-1 (TTF-1). Importantly, these two groups of cell lines showed different sensitivities to gefitinib and chemotherapeutic agents. We have validated this classification by analyzing the gene expression pattern and survival of 442 lung adenocarcinoma patients. Thus, co-activation of EGFR and MET defines a distinct subgroup of lung carcinoma with characteristic genetic abnormalities, gene expression pattern, and response to chemotherapeutic reagents.
Materials and Methods
Reagents
PHA-665752 was kindly provided from Dr. J. Christensen, Pfizer. Gefitinib was generously provided by AstraZeneca. CL387,785 was purchased from Calbiochem (now Merck KGaA, Darmstadt, Germany). Paclitaxel and cisplatin were purchased from Enzo Life Sciences (Plymouth Meeting, PA) and LKT Laboratories (St. Paul, MN), respectively. Stock solutions of gefitinib and paclitaxel were prepared in dimethyl sulfoxide and stored at −80°C until use. Stock solution of cisplatin was freshly prepared in 0.9% NaCl for each experiment.
Cell Lines and Medium
We used 40 lung cancer cell lines. The sources and histological types of these cell lines are shown in Table 1.22 All cell lines were maintained in RPMI 1640 supplemented with 10% fetal calf serum, glutamine, and antibiotics in a humidified atmosphere with 5% CO2 and 95% air.
Table 1.
Histological Types and Sources of 40 Lung Carcinoma Cell Lines
| Cell line | Histological type | Source |
|---|---|---|
| H23 | Adenocarcinoma cell line | ATCC* |
| H292 | Adenocarcinoma cell line | ATCC |
| H358 | Adenocarcinoma cell line | ATCC |
| H441 | Adenocarcinoma cell line | ATCC |
| H522 | Adenocarcinoma cell line | ATCC |
| H650 | Adenocarcinoma cell line | ATCC |
| H1395 | Adenocarcinoma cell line | ATCC |
| H1648 | Adenocarcinoma cell line | ATCC |
| H1650 | Adenocarcinoma cell line | ATCC |
| H1651 | Adenocarcinoma cell line | ATCC |
| H1703 | Adenocarcinoma cell line | ATCC |
| H1781 | Adenocarcinoma cell line | ATCC |
| H1793 | Adenocarcinoma cell line | ATCC |
| H1838 | Adenocarcinoma cell line | ATCC |
| H1975 | Adenocarcinoma cell line | ATCC |
| H1993 | Adenocarcinoma cell line | ATCC |
| H2009 | Adenocarcinoma cell line | ATCC |
| H2087 | Adenocarcinoma cell line | ATCC |
| H2228 | Adenocarcinoma cell line | ATCC |
| H2405 | Adenocarcinoma cell line | ATCC |
| HCC827 | Adenocarcinoma cell line | ATCC |
| HCC4006 | Adenocarcinoma cell line | ATCC |
| Calu3 | Adenocarcinoma cell line | ATCC |
| A549 | Adenocarcinoma cell line | Japanese Cancer Research Resources Bank (Osaka, Japan) |
| ABC-1 | Adenocarcinoma cell line | Japanese Cancer Research Resources Bank (Osaka, Japan) |
| PC3 | Adenocarcinoma cell line | Japanese Cancer Research Resources Bank (Osaka, Japan) |
| VMRC-LCD | Adenocarcinoma cell line | Japanese Cancer Research Resources Bank (Osaka, Japan) |
| RELF-LC-Ad1 | Adenocarcinoma cell line | Japanese Cancer Research Resources Bank (Osaka, Japan) |
| RELF-LC-Ad2 | Adenocarcinoma cell line | Japanese Cancer Research Resources Bank (Osaka, Japan) |
| RELF-LC-MS | Adenocarcinoma cell line | Japanese Cancer Research Resources Bank (Osaka, Japan) |
| HLC-1 | Adenocarcinoma cell line | RIKEN Cell Bank (Tsukuba, Japan) |
| LC-2/ad | Adenocarcinoma cell line | RIKEN Cell Bank (Tsukuba, Japan) |
| PC14 | Adenocarcinoma cell line | RIKEN Cell Bank (Tsukuba, Japan) |
| RERF-LC-KJ | Adenocarcinoma cell line | Japanese Cancer Research Resources Bank (Osaka, Japan) |
| L27 | Adenocarcinoma cell line | A kind gift from Dr. S. Hirohashi, National Cancer Center Institute, Tokyo, Japan, as described in a previous study (22) |
| H596 | Adenosquamous cell line | ATCC |
| H460 | Large cell carcinoma cell line | ATCC |
| H661 | Large cell carcinoma cell line | ATCC |
| H1299 | Large cell carcinoma cell line | ATCC |
| Lu65 | Large cell carcinoma cell line | Japanese Cancer Research Resources Bank (Osaka, Japan) |
ATCC, American Type Culture Collection.
Antibodies
The sources of the antibodies used in this study are summarized in Table 2.23
Table 2.
Antibodies Used in Western Blot Analysis
| Antibodies | Clone | Sources |
|---|---|---|
| c-MET | Rabbit polyclonal | IBL (Gunma, Japan) |
| COX2 | Rabbit polyclonal | IBL (Gunma, Japan) |
| EGFR (#2232) | Rabbit polyclonal | Cell Signaling Technology (Danvers, MA) |
| Phospho-EGFR (Y1068) (#2234) | Rabbit polyclonal | Cell Signaling Technology (Danvers, MA) |
| Phospho-c-MET(Y1234/Y1235) | Rabbit polyclonal | Cell Signaling Technology (Danvers, MA) |
| TTF-1 (clone 8G7G3/1) | Mouse monoclonal | DAKO (Glostrup, Denmark) |
| Cytokeratin 7 (clone OV-TL 12/30) | Mouse monoclonal | DAKO (Glostrup, Denmark) |
| Vimentin (clone V9) | Mouse monoclonal | DAKO (Glostrup, Denmark) |
| E-cadherin (clone 36) | Mouse monoclonal | BD Biosciences (Franklin Lakes, NJ) |
| P-cadherin (clone 56) | Mouse monoclonal | BD Biosciences (Franklin Lakes, NJ) |
| MUC1 smaller cytoplasmic subunit | Hamster monoclonal | Lab Vision (Cheshire, UK) |
| Laminin gamma2 | Mouse monoclonal | Described in a previous study (23) |
| Anti-rabbit IgG peroxidase conjugate | Amersham (Arlington Heights, IL) | |
| Anti-mouse IgG peroxidase conjugate | Amersham (Arlington Heights, IL) | |
| Anti-Armenian hamster IgG peroxidase conjugate | Jackson Immunoresearch (West Grove, PA) |
DNA Sequencing
The DNA was extracted from cell lines by standard procedures. The PCR primers and conditions for amplifying and sequencing exon 18 through exon 21 of the EGFR gene are described in the previous literature.24 The PCR primers and the conditions for mutational analysis of KRAS (codon 12, 13, and 61); MET (exon 2 and 3 encoding the Sema domain, and exon 14); and human epidermal growth factor-2 (HER2; exon 19 and 20) are not shown. The PCR products were sent to Macrogen Inc. (Seoul, Korea) and sequenced.
Gene Expression Profile and Single Nucleotide Polymorphism Array Analyses
A comprehensive gene expression analysis was performed using an oligonucleotide microarray (GeneChip Human Genome U133A, Affymetrix, Santa Clara, CA) as described previously.25,26,27 Single nucleotide polymorphism array (Affymetrix human mapping 50K XbaI array) analysis was performed using GIM (Genome Imbalance Map) algorithm as described previously.28
Western Blot Analysis
Cells were lysed in a lysis buffer consisting of 20mmol/L Tris-HCl (pH7.4), 150 mmol/L NaCl, 50 mmol/L NaF, and 1 mmol/L Na3VO4 with a cocktail of proteinase inhibitors. After sonication, lysates were boiled at 98°C for 5 minutes and cleared by centrifugation. Protein concentrations were determined by the DC Protein Assay kit (BioRad). For Western blotting, equal amounts of protein samples were size-separated on 8% polyacrylamide gels and electroblotted onto nitrocellulose membrane. Nonspecific binding was blocked by immersion of the membranes for 20 minutes in 5% skim milk in Tris-buffer saline at room temperature. The membranes were washed with Tris-buffer saline buffer containing 0.1% Tween 20, incubated for 1 hour at room temperature with primary antibodies, washed, and then reacted with peroxidase-conjugated secondary antibodies. The antigen was detected using ECL Western Blotting Detection Reagents (Amersham) following the manufacture’s instructions.
