Abstract
Various studies in cell lines have previously demonstrated that sphingosine kinase 1 (SK1) and extracellular signal-regulated kinase 1/2 (ERK-1/2) interact in an estrogen receptor (ER)-dependent manner to influence both breast cancer cell growth and migration. A cohort of 304 ER-positive breast cancer patients was used to investigate the prognostic significance of sphingosine 1-phosphate (S1P) receptors 1, 2, and 3 (ie, S1P1, S1P2, and S1P3), SK1, and ERK-1/2 expression levels. Expression levels of both SK1 and ERK-1/2 were already available for the cohort, and S1P1, S1P2, and S1P3 levels were established by immunohistochemical analysis. High membrane S1P1 expression was associated with shorter time to recurrence (P = 0.008). High cytoplasmic S1P1 and S1P3 expression levels were also associated with shorter disease-specific survival times (P = 0.036 and P = 0.019, respectively). Those patients with tumors that expressed high levels of both cytoplasmic SK1 and ERK-1/2 had significantly shorter recurrence times than those that expressed low levels of cytoplasmic SK1 and cytoplasmic ERK-1/2 (P = 0.00008), with a difference in recurrence time of 10.5 years. Similarly, high cytoplasmic S1P1 and cytoplasmic ERK-1/2 expression levels (P = 0.004) and high cytoplasmic S1P3 expression and cytoplasmic ERK-1/2 expression levels (P = 0.004) were associated with shorter recurrence times. These results support a model in which the interaction between SK1, S1P1, and/or S1P3 and ERK-1/2 might drive breast cancer progression, and these findings, therefore, warrant further investigation.
The incidence of breast cancer is increasing and the lifetime odds of a woman developing breast cancer are 1 in 9 (http://www.uicc.org, last accessed February 1, 2010). It is the second major cause of cancer-related death among women in the United States and Europe (http://www.statistics.gov.uk, last accessed February 1, 2010). Early identification and appropriate treatment of the more aggressive tumors could dramatically reduce this figure. Over the past two decades, it has been demonstrated that women diagnosed with estrogen receptor (ER)α expressing breast cancer have a better prognosis than women diagnosed with ER-negative breast cancer.1 The ER is a member of the steroid receptor family and is activated by estrogen (E2), which induces nuclear translocation, allowing the ER to function as a transcription factor to increase cell proliferation and decrease cell apoptosis.1,2 ER-positive breast cancers are therefore treated with endocrine therapy to abolish the proliferative and antiapoptotic effect of E2 on breast tumors.2
Tamoxifen is one of the most widely used selective ER modulators and acts by inhibiting the interaction between E2 and ER, thus preventing ER ligand-dependent activation. Patients receiving adjuvant tamoxifen for 5 years have on average a 50% reduction in their recurrence rate and a 28% reduction in the rate of mortality.3 Unfortunately, approximately a quarter of ER-positive breast cancers exhibit either de novo or acquired resistance during the course of therapy.4 Those patients that relapse on tamoxifen therapy generally retain their ER, with the mechanisms underlying tamoxifen resistance not currently fully understood.
There is a accumulating evidence of aberrant sphingosine 1-phosphate (S1P) signaling in many types of cancer.5 Recently, it has been suggested that sphingosine kinase 1 (SK1), a lipid kinase, induces breast cancer progression6,7 via an ER-dependent pathway.6 Breast cancer cell line data suggest that E2 can activate SK1 in both a rapid, transient manner and a delayed, more prolonged manner.8,9 E2 induces activation of SK1 and the production of S1P,10 which is released from cells and activates the S1P3 receptor and ERK-1/2.7,10 S1P3 is a member of a family of five G protein-coupled receptors termed S1Pn (where n = 1–5).11 ERK-1/2 is a serine/threonine kinase, whose activation results in the phosphorylation of >50 substrates in the cytosol and nucleus. Activation of ERK-1/2 can promote proliferation and antiapoptotic effects. In addition to activation of ERK-1/2 via E2 and SK1, breast cancer cell line studies have also demonstrated that ERK-1/2 can induce SK1 phosphorylation and activation in an E2-dependent manner.10 These results suggest that an E2-dependent feedback mechanism might exist between SK1 and ERK-1/2 in ER-positive breast cancer cells.