Bioinformatic Analyses
We used the Cluster program (http://rana.lbl.gov/EisenSoftware.htm, last accessed on March 19, 2008) for cluster analysis of the gene expression data of cell lines and lung adenocarcinoma cases. In brief, we performed average linkage hierarchical clustering of 40 cell lines, using median centering, mean centering and normalization of genes, and we performed average linkage hierarchical clustering of 442 lung adenocarcinoma cases, using median centering and mean centering of genes. Next we displayed the results with the aid of TreeView software (http://rana.lbl.gov/EisenSoftware.htm, last accessed on March 21, 2008). The image used a color code to represent relative expression levels. Red represents expression levels greater than the mean for a given gene across all samples. Green represents expression levels less than the mean across samples.
Cell Proliferation Assay
Cell viability was measured by the Cell Counting Kit (CCK)−8 assay (Dojindo, Tokyo, Japan) according to the manufacturer’s instructions. Cells (4 ∼ 8 × 103 cells) were plated in the wells of 96-well microtiter plates. After 24 hours, paclitaxel, cisplatin, and gefitinib were added respectively to the wells at the following final concentrations: 3.3 μmol/L, 1 μmol/L, 0.1 μmol/L, 0.01 μmol/L, and 0.01 μmol/L for paclitaxel; 50 μmol/L, 10 μmol/L , 3.3 μmol/L , 1 μmol/L , 0.1 μmol/L , and 0.01 μmol/L, for cisplatin; and 10 μmol/L, 3.3 μmol/L, 1 μmol/L, 0.1 μmol/L, 0.01 μmol/L, and 0.001 μmol/L, for gefitinib. The cells were then incubated for another 4 days at 37°C. The absorbance of each well at 450 nm was measured with a reference at 630 nm using a BIO-RAD model 680XR microplate reader (Bio-Rad, Hercules, CA). The percentage of cell viability was calculated by following formula: %cell viability = (mean absorbance in test wells)/(mean absorbance in control well) × 100. Results were plotted as cell viability versus log10 (concentration of reagents) and IC50 value was calculated using a software GraphPad Prism 5 (GraphPad Software, Inc., CA).
Statistics
Clinicopathological correlations were analyzed by one-factor analysis of variance and Fisher’s PLSD (post hoc) tests after converting T-stage, N-stage, pathological stage, differentiation grade, and smoking history to ordinal scale data. The calculations were done with StatView (Abacus Concepts, Berkeley, CA). P values of less than 0.05 were considered statistically significant. Survival curves were generated using the Kaplan–Meier method and differences in survival were analyzed by the Wilcoxon method.
Results
Activation of EGFR and MET in Cell Lines of Lung Adenocarcinoma
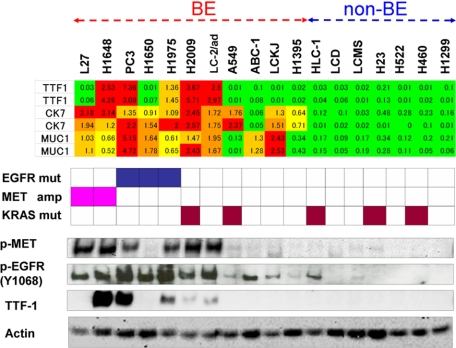
First, we examined a panel of 18 cell lines of lung carcinoma by Western blot analysis to determine the activation of EGFR and MET. The cell lines consisted of 16 adenocarcinomas and 2 large cell carcinomas, derived from cell banks in the United States and Japan. The results are shown in Figure 1 (lower panel). MET was activated at high levels in 6 cell lines (L27, H1648, PC3, H1975, H2009, and LC-2/ad) and at weak levels in several other lines. Similarly, EGFR was activated at high levels in 7 cell lines (L27, H1648, PC3, H1650, H1975, H2009, and LC-2/ad) and at modest levels in several others. Close examination of genetic status of EGFR, MET and KRAS (middle panel) and the activation profiles of EGFR and MET (lower panel) in Figure 1 revealed a number of interesting findings. First, although EGFR and MET were highly activated in the cell lines with EGFR mutation and MET amplification, respectively, these gene abnormalities were not the sole determinants of the activation status of the two receptors. Second, EGFR mutation, KRAS mutation, and MET amplification were mutually exclusive. Third, a striking overlap was noted between activation profiles of EGFR and MET: all of the five cell lines with high MET activation showed high activation of EGFR. Fourth, as a result of this overlap, the cell lines with activation of EGFR and MET appeared to constitute a subgroup among the cell lines examined. These findings were largely confirmed through analyses of another panel of lung cancers consisting of 19 cell lines of adenocarcinoma, 2 cell lines of large cell carcinoma, and 1 cell line of adenosquamous carcinoma (See Supplemental Figure S1A at http://ajp.amjpathol.org).
Figure 1.
Comparison of MUC1, CK7, and TTF-1 expression levels with the status of EGFR, MET, and KRAS and the activation profiles of EGFR and MET in a panel of lung carcinoma cell lines. Upper panel shows the gene expression levels of MUC1, CK7, and TTF-1 expressed to relative to the average (= 1.0) of 18 cell lines, and color indication is as described for supplemental Table S1 at http://ajp.amjpathol.org. Middle panel shows cell lines harboring EGFR mutation (blue box), MET amplification (pink box), and KRAS mutation (brown box). Lower panel shows the protein levels of phospho-MET, phospho-EGFR (Y1068), and TTF-1 expression (This lower panel is rearranged in Figure 3C, Figure 3D, and supplemental Figure S1B at http://ajp.amjpathol.org.). MET was highly phosphorylated in 6 cell lines (L27, H1648, PC3, H1975, H2009, and LC-2/ad), and EGFR was highly phosphorylated in seven cell lines (L27, H1648, PC3, H1650, H1975, H2009, and LC-2/ad). Thus, a striking overlap was noted between activation profiles of EGFR and MET. TTF-1 expression was correlated with EGFR and MET activation, but the cell lines that express TTF-1 were more restricted than those that express phospho-MET or phospho-EGFR (Y1068) at high levels. Thus, the cell lines may be broadly divided into two groups; bronchial epithelial phenotype (BE) and non-bronchial epithelial phenotype (non-BE) based on expression levels of MUC1, CK7, and TTF-1 and the activation profiles of EGFR and MET.
We were especially interested in the striking overlap between the activation profiles of EGFR and MET, as it implied the existence of a molecularly defined subgroup of lung adenocarcinomas. According to a recently proposed molecular classification of lung adenocarcinomas based on TTF-1 expression, TTF-1-positive tumors have the features of terminal respiratory units (TRUs) and are associated with EGFR mutation and sensitivities to EGFR inhibitor gefitinib.9 To investigate further, we examined TTF-1 expression in our panel of cell lines by Western blot analysis. The results are shown in Figure 1 (lower panel) and supplemental Figure S1B at http://ajp.amjpathol.org. Though TTF-1 expression was clearly correlated with EGFR and MET activation, the cell lines defined by TTF-1 expression did not completely match those defined by EGFR and MET activation.
These results prompted us to search for markers that may correlate with high activation of EGFR and MET. In combination with TTF-1, the markers that correlate with high activation of EGFR and MET may turn out to be useful markers in the molecular classification of lung adenocarcinoma cell lines.
The Expressions of TTF-1, Cytokeratin 7, and MUC1 Genes Correlate with the Activation of EGFR and MET
In our search for markers correlated with EGFR and MET activation, we turned to classical histopathological markers, ie, the cytokeratin and MUC families.29,30 Both of these gene families have been used in numerous histopathological studies to characterize various types of cancer, including lung adenocarcinomas.31,32,33 Noting this, we reasoned that expression data on these families, together with data on TTF-1 expression, would provide fundamental information on the properties of lung adenocarcinoma cells. Toward this end, we extracted expression profile data for the cytokeratin and MUC family genes and examined the relative expression levels of these genes in the cell lines. The results are shown in the heat-map in supplemental Table S1 at http://ajp.amjpathol.org. According to the correlation coefficients, the expressions of MUC1, MUC4, cytokeratin 7 (CK7), cytokeratin 16 (CK16), cytokeratin 17 (CK17), and cytokeratin 19 (CK19) were high in the six cell lines in which co-activation of EGFR and MET was detected (L27, H1648, PC3, H1975, H2009, and LC-2/ad). We chose MUC1 and CK7, a pair of histopathological markers frequently used for lung adenocarcinomas, for further analysis. The gene expressions of TTF-1, CK7, and MUC1 are shown in Figure 1 (upper panel). Seven cell lines with high levels of EGFR and MET activation and high level expression of TTF-1, CK7, and MUC1 (L27, H1648, PC3, H1650, H1975, H2009, and LC-2/ad) and 7 cell lines with negligible activation of EGFR and MET and low level expression of TTF-1, CK7, and MUC1 (HLC-1, VMRC-LCD, RELF-LCMS, H23, H522, H460, and H1299) appeared to form as two distinct groups. Another four cell lines (A549, ABC1, RELF-LCKJ, and H1395), meanwhile, were intermediate between the former two. The first seven cell lines with high level expression of TTF-1, CK7, and MUC1, and four intermediate cell lines with modest level expression of them were tentatively combined and designated as bronchial epithelial phenotype, and the remainder with negligible level expression of them were designated as nonbronchial epithelial phenotype. High level activation and genetic abnormalities of MET and EGFR were observed only in bronchial epithelial phenotype. The broad separation of cell lines into two groups was largely confirmed using another panel of 22 lines of lung cancer cells. The results on the entire panel of 40 cell lines are shown in supplemental Figure S1B at http://ajp.amjpathol.org.
Genes Expression Pattern Characterizing the Two Phenotypes of Cell Lines
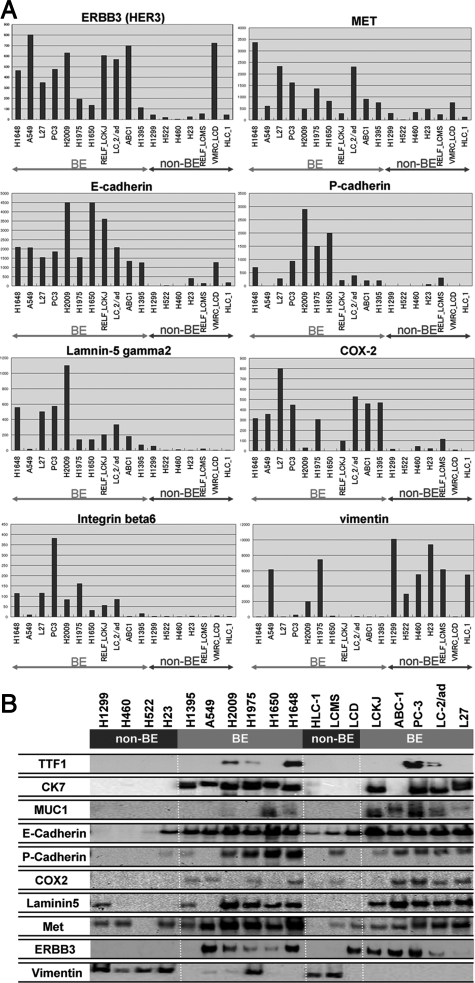
Previous studies have documented a list of genes whose expressions correlate with the classifications for lung adenocarcinomas 34, 35. Yet many of the genes included in lists of this type have functions heretofore unknown. For this reason, it remains to be seen whether gene lists will improve our understanding of the biological properties of lung adenocarcinomas. As an alternative, we focused on a family of genes whose functions have been well characterized in cancer cell biology: tyrosine kinase receptors, cadherins, integrins, proteinases, molecules related to prostaglandin synthesis, extracellular matrix molecules such as collagens and laminins, and genes related to epithelial–mesenchymal transition. The numbers of genes and probes sets retrieved for analysis are shown in supplemental Table S2 at http://ajp.amjpathol.org, and the comprehensive dataset for the expression profiles of these families is shown in supplemental Table S3 at http://ajp.amjpathol.org. Next, we calculated the correlation coefficients of TTF-1, CK7, and MUC1, as well as the mean value of the three coefficients, for each gene. We also determined the mean expression ratio between the cell lines in bronchial epithelial phenotype and nonbronchial epithelial phenotype for each gene. Finally, we selected the genes which met the following two requirements: (A) the absolute value of the mean correlation coefficient exceeds 0.3, and (B) the mean expression ratio of bronchial epithelial phenotype versus nonbronchial epithelial phenotype is greater than 3 or less than 1/3. The results are shown in Table 3. To illustrate the differential expression of these genes, the expression levels of the several selected genes are shown in the bar graphs in Figure 2A. Bronchial epithelial phenotype was characterized by high expressions of MET, human epidermal growth factor receptor-3 (HER3), integrin beta6, E-cadherin, P-cadherin, urokinase-type plasminogen activator (u-PA), cyclooxygenase-2 (Cox-2), and laminin gamma2. In contrast, non-bronchial epithelial phenotype showed a high expression of vimentin, fibroblast growth factor receptor-1 (FGFRI), and transcription factor 8 (TCF-8) (also called Zeb1).
Table 3.
Genes Selectively Expressed as Bronchial Epithelial Phenotype or Nonbronchial Epithelial Phenotype (BE/non-BE >3 or <1/3), Showing Positive or Negative Correlations with the Expression of CK7, TTF1, and MUC1 (Correlation Coefficient >0.3 or <−0.3)
| Gene | CORREL | BE/non-BE | BE (avg) | Non-BE (avg) |
|---|---|---|---|---|
| TKRs | ||||
| MET | 0.46 | 4.6 | 1460 | 316 |
| ERBB3 | 0.39 | 3.6 | 470 | 132 |
| ROR1 | 0.64 | 5.7 | 125 | 22 |
| EPHB2 | 0.55 | 4.4 | 138 | 31.6 |
| EPHB3 | 0.71 | 8.1 | 61.7 | 7.59 |
| EPHA1 | 0.63 | 6.5 | 120 | 18.6 |
| FGFR1 | −0.32 | 0.31 | 54 | 174 |
| MUCs | ||||
| MUC1 | 0.71 | 14 | 770 | 57.1 |
| MUC4 | 0.50 | 40 | 173 | 4.32 |
| MUC16 | 0.61 | 5.3 | 88.5 | 16.8 |
| MUC20 | 0.38 | 36 | 283 | 7.81 |
| Keratins | ||||
| KRT4 | 0.50 | 29 | 349 | 12.2 |
| KRT7 | 0.68 | 80 | 2910 | 36.4 |
| KRT13 | 0.53 | 33 | 95.6 | 2.89 |
| KRT15 | 0.62 | 7.9 | 135 | 17.1 |
| KRT19 | 0.59 | 5.4 | 4920 | 920 |
| Growth factors | ||||
| TGFA | 0.57 | 6.6 | 413 | 62.4 |
| VEGFC | 0.54 | 4.1 | 250 | 60.8 |
| INHBB | 0.53 | 19 | 414 | 21.7 |
| IGFBP6 | 0.46 | 6.8 | 466 | 68.5 |
| TGFBI | 0.43 | 4.4 | 4020 | 910 |
| AREG | 0.33 | 7.4 | 738 | 99.7 |
| BMP4 | 0.42 | 7.7 | 63.2 | 8.2 |
| WNT7B | 0.57 | 6.8 | 50.8 | 7.51 |
| Eicosanoids | ||||
| PTGES | 0.40 | 5.5 | 419 | 76.6 |
| PTGFRN | 0.39 | 4.8 | 403 | 83.3 |
| PTGS2 (COX2) | 0.33 | 11 | 319 | 29.9 |
| Rho-Racs | ||||
| RAC2 | 0.55 | 56 | 663 | 11.8 |
| RHOD | 0.48 | 3.3 | 431 | 131 |
| Proteinases | ||||
| MMP1 | 0.31 | 8.3 | 810 | 97.1 |
| CTSS | 0.66 | 30 | 152 | 5.07 |
| PLAT | 0.61 | 21 | 1360 | 63.9 |
| PLAU | 0.54 | 4.3 | 1140 | 264 |
| Cadherin-associated | ||||
| CDH1 | 0.54 | 9.3 | 2640 | 285 |
| CDH6 | 0.52 | 3 | 57.9 | 19.3 |
| CDH3 | 0.51 | 19 | 987 | 52.6 |
| PCDH1 | 0.53 | 6.6 | 68.7 | 10.4 |
| Integrins | ||||
| ITGB2 | 0.73 | 13 | 75.1 | 5.8 |
| ITGB4 | 0.61 | 5.1 | 280 | 55.2 |
| ITGB6 | 0.68 | 43 | 116 | 2.67 |
| Laminins | ||||
| LAMA3 | 0.60 | 6.8 | 241 | 35.5 |
| LAMC2 | 0.70 | 28 | 395 | 8.1 |
| LAMB3 | 0.53 | 5.8 | 749 | 129 |
| LAMA1 | −0.30 | 0.018 | 6.63 | 372 |
| Collagens | ||||
| COL18A1 | 0.43 | 3.8 | 51.5 | 13.7 |
| COL5A2 | −0.34 | 0.022 | 30.4 | 1350 |
| EMTs | ||||
| JUP | 0.55 | 3.1 | 857 | 278 |
| SNAI2 | 0.47 | 7.6 | 210 | 27.7 |
| SMAD3 | 0.46 | 3.3 | 216 | 64.7 |
| TGFBR2 | 0.44 | 5.4 | 694 | 128 |
| CLDN4 | 0.71 | 8.5 | 354 | 41.8 |
| CLDN7 | 0.46 | 14 | 991 | 72 |
| VIM | −0.38 | 0.32 | 1780 | 5660 |
| TCF8 | −0.56 | 0.161 | 15.4 | 95.6 |
Figure 2.