It has already been established that activation of the ERK-1/2 cascade is associated with development of tamoxifen resistance in ER-positive breast cancer patients.12 We therefore hypothesized that SK1/S1P1–3/ERK-1/2 may also be involved in breast cancer progression and the development of tamoxifen resistance in the clinical setting. Associations between SK1 expression and survival using clinical breast cancer specimens have previously been observed by ourselves7 and others.6 A reduced disease-specific survival occurs in ER-positive breast cancer patients with tumors that express high levels of SK1,6,7 together with increased tamoxifen resistance.7 However, no correlations have previously been made for interactions between SK1/S1P1-3 and ERK-1/2 with regard to tamoxifen resistance and disease specific survival. The aim of this study was to establish whether SK1/S1P1-3/ERK-1/2 expression is associated with tamoxifen resistance and reduced disease-specific survival in a cohort of tamoxifen-treated ER-positive breast cancer patients. Elucidation of these interactions may provide new options for future therapeutic intervention in ER-positive endocrine-resistant breast cancer patients.
Materials and Methods
Cell Culture
MCF-7 cells and HEK 293 cells were grown in a monolayer culture in high glucose Dulbecco’s modified Eagle’s medium or minimum essential medium, respectively, with 10% European fetal calf serum and 1% Pen-Strep (10,000 U/ml penicillin G Sodium and 10,000 μg/ml streptomycin sulfate), 0.4% geneticin, and 15 μg/ml insulin at 37°C with 5% CO2.
Small Interfering RNA Treatment
SK1 and S1P3 down-regulation was achieved using sequence-specific SK1 and S1P3 small interfering RNA (siRNA), respectively. SK1 siRNA and DharmaFECT 2 reagent were from Dharmacon (Cromlington, UK). S1P3 siRNA was from Santa Cruz Biotechnology (Santa Cruz, CA). siRNA transfection was performed according to the protocol detailed by Dharmacon. MCF-7 cells grown on 24-well plates were transfected with 100 nmol/L sequence-specific siRNA or scrambled siRNA prepared in a mix with DharmaFECT 2 reagent and Dulbecco’s modified Eagle’s medium containing 10% serum. The cells were then cultured for 48 hours before serum starvation for 24 hours before tamoxifen treatment and measurement of DNA synthesis. The cells were grown in 75-cm3 flasks for the preparation of cell pellets.
Western Blotting
Analysis of proteins by SDS-PAGE and Western blotting was performed as previously described by us with anti-SK1, anti-hemagglutinin (HA), and anti-myc tag antibodies.13
DNA Synthesis
Cells were treated with and without tamoxifen (5 μmol/L) for 20 hours and [3H]thymidine (1 μCi/well) for 4 hours, after which cells were washed in ice-cold 10% (w/v) trichloracetic acid (three times, 10 minutes each), and nuclear material was harvested in 0.3 M NaOH/0.1% (w/v) SDS. [3H]Thymidine incorporation into DNA was quantified using liquid scintillation counting.
Patients
A total of 304 patients were recruited. All patients were diagnosed with operable invasive breast carcinoma between 1980 and 1999 in the Greater Glasgow area. These patients received standard adjuvant treatment according to protocols at the time of diagnosis. Patient follow-up details included information on clinical attendances, recurrence and metastasis, date and cause of death as well as adjuvant therapy details. All patients were treated with tamoxifen for a minimum of 5 years. In addition to receiving tamoxifen, 95 patients (31%) received adjuvant chemotherapy (3 unknown) and 98 (32%) received adjuvant radiotherapy (3 unknown). Ethics approval was granted by the local ethics committee.
Transfection
HEK 293 cells were transfected with myc-tagged or HA-tagged versions of SK1, S1P1, S1P2 and S1P3 plasmid constructs using Lipofectamine2000 (Invitrogen, Paisley, UK) reagent according to the manufacturer’s instruction. Transfection was performed for 24 hours at 37°C before serum starvation for an additional 24 hours before harvesting in Laemmli buffer and analysis for tagged proteins by SDS-PAGE and Western blotting. Parallel samples of transfected cells were prepared and collected as cell pellets (by rinsing twice in PBS and centrifugation (180 × g, 3 minutes)) before being formalin fixed and paraffin embedded and processed by immunohistochemistry (IHC) (see below).
Tissue Microarray Construction
Tissue microarrays were already available for use in the current study. A total of 0.6 mm2 cores of breast cancer tissue, identified by the pathologist, were removed from representative areas of the tumor taken from breast cancer patients at the time of surgical resection. All tissue microarray blocks were constructed in triplicate.