A: Genes selectively expressed in the bronchial epithelial phenotype (BE) and nonbronchial epithelial phenotype (non-BE). The expression levels of the indicated genes are shown in the bar graphs. B: Western blot analysis of the gene products selectively expressed in the bronchial epithelial phenotype (BE) and non-bronchial epithelial phenotype (non-BE). The results largely confirmed the data obtained at the mRNA level by oligonucleotide array analysis.
Next, we tried to confirm whether these genes expression patterns of bronchial epithelial phenotype and nonbronchial epithelial phenotype cell lines were reflected in corresponding protein levels. For this purpose, we chose MET, HER3, E-cadherin, P-cadherin, Cox-2, and laminin gamma2, the genes with antibodies available for Western blotting. As shown in Figure 2B, Western blot analysis largely confirmed the data obtained at the mRNA level by oligonucleotide array analysis.
Hierarchical Cluster Analysis of Cell Lines
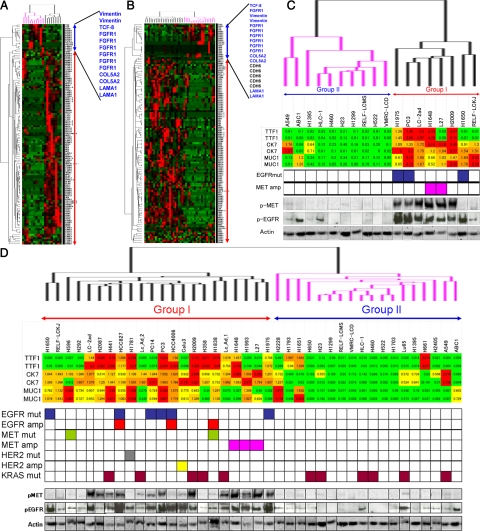
We performed a hierarchical cluster analysis of the initial 18 lung cancer cell lines (16 adenocarcinoma and 2 large cell carcinoma) using the genes selectively expressed in the bronchial epithelial phenotype and nonbronchial epithelial phenotype cell lines. The results are shown in Figure 3A and C. Eighteen cell lines were classified into two groups; Group I and Group II. Group I and Group II nearly correspond to bronchial epithelial phenotype and nonbronchial epithelial phenotype, respectively, but the former classification seems to be more closely correlated with the genetic abnormality and the activation of EGFR and MET than the latter. As expected, the genes selectively expressed in the bronchial epithelial phenotype and non-bronchial epithelial phenotype cell lines of our initial panel clustered with the corresponding groups. These results were mostly confirmed using the entire panel of 40 cell lines (35 cell lines of adenocarcinoma, 4 cell lines of large cell carcinoma, and 1 cell line of adenosquamous carcinoma) (Figure 3, B and D). The gene expression data of another panel of 22 cell lines for hierarchical cluster analysis is shown in supplemental Table S4 at http://ajp.amjpathol.org. Interestingly, not only EGFR mutation and MET amplification, but also EGFR amplification, MET mutation, HER2 amplification, and HER2 mutation were concentrated in Group I. MET was reported to interact with both EGFR and HER2.36 These results suggest that cross talk between the EGFR pathway and the MET pathway might occur in Group I cell lines, and that co-activation of EGFR and MET might be associated with genetic abnormalities of EGFR, MET, and HER2.
Figure 3.
A: Hierarchical cluster analysis of the initial 18 lung cancer cell lines using the genes selectively expressed in the bronchial epithelial phenotype and nonbronchial epithelial phenotype cell lines. The blue bar indicates genes selectively expressed in nonbronchial epithelial phenotype, and the red bar indicates genes selectively expressed in bronchial epithelial phenotype. B: Hierarchical cluster analysis of the entire panel of 40 cancer cell lines using the genes selectively expressed in the bronchial epithelial phenotype and nonbronchial epithelial phenotype cell lines. C: Enlarged view of the array; dendrogram shown in panel A along with sample identification (upper panel). Under the array dendrogram, TTF-1, CK7, and MUC-1 expressions, the status of EGFR and MET and the activation profiles of EGFR and MET of the 18 cell lines are shown. D: Enlarged view of the array dendrogram shown in panel B along with sample identification (upper panel). Under the array dendrogram, TTF-1, CK7, and MUC-1 expressions, the status of EGFR, MET, HER2, and KRAS, and the activation profiles of EGFR and MET of the 40 cell lines are shown.
Effects of PHA665752 (MET Inhibitor) and CL387,785 (Dual EGFR/HER2 Inhibitor) on Phospho-MET and Phospho-EGFR
We tested the effects of PHA665752 (MET inhibitor) treatment and CL387,785 (dual EGFR/HER2 inhibitor) treatment on the phosphorylation of MET and EGFR in three Group I cell lines: H1993 with MET amplification, HCC827 with EGFR mutation/amplification, and Calu-3 with HER2 amplification. The results are shown in supplemental Figure 2 at http://ajp.amjpathol.org. PHA665752 (1 × 10−6 mol/L) suppressed the constitutive MET phosphorylation in the three cell lines and abolished the baseline phosphorylation of EGFR in H1993. It was significant that PHA665752 at this concentration conferred the latter effect. Conversely, CL387,785 (1 × 10−6 mol/L) suppressed the expressions and phosphorylation of both EGFR and MET in HCC827 and Calu-3. These results suggest that the MET and EGFR activations may occur reciprocally via either MET amplification, EGFR mutation, or HER2 amplification. The baseline phosphorylation of EGFR in HCC827 and Calu-3 was unaffected by PHA665752. This was expected, as EGFR activation is dependent on either EGFR or HER2 in these two cell lines.
Hierarchical Cluster Analysis of Primary Tumors
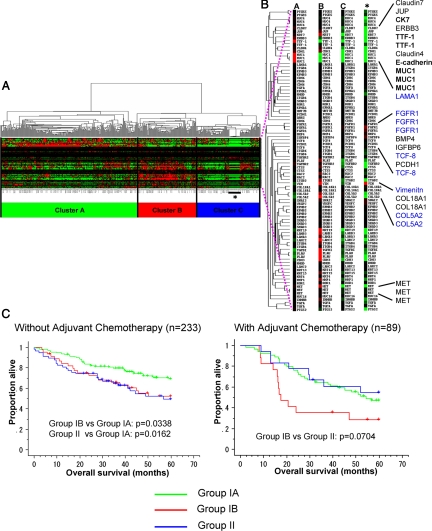
To further validate the relevance of this classification in primary tumors, we performed hierarchical cluster analysis using publicly available data of 442 primary lung adenocarcinoma cases,37 using the genes selectively expressed in Group I and Group II. As shown in Figure 4A, primary lung adenocarcinoma cases were divided into three clusters (Cluster A, B, and C), reflecting the intertumoral heterogeneity of primary tumors as compared to cell lines. Cluster A and Cluster B differentially expressed a subset of genes selectively expressed in Group I. Cluster A showed high expression of bronchial epithelial markers (CK7, TTF-1, and E-cadherin), whereas Cluster B showed modest expression of bronchial epithelial markers (CK7, TTF-1, and E-cadherin) and high expression of a subset of genes (u-PA, P-cadherin, laminin beta3, laminin gamma2, integrin beta4, etc) that is associated with cancer cell invasion (see Discussion) (Figure 4B). Thus, Cluster A and Cluster B represent different subsets of Group I primary tumors, and they were designated as Group IA and Group IB, respectively. In contrast, Cluster C showed very low expression of both bronchial epithelial markers and cancer invasion-associated genes that characterized Group I (Figure 4B). Thus, Cluster C represents primary lung adenocarcinomas that correspond to Group II. In this analysis of primary tumors, the genes selectively expressed in Group II were not clustered together. However, this is due to stromal contamination in primary tumors, as evidenced by the high expression of FGFR1 and vimentin in normal control lung tissue (Figure 4B).
Figure 4.
Analysis of publicly available data of 442 primary lung adenocarcinoma cases. A: Hierarchical cluster analysis using the genes selectively expressed in bronchial epithelial phenotype and nonbronchial epithelial phenotype. B: Cluster A showed high expression of terminal bronchial epithelial markers (CK7, TTF-1, and E-cadherin), whereas Cluster B showed modest expression of bronchial epithelial markers (CK7, TTF-1, and E-cadherin) and high expression of a subset of genes (u-PA, P-cadherin, LAMB3, LAMC2, ITGB4, etc) that is associated with cancer cell invasion (see Discussion). In contrast, Cluster C showed very low expression of both bronchial epithelial markers and cancer invasion-associated genes that characterized Group I. The genes selectively expressed in Group II were not clustered together. However, this is due to stromal contamination in primary tumors, as evidenced by the high expression of FGFR1 and vimentin in normal control lung tissue (asterisk). C: Patient survival curves of Group IA (green line), Group IB (red line), and Group II (blue line). Patients without adjuvant chemotherapy (left panel), and those with adjuvant chemotherapy (right panel) were separately analyzed.
We examined the correlations between the groups defined by gene expression patterns (Group IA, Group IB, and Group II) and clinicopathological factors (Table 4). T-stage was significantly higher in Group IB than Group IA (Group IB versus Group IA: P = 0.0057). Pathological stage was significantly higher in Group IB than Group IA (Group IB versus Group IA: P = 0.0123). Histologically, Group IB and Group II showed significantly poorer differentiation than Group IA (Group IB versus Group IA: P < 0.0001, Group II versus Group IA: P < 0.0001). The ever-smoker rates were significantly higher in Group IB and Group II than in Group IA (Group IB versus Group IA: P = 0.0086, Group II versus Group IA: P = 0.0015). Figure 4C shows the 5-year survival curves for Group IA, Group IB, and Group II without adjuvant chemotherapy (left panel) and with adjuvant chemotherapy (right panel). Among the cases without adjuvant chemotherapy, Group IB and Group II showed almost the same survival curves as each other and significantly poorer prognosis than Group IA (Group IB versus Group IA, P = 0.0332; Group II versus Group IA, P = 0.0162; Wilcoxon method). The relatively favorable prognosis of Group IA may explain why this group is underrepresented in cell lines. Among the patients who received adjuvant therapy, however, the Group II patients showed a better prognosis than the Group IB patients (P = 0.0704, Wilcoxon method). It thus appeared that Group II was more sensitive to chemotherapy than Group I.
Table 4.
Correlations between Clinicopathological Factors and Groups Defined by Gene Expression Pattern
| Clinical feature | n | Group IA | Group IB | Group II | P value |
|---|---|---|---|---|---|
| Pathological T stage (UICC fifth ed.) | |||||
| T1/T2 | 401 | 227 | 89 | 85 | Group IB versus Group IA: 0.0057 |
| T3 | 28 | 9 | 11 | 8 | Group II versus Group IA: 0.1193 |
| T4 | 12 | 4 | 5 | 3 | Group II versus Group IB: 0.3333 |
| Nodal involvement (UICC fifth ed.) | |||||
| pN0 | 299 | 171 | 65 | 63 | NS |
| pN1 | 88 | 41 | 22 | 25 | |
| pN2 | 53 | 27 | 18 | 8 | |
| Pathological stage (UICC fifth ed.) | |||||
| Stage IA | 114 | 65 | 27 | 22 | Group IB versus Group IA: 0.0123 |
| Stage IB | 162 | 98 | 32 | 32 | Group II versus Group IA: 0.0750 |
| Stage IIA | 24 | 15 | 5 | 4 | Group II versus Group IB: 0.5772 |
| Stage IIB | 71 | 31 | 14 | 26 | |
| Stage IIIA | 57 | 26 | 22 | 9 | |
| Stage IIIB | 12 | 4 | 5 | 3 | |
| Differentiation | |||||
| Well | 60 | 50 | 5 | 5 | Group IB versus Group IA: <0.0001 |
| Moderate | 209 | 133 | 46 | 30 | Group II versus Group IA: <0.0001 |
| Poor | 166 | 56 | 54 | 56 | Group II versus Group IB: 0.2979 |
| Smoking history | Group IB versus Group IA: 0.0086 | ||||
| Never smoked | 49 | 40 | 5 | 4 | Group II versus Group IA: 0.0015 |
| Smoker | 300 | 158 | 64 | 78 | Group II versus Group IB: 0.7177 |
Sensitivities of 40 Lung Cancer Cell Lines to Gefitinib, Cisplatin, and Paclitaxel
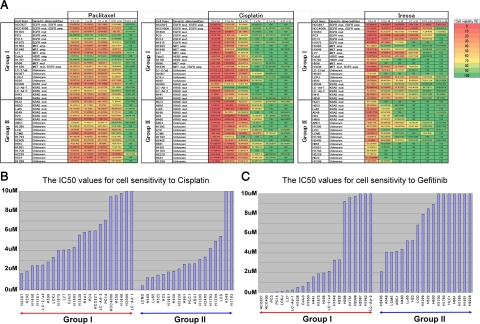
Finally, we examined the sensitivities to gefitinib, cisplatin, and paclitaxel in a panel of 40 non-small cell lung cancer cell lines classified into Group I and Group II (Figure 5A). A high sensitivity to gefitinib was observed in the EGFR-mutated and EGFR-amplified cancer cells, while less pronounced but appreciable sensitivities to gefitinib were observed in a MET-amplified cell line (L27) and HER2-amplified cell line (Calu3). A close examination of the responses of KRAS-mutated cancer cells to cisplatin and gefitinib (Figure 5A) revealed that the KRAS-mutated Group I cancer cells (H2009, LC-Ad-1, LC-Ad-2, H441, and H358) were more sensitive to gefitinib and more resistant to cisplatin than the KRAS-mutated Group II cancer cells (HLC-1, H650, Lu65, A549, H23, and H460). In comparing the IC 50 values for cisplatin and gefitinib between Group I and Group II, we found that the Group II cancer cells were significantly more sensitive to cisplatin (P = 0.0115, Mann-Whitney U-test) and more resistant to gefitinib than the Group I cancer cells (P = 0.0017, Mann-Whitney U-test) (Figure 5, B and C). Paclitaxel was relatively effective against most of the cell lines of Group I and Group II at low concentration (0.1 μmol/L), but four cell lines (PC3, PC14, Calu3, and H1781) from Group I were highly resistant to paclitaxel. Of these four cell lines, two (PC3 and PC14) harbored EGFR mutation and the other two, Calu3 and H1781, harbored HER2 amplification and HER2 mutation, respectively (Figure 5A).
Figure 5.
A. Sensitivities of 40 lung cancer cell lines to paclitaxel (left), cisplatin (middle), and gefitinib (right). The viability of cells with each concentration is shown by color gradation (color scale is shown in extreme right panel). Paclitaxel was relatively effective against most of the cell lines at 0.1 μmol/L , but four cell lines (PC3, PC14, Calu3, and H1781), belonging to Group I, were resistant even at high concentration. Cisplatin needed higher concentration to be effective as compared with paclitaxel. Cisplatin was more effective against Group II than Group I at about 1 μmol/L. Gefitinib was more effective against Group I than Group II at any concentration tested. It was also of note that KRAS-mutated Group I cell lines were more sensitive to gefitinib than KRAS-mutated Group II cell lines. B and C: Comparisons of the IC50 values for cisplatin (B) and gefitinib (C) between Group I and Group II cell lines. Group II cancer cells were more sensitive to cisplatin than Group I cancer cells (B, P = 0.0115, Mann-Whitney U-test). Group I cancer cells were more sensitive to gefitinib than Group II cancer cells (C, P = 0.0017, Mann-Whitney U-test). Upper-limit values of the graphs are set to be 10 μmol/L.
Discussion
In this study, we began by examining the expression and activation levels of EGFR and MET in a panel of cell lines of lung adenocarcinoma. After comparing EGFR and MET activation, we compared the respective levels of activation with our results on EGFR mutation and MET amplification. While EGFR and MET were highly activated in cell lines with EGFR mutation and MET amplification, respectively, these gene abnormalities were not the sole determinants of the activation status of these two receptors. As reported for EGFR mutation and RAS mutation,5,6 MET amplification had a mutually exclusive relationship with EGFR and KRAS mutations. Importantly, we found an extensive overlap between EGFR activation and MET activation, a finding which suggests that the cell lines manifesting EGFR and MET activation may constitute a distinct subgroup among the lung adenocarcinoma cells. On detecting high levels of EGFR and MET activation in two cell lines negative for TTF-1 expression, we set out to see if there were other markers that could help to define this distinct group of cell lines with EGFR and MET activation. We found, as a result, that lung adenocarcinoma cells with high EGFR and MET activation can be categorized as bronchial epithelial type or Group I; this type of tumors characteristically express the histopathological markers (TTF-1, CK7, and MUC1) and several gene products frequently involved in cancer cell invasion (integrin beta6, P-cadherin, urokinase-type plasminogen activator, Cox-2, and laminin gamma2). According to a hierarchical cluster analysis, Group I and Group II largely corresponded to bronchial epithelial and nonbronchial epithelial types, respectively, just as we had designated at the outset of this study. Earlier papers have proposed that lung adenocarcinoma can be classified into TRU type and non-TRU type, mainly based on the expression of TTF-1.9 According to our classification, Group I defines a subset of lung adenocarcinomas which includes TRU type in addition to non-TRU type tumors with high expression of bronchial epithelial markers such as CK7 and MUC1. With regard to genetic correlation, a significantly higher frequency of EGFR mutation is observed in the TRU type than in the non-TRU type. The relationship between TRU-type tumors and MET abnormalities remains unclear, however. We propose that our classification better reflects genetic abnormalities and activation profiles of EGFR and MET, as has been shown in this study.
The extensive overlap between the activation profiles of EGFR and MET raises several intriguing questions. What is the molecular basis for this overlap? Could it be attributable to cross talk between these two tyrosine kinase receptors on the cell surface? To answer these questions we tested the effects of PHA665752 (MET inhibitor) and CL387,785 (dual EGF/HER2 inhibitor) on phospho-MET and phospho-EGFR in three representative cell lines harboring genetic abnormalities of EGFR, MET or HER2. PHA665752 suppressed both phospho-MET and phospho-EGFR in a MET-amplified cell line but not in EGFR-mutated or HER2-amplificed cell lines. Conversely, CL387,785 suppressed both phospho-MET and phospho-EGFR in EGFR-mutated or HER2-amplificed cell lines, but not in a MET-amplified cell line. These results suggested that cross talk between MET and EGFR may occur in a reciprocal manner, and that the co-activation of EGFR and MET may be caused by the genetic abnormalities of EGFR, MET or HER2 in some Group I cell lines. CL387,785 suppressed MET expression as well as phospho-MET expression, suggesting that EGFR activation leads to increased transcription of the MET gene in addition to MET activation. In fact, two recent papers point to the presence of an activation network involving MET and EGFR.38,39 Specifically, these papers have shown that MET activation leads to increased gene transcription and secretion of two EGFR ligands, transforming growth factor-α and heparin-binding epidermal growth factor, which in turn activates EGFR. Conversely, studies in mutant cell lines HCC827 and H3255 by Guo et al40 have shown that MET is highly phosphorylated and co-immunoprecipitated with mutant EGFR, and that the expression and phosphorylation of MET decreases following treatment with gefitinib. They also noted an extensive overlap in the phosphoproteome profiles between EGFR mutant lung adenocarcinoma cell lines and the MET-amplified gastric cell line MKN45.40
In this study, we sought to determine gene expression patterns capable of characterizing the biological properties of the cells lines known to overexpress and activate EGFR and MET. Several overexpressed genes of possible biological relevance were identified in the Group I cell lines: MET, HER3, integrin beta6, E-cadherin and P-cadherin, Cox-2, and laminin-5 gamma2 chain. In contrast, Group II was characterized by overexpression of vimentin, FGFR1, and TCF8. In cell lines with MET amplification, MET associates with HER3, an adapter molecule for downstream signaling.41 Integrin beta6 forms a β subunit of α(v)beta6 integrin, which plays a critical role in the activation of transforming growth factor-β.42 E-cadherin and P-cadherin are involved in homophilic cell adhesion in epithelial cells. A loss of E-cadherin is a hallmark of epithelial–mesenchymal transition, a process implicated in cell invasion and metastasis of many epithelial malignancies.43 The significance of P-cadherin expression in cancer is less understood. Studies have shown the upregulation of P-cadherin during wound healing and the early stages of colon cancer, but the expression of this molecule seems to disappear in poorly differentiated tumors.44 P-cadherin has also been reported to promote the motility of pancreas cancer,45 and its expression is an indicator of poor outcome in breast cancer.46 Cox-2 is a rate-limiting enzyme in the production of prostaglandins.23 Overexpression of cox-2 in cancer has been reported in numerous studies, and the enzyme plays an important role in tumor angiogenesis and survival.23 u-PA and laminin-5 gamma2 chain are molecular markers frequently overexpressed at the invasive front lines of cancer.47 Cox-2 and laminin-5 gamma2 chains are up-regulated by activation of EGFR and MET.22 MUC1 regulates the expression of EGFR and modulates transforming growth factor-α/EGFR-dependent cancer progression.48,49 Taken together, these data suggest that the cell lines with high levels of EGFR and MET activation (Group I) rely on this set of molecules (MET, EGFR, HER3, integrin beta6, E-cadherin and P-cadherin, Cox-2, laminin-5 gamma2 chain) for their malignant properties (eg, cell proliferation, migration, and metastasis). Supplemental Figure S3 at http://ajp.amjpathol.org illustrates these regulatory networks operating in the Group I cell lines. In contrast, cell lines without EGFR and MET overexpression (Group II) are characterized by an overexpression of vimentin, a classical marker of epithelial–mesenchymal transition, and they may rely on molecules other than MUC1, Cox-2, integrin beta6, etc for their malignant behaviors. These features of Group I and II cancer cells may need to be considered in the planning of molecular target therapies for the various types of lung adenocarcinoma.
In this study we further investigated the sensitivities of Group I and Group II cancer cells to gefitinib, cisplatin, and paclitaxel. Group I cancer cells were more sensitive to gefitinib, but more resistant to cisplatin, than Group II cancer cells. As we expected, EGFR-mutated or EGFR-amplified cancer cells were highly sensitive to gefitinib. The KRAS mutation appeared in both Group I and Group II. And intriguingly, the KRAS-mutated Group I cancer cells were more sensitive to gefitinib than the KRAS-mutated Group II cancer cells. This suggests that our classification will be useful to extract a subset of KRAS-mutated lung cancer cases against which gefitinib can be effective. It may be that the KRAS mutation is somewhat less strong than MET amplification or EGFR mutation as a growth driver, and that the maintenance of optimal cell growth and survival in KRAS-mutated Group I cell lines depends on not only the oncogenic mutant KRAS, but also MET activation or EGFR activation. We have recently reported that KRAS-mutated cell lines with high levels of phospho-MET were modestly sensitive to PHA665752, while KRAS-mutated cell lines with low levels of phospho-MET were wholly resistant to PHA665752.50 The differences of biological properties and drug sensitivity between KRAS-mutated Group I cell lines and KRAS-mutated Group II cell lines may be explained by additional genetic abnormalities acquired in the latter. This hypothesis will be tested in future studies.
Paclitaxel was effective against most of the 40 cell lines at low concentration, but four Group I cancer cells harboring EGFR mutation, HER2 amplification or HER2 mutation were quite resistant to paclitaxel. Group I cancer cells frequently harbored genetic abnormalities of EGFR, MET, and HER2. Three MET-amplified lung adenocarcinoma cell lines (H1648, H1993, and L27) also belonged to Group I, and these lines were quite sensitive to the MET inhibitor PHA665752.50 On this basis, we believe that molecular-targeted therapy against these strong growth drivers should be the first line of treatment for Group I lung cancer cases. Chemotherapy should be the first line of treatment for Group II cases, unless strong growth drivers of the cancers are revealed.
Our classification will be applicable to lung adenocarcinomas from both Japanese and Western populations. An analysis of primary adenocarcinomas from four institutions in the United States has validated the classification, and lung adenocarcinomas surgically resected at Jichi University Hospital are also classifiable into Group I or Group II according to the expression levels of MET, TTF-1, laminin-5, Cox-2, etc (unpublished observation). Yet the ratio of Group I to Group II in our institution was higher than in the four institutions in the United States (not published). One possible explanation for this may be the higher frequency of EGFR mutations in the lung adenocarcinomas of East-Asians compared to those of other ethnic groups.51 Our classification may also be useful for elucidating the discrepancies in the prognostic factors found among different institutions and geographical areas. The different proportions of Group I and Group II will influence the prognostic significance of some molecular biomarkers. Cox-2 and laminin-5, for example, will be assessed as unfavorable prognostic factors in Group I, but not in Group II. This hypothesis will be tested in future studies.
In summary, we have shown that co-activation of EGFR and MET defines a distinct subgroup of lung carcinoma with characteristic genetic abnormalities, gene expression pattern, and response to chemotherapeutic reagents. We propose that this classification based on integrated information on EGFR and MET abnormalities will not only be useful for the understanding of the biological properties of lung adenocarcinoma, but also for the clinical decision making between molecular-targeted therapy and chemotherapy.
Footnotes
Address reprint requests to Toshiro Niki, M.D., Ph.D., Department of Integrative Pathology, Jichi Medical University, 3311-1 Yakushiji, Shimotsuke-shi, Tochigi, 329-0498, Japan. E-mail: tniki@jichi.ac.jp.
Supported in part by the Smoking Research Foundation, the Foundation for Development of Community, the Vehicle Racing Commemorative Foundation, and the Ministry of Health, Labor and Welfare, and the Ministry of Education, Culture, Sports, Science and Technology of Japan. Daisuke Matsubara is a recipient of Jichi Medical University Young Investigator Award. This publication was subsidized by Japan Keirin Association through its promotion funds from KEIRIN RACE.
T.N. and D.M. are recipients of the Astra-Zeneca research award 2007.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Tokyo: Ministry of Health, Labor, and Welfare; Statistics and Information Department. Vital Statistics, 2000. 2001 [Google Scholar]
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer Statistics, 2006. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- Naruke T, Tsuchiya R, Kondo H, Asamura H. Prognosis and survival after resection for bronchogenic carcinoma based on the 1997 TNM-staging classification: the Japanese experience. Ann Thorac Surg. 2001;71:1759–1764. doi: 10.1016/s0003-4975(00)02609-6. [DOI] [PubMed] [Google Scholar]
- Janssen-Heijnen ML, Coebergh JW. Trends in incidence and prognosis of the histological subtypes of lung cancer in North America. Australia, New Zealand and Europe. Lung Cancer. 2001;31:123–137. doi: 10.1016/s0169-5002(00)00197-5. [DOI] [PubMed] [Google Scholar]
- Riely GJ, Politi KA, Miller VA, Pao W. Update on epidermal growth factor receptor mutations in non-small cell lung cancer. Clin Cancer Res. 2006;12:7232–7241. doi: 10.1158/1078-0432.CCR-06-0658. [DOI] [PubMed] [Google Scholar]
- Mitsudomi T, Yatabe Y. Mutations of the epidermal growth factor receptor gene and related genes as determinants of epidermal growth factor receptor tyrosine kinase inhibitors sensitivity in lung cancer. Cancer Sci. 2007;98:1817–1824. doi: 10.1111/j.1349-7006.2007.00607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota J, Kohno T. Molecular footprints of human lung cancer progression. Cancer Sci. 2004;95:197–204. doi: 10.1111/j.1349-7006.2004.tb02203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatabe Y, Mitsudomi T. Epidermal growth factor receptor mutations in lung cancers. Pathol Int. 2007;57:233–244. doi: 10.1111/j.1440-1827.2007.02098.x. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Tomida S, Yatabe Y, Kosaka T, Osada H, Yanagisawa K, Mitsudomi T, Takahashi T. Expression profile-defined classification of lung adenocarcinoma shows close relationship with underlying major genetic changes and clinicopathologic behaviors. J Clin Oncol. 2006;24:1679–1688. doi: 10.1200/JCO.2005.03.8224. [DOI] [PubMed] [Google Scholar]
- Motoi N, Szoke J, Riely GJ, Seshan VE, Kris MG, Rusch VW, Gerald WL, Travis WD. Lung adenocarcinoma: modification of the 2004 WHO mixed subtype to include the major histologic subtype suggests correlations between papillary and micropapillary adenocarcinoma subtypes. EGFR mutations and gene expression analysis. Am J Surg Pathol. 2008;32:810–827. doi: 10.1097/PAS.0b013e31815cb162. [DOI] [PubMed] [Google Scholar]
- Ninomiya H, Hiramatsu M, Inamura K, Nomura K, Okui M, Miyoshi T, Okumura S, Satoh Y, Nakagawa K, Nishio M, Horai T, Miyata S, Tsuchiya E, Fukayama M, Ishikawa Y. Correlation between morphology and EGFR mutations in lung adenocarcinomas Significance of the micropapillary pattern and the hobnail cell type. Lung Cancer. 2009;63:235–240. doi: 10.1016/j.lungcan.2008.04.017. [DOI] [PubMed] [Google Scholar]
- Miyoshi T, Satoh Y, Okumura S, Nakagawa K, Shirakusa T, Tsuchiya E, Ishikawa Y. Early-stage lung adenocarcinomas with a micropapillary pattern, a distinct pathologic marker for a significantly poor prognosis. Am J Surg Pathol. 2003;27:101–109. doi: 10.1097/00000478-200301000-00011. [DOI] [PubMed] [Google Scholar]
- Amin MB, Tamboli P, Merchant SH, Ordóñez NG, Ro J, Ayala AG, Ro JY. Micropapillary component in lung adenocarcinoma: a distinctive histologic feature with possible prognostic significance. Am J Surg Pathol. 2002;26:358–364. doi: 10.1097/00000478-200203000-00010. [DOI] [PubMed] [Google Scholar]
- Sakuma Y, Matsukuma S, Yoshihara M, Nakamura Y, Noda K, Nakayama H, Kameda Y, Tsuchiya E, Miyagi Y. Distinctive evaluation of nonmucinous and mucinous subtypes of bronchioloalveolar carcinomas in EGFR and K-ras gene-mutation analyses for Japanese lung adenocarcinomas: confirmation of the correlations with histologic subtypes and gene mutations. Am J Clin Pathol. 2007;128:100–108. doi: 10.1309/WVXFGAFLAUX48DU6. [DOI] [PubMed] [Google Scholar]
- De Oliveira Duarte Achcar R, Nikiforova MN, Yousem SA. Micropapillary lung adenocarcinoma: eGFR, K-ras, and BRAF mutational profile. Am J Clin Pathol. 2009;131:694–700. doi: 10.1309/AJCPBS85VJEOBPDO. [DOI] [PubMed] [Google Scholar]
- Takanami I, Tanana F, Hashizume T, Kikuchi K, Yamamoto Y, Yamamoto T, Kodaira S. Hepatocyte growth factor and c-Met/hepatocyte growth factor receptor in pulmonary adenocarcinomas: an evaluation of their expression as prognostic markers. Oncology. 1996;53:392–397. doi: 10.1159/000227594. [DOI] [PubMed] [Google Scholar]
- Tsao MS, Liu N, Chen JR, Pappas J, Ho J, To C, Viallet J, Park M, Zhu H. Differential expression of Met/hepatocyte growth factor receptor in subtypes of non-small cell lung cancers. Lung Cancer. 1998;20:1–16. doi: 10.1016/s0169-5002(98)00007-5. [DOI] [PubMed] [Google Scholar]
- Masuya D, Huang C, Liu D, Nakashima T, Kameyama K, Haba R, Ueno M, Yokomise H. The tumour-stromal interaction between intratumoral c-Met and stromal hepatocyte growth factor associated with tumour growth and prognosis in non-small-cell lung cancer patients. Br J Cancer. 2004;90:1555–1562. doi: 10.1038/sj.bjc.6601718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Niki T, Goto A, Morikawa T, Miyazawa K, Nakajima J, Fukayama M. c-Met activation in lung adenocarcinoma tissues: an immunohistochemical analysis. Cancer Sci. 2007;98:1006–1013. doi: 10.1111/j.1349-7006.2007.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Matsubara D, Goto A, Ota S, Sachiko O, Ishikawa S, Aburatani H, Miyazawa K, Fukayama M, Niki T. Constitutive activation of c-Met is correlated with c-Met overexpression and dependent on cell-matrix adhesion in lung adenocarcinoma cell lines. Cancer Sci. 2008;99:14–22. doi: 10.1111/j.1349-7006.2007.00640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T, Ohe Y, Sakamoto H, Tsuta K, Matsuno Y, Tateishi U, Yamamoto S, Nokihara H, Yamamoto N, Sekine I, Kunitoh H, Shibata T, Sakiyama T, Yoshida T, Tamura T. Epidermal growth factor receptor gene mutations and increased copy numbers predict gefitinib sensitivity in patients with recurrent non-small-cell lung cancer. J Clin Oncol. 2005;23:6829–6837. doi: 10.1200/JCO.2005.01.0793. [DOI] [PubMed] [Google Scholar]
- Niki T, Kohno T, Iba S, Moriya Y, Takahashi Y, Saito M, Maeshima A, Yamada T, Matsuno Y, Fukayama M, Yokota J, Hirohashi S. Frequent co-localization of Cox-2 and laminin-5 gamma2 chain at the invasive front of early-stage lung adenocarcinomas. Am J Pathol. 2002;160:1129–1141. doi: 10.1016/s0002-9440(10)64933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MT, Honn KV, Nie D. Cyclooxygenases, prostanoids, and tumor progression. Cancer Metastasis Rev. 2007;26:525–534. doi: 10.1007/s10555-007-9096-5. [DOI] [PubMed] [Google Scholar]
- Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, Louis DN, Christiani DC, Settleman J, Haber DA. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- Ishii M, Hashimoto S, Tsutsumi S, Wada Y, Matsushima K, Kodama T, Aburatani H. Direct comparison of GeneChip and SAGE on the quantitative accuracy in transcript profiling analysis. Genomics. 2000;68:136–143. doi: 10.1006/geno.2000.6284. [DOI] [PubMed] [Google Scholar]
- Midorikawa Y, Yamamoto S, Ishikawa S, Kamimura N, Igarashi H, Sugimura H, Makuuchi M, Aburatani H. Molecular karyotyping of human hepatocellular carcinoma using single-nucleotide polymorphism arrays. Oncogene. 2006;25:5581–5590. doi: 10.1038/sj.onc.1209537. [DOI] [PubMed] [Google Scholar]
- Wang T, Niki T, Goto A, Ota S, Morikawa T, Nakamura Y, Ohara E, Ishikawa S, Aburatani H, Nakajima J, Fukayama M. Hypoxia increases the motility of lung adenocarcinoma cells A549 via activation of the epidermal growth factor receptor pathway. Cancer Sci. 2007;98:506–511. doi: 10.1111/j.1349-7006.2007.00428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa S, Komura D, Tsuji S, Nishimura K, Yamamoto S, Panda B, Huang J, Fukayama M, Jones KW, Aburatani H. Allelic dosage analysis with genotyping microarrays. Biochem Biophys Res Commun. 2005;333:1309–1314. doi: 10.1016/j.bbrc.2005.06.040. [DOI] [PubMed] [Google Scholar]
- Coulombe PA, Omary MB. ‘Hard’ and ‘soft’ principles defining the structure, function and regulation of keratin intermediate filaments. Curr Opin Cell Biol. 2002;14:110–122. doi: 10.1016/s0955-0674(01)00301-5. [DOI] [PubMed] [Google Scholar]
- Moniaux N, Escande F, Porchet N, Aubert JP, Batra SK. Structural organization and classification of the human mucin genes. Front Biosci. 2001;6:D1192–D1206. doi: 10.2741/moniaux. [DOI] [PubMed] [Google Scholar]
- Inamura K, Satoh Y, Okumura S, Nakagawa K, Tsuchiya E, Fukayama M, Ishikawa Y. Pulmonary adenocarcinomas with enteric differentiation: histologic and immunohistochemical characteristics compared with metastatic colorectal cancers and usual pulmonary adenocarcinomas. Am J Surg Pathol. 2005;29:660–665. doi: 10.1097/01.pas.0000160438.00652.8b. [DOI] [PubMed] [Google Scholar]
- Rossi G, Marchioni A, Milani M, Scotti R, Foroni M, Cesinaro A, Longo L, Migaldi M, Cavazza A. TTF-1, cytokeratin 7, 34betaE12, and CD56/NCAM immunostaining in the subclassification of large cell carcinomas of the lung. Am J Clin Pathol. 2004;122:884–893. [PubMed] [Google Scholar]
- Tsuta K, Ishii G, Nitadori J, Murata Y, Kodama T, Nagai K, Ochiai A. Comparison of the immunophenotypes of signet-ring cell carcinoma, solid adenocarcinoma with mucin production, and mucinous bronchioloalveolar carcinoma of the lung characterized by the presence of cytoplasmic mucin. J Pathol. 2006;209:78–87. doi: 10.1002/path.1947. [DOI] [PubMed] [Google Scholar]
- Garber ME, Troyanskaya OG, Schluens K, Petersen S, Thaesler Z, Pacyna-Gengelbach M, van de Rijn M, Rosen GD, Perou CM, Whyte RI, Altman RB, Brown PO, Botstein D, Petersen I. Diversity of gene expression in adenocarcinoma of the lung. Proc Natl Acad Sci USA. 2001;98:13784–13789. doi: 10.1073/pnas.241500798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee A, Richards WG, Staunton J, Li C, Monti S, Vasa P, Ladd C, Beheshti J, Bueno R, Gillette M, Loda M, Weber G, Mark EJ, Lander ES, Wong W, Johnson BE, Golub TR, Sugarbaker DJ, Meyerson M. Classification of human lung carcinomas by mRNA expression profiling reveals distinct adenocarcinoma subclasses. Proc Natl Acad Sci USA. 2001;98:13790–13795. doi: 10.1073/pnas.191502998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal S, Zerillo C, Kolmakova J, Christensen JG, Harris LN, Rimm DL, Digiovanna MP, Stern DF. Association of constitutively activated hepatocyte growth factor receptor (Met) with resistance to a dual EGFR/Her2 inhibitor in non-small-cell lung cancer cells. Br J Cancer. 2009;100:941–949. doi: 10.1038/sj.bjc.6604937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shedden K, Taylor JM, Enkemann SA, Tsao MS, Yeatman TJ, Gerald WL, Eschrich S, Jurisica I, Giordano TJ, Misek DE, Chang AC, Zhu CQ, Strumpf D, Hanash S, Shepherd FA, Ding K, Seymour L, Naoki K, Pennell N, Weir B, Verhaak R, Ladd-Acosta C, Golub T, Gruidl M, Sharma A, Szoke J, Zakowski M, Rusch V, Kris M, Viale A, Motoi N, Travis W, Conley B, Seshan VE, Meyerson M, Kuick R, Dobbin KK, Lively T, Jacobson JW, Beer DG. Gene expression-based survival prediction in lung adenocarcinoma: a multi-site, blinded validation study. Nat Med. 2008;14:822–827. doi: 10.1038/nm.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznik TE, Sang Y, Ma Y, Abounader R, Rosen EM, Xia S, Laterra J. Transcription-dependent epidermal growth factor receptor activation by hepatocyte growth factor. Mol Cancer Res. 2008;6:139–150. doi: 10.1158/1541-7786.MCR-07-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegfried JM, Gubish CT, Rothstein ME, Queiroz de Oliveira PE, Stabile LP. Signaling pathways involved in cyclooxygenase-2 induction by hepatocyte growth factor in non small-cell lung cancer. Mol Pharmacol. 2007;72:769–779. doi: 10.1124/mol.107.034215. [DOI] [PubMed] [Google Scholar]
- Guo A, Villén J, Kornhauser J, Lee KA, Stokes MP, Rikova K, Possemato A, Nardone J, Innocenti G, Wetzel R, Wang Y, MacNeill J, Mitchell J, Gygi SP, Rush J, Polakiewicz RD, Comb MJ. Signaling networks assembled by oncogenic EGFR and c-Met. Proc Natl Acad Sci USA. 2008;105:692–697. doi: 10.1073/pnas.0707270105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, Lindeman N, Gale CM, Zhao X, Christensen J, Kosaka T, Holmes AJ, Rogers AM, Cappuzzo F, Mok T, Lee C, Johnson BE, Cantley LC, Jänne PA. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- Koth LL, Alex B, Hawgood S, Nead MA, Sheppard D, Erle DJ, Morris DG. Integrin beta6 mediates phospholipid and collectin homeostasis by activation of latent TGF-beta1. Am J Respir Cell Mol Biol. 2007;37:651–659. doi: 10.1165/rcmb.2006-0428OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirohashi S, Kanai Y. Cell adhesion system and human cancer morphogenesis. Cancer Sci. 2003;94:575–581. doi: 10.1111/j.1349-7006.2003.tb01485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders DS, Perry I, Hardy R, Jankowski J. Aberrant P-cadherin expression is a feature of clonal expansion in the gastrointestinal tract associated with repair and neoplasia. J Pathol. 2000;190:526–530. doi: 10.1002/(SICI)1096-9896(200004)190:5<526::AID-PATH564>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Taniuchi K, Nakagawa H, Hosokawa M, Nakamura T, Eguchi H, Ohigashi H, Ishikawa O, Katagiri T, Nakamura Y. Overexpressed P-cadherin/CDH3 promotes motility of pancreatic cancer cells by interacting with p120ctn and activating rho-family GTPases. Cancer Res. 2005;65:3092–3099. doi: 10.1158/0008.5472.CAN-04-3646. [DOI] [PubMed] [Google Scholar]
- Paredes J, Correia AL, Ribeiro AS, Albergaria A, Milanezi F, Schmitt FC. P-cadherin expression in breast cancer: a review. Breast Cancer Res. 2007;9:214. doi: 10.1186/bcr1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyke C, Salo S, Ralfkiaer E, Rømer J, Danø K, Tryggvason K. Laminin-5 is a marker of invading cancer cells in some human carcinomas and is coexpressed with the receptor for urokinase plasminogen activator in budding cancer cells in colon adenocarcinomas. Cancer Res. 1995;55:4132–4139. [PubMed] [Google Scholar]
- Pochampalli MR, Bitler BG, Schroeder JA. Transforming growth factor alpha dependent cancer progression is modulated by Muc1. Cancer Res. 2007;67:6591–6598. doi: 10.1158/0008-5472.CAN-06-4518. [DOI] [PubMed] [Google Scholar]
- Li X, Wang L, Nunes DP, Troxler RF, Offner GD. Suppression of MUC1 synthesis downregulates expression of the epidermal growth factor receptor. Cancer Biol Ther. 2005;4:968–973. doi: 10.4161/cbt.4.9.1913. [DOI] [PubMed] [Google Scholar]
- Matsubara D, Ishikawa S, Oguni S, Aburatani H, Fukayama M, Niki T. Molecular predictors of sensitivity to the MET inhibitor PHA665752 in lung carcinoma cells. J Thorac Oncol. 2010;5:1317–1324. doi: 10.1097/JTO.0b013e3181e2a409. [DOI] [PubMed] [Google Scholar]
- Shigematsu H, Lin L, Takahashi T, Nomura M, Suzuki M, Wistuba II, Fong KM, Lee H, Toyooka S, Shimizu N, Fujisawa T, Feng Z, Roth JA, Herz J, Minna JD, Gazdar AF. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005;97:339–346. doi: 10.1093/jnci/dji055. [DOI] [PubMed] [Google Scholar]