Immunohistochemistry
Staining for ER, progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2), SK1, ERK-1/2 and phosphorylated ERK-1/2 had been previously performed for the cohort.12,14 ER, PR, SK1, ERK-1/2, and phosphorylated ERK-1/2 protein expression were scored using the weighted histoscore technique.14,15 Tumors were classified as ER and PR positive if they had a weighted histoscore of >10. All tumors used in the current study were ER positive (expressed nuclear ER). Weak membrane ER staining was observed for 45 patients, although no correlations were observed between this and SK1 or receptors and tamoxifen resistance in this cohort. ERK-1/2 or phosphorylated ERK-1/2 expression was classified as high if levels as defined by IHC scores were equal to or above the upper quartile value. All other levels of expression were classified as low.
The tissue microarray slides were first dewaxed and rehydrated through a series of xylene and alcohol washes. Antigen retrieval was performed by microwaving the slides under pressure in a citrate buffer for 5 minutes (pH 6.0). Endogenous peroxidase was blocked using 3% hydrogen peroxide for 20 minutes, and nonspecific background staining was reduced by blocking with a 1:10 concentration of casein ×10 diluted in Tris-buffered saline for 20 minutes. The sections were incubated with the primary antibody. The antibodies for the following proteins were used: SK1 (Abgent, Abingdon, UK), S1P1, S1P2, and S1P3 (ExAlpha, Shirley, MA). Antibodies were used at dilutions of 1/40 (S1P1), 1/70 (S1P2) and 1/50 (S1P3) and incubated at 4°C overnight. Anti-SK1 antibody was used at a dilution of 1/50 and incubated at 25°C in a humidified incubator for 60 minutes. EnVision-HRP conjugate (DakoCytomation, Cambridgeshire, UK) was used for signal amplification, and positive staining was identified using 3,3′-diaminobenzidine chromagen (Vector Laboratories, Burlingame, CA). The slides were then counterstained with hematoxylin and Scott’s Tap Water Substitute before dehydration and mounting.
Scoring
Protein expression of each core (three per tumor specimen) was assessed using the weighted histoscore method (H score method). The weighted histoscore grades staining intensity as negative (0), weak (1), moderate (2), and strong (3)14,15 and then multiplies the percentage of tumor cells within each category. The histoscore range is from 0 (minimum) to 300 (maximum). Agreement between observers was calculated using interclass correlation coefficient (ICCC). All interclass correlation coefficient scores were above 0.84.
Statistical Analysis
Statistics were performed on cell culture experiments using Student’s t-test (n = 3 samples for each treatment). Disease-specific survival rates were generated using the Kaplan-Meier method. The log-rank test was used to compare significant differences between subgroups using univariate analysis. On the basis of the results of the univariate analysis a multivariate analysis was then carried out. The multivariate stepwise Cox regression analysis was performed to identify factors that were independently associated with disease-specific death. A stepwise backward procedure was used to derive a final model of the variables that had a significant independent relationship with survival. To remove a variable from the model, the corresponding P value had to be >0.05. Interrelationships between clinical parameters, PR and HER2 status, were calculated using the χ2 test. Correlations between SK1, S1P1–3, and ERK-1/2 were performed using Spearman Rank correlations. Data are expressed as median and range. The statistical analyses were performed using a statistical software package (15.0; SPSS, Chicago, IL).
Results
MCF-7 Cell Studies
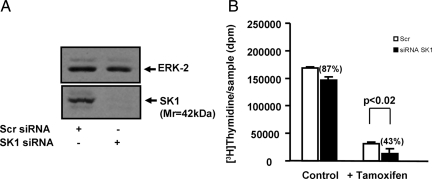
To test the role of SK1 in the acquisition of tamoxifen resistance, we used an siRNA approach. In this regard, the treatment of MCF-7 cells with SK1 siRNA substantially reduced the expression of SK1 (Mr 42,000) without affecting ERK-1/2 levels (Figure 1A).
Figure 1.
A: Western blot demonstrating that treatment of MCF-7 cells with SK1 siRNA substantially reduces the expression of SK1 (Mr 42,000) compared with scrambled siRNA-treated MCF-7 cells, and Western blot showing ERK-2 levels do not change in scrambled versus SK1 siRNA-treated cells. B: Graph demonstrating that tamoxifen significantly reduces basal [3H]thymidine incorporation in scrambled and SK1 siRNA-treated cells. Treatment with siRNA SK1 did not significantly reduce basal DNA synthesis compared with scrambled siRNA-treated cells. Treatment with siRNA SK1 increased tamoxifen-inhibited DNA synthesis by 57% versus scrambled siRNA-treated cells.
We also found that tamoxifen significantly reduced basal [3H]thymidine incorporation in scrambled and SK1 siRNA-treated cells (Figure 1B). However, in the presence of tamoxifen, the treatment of cells with SK1 siRNA enhanced the inhibition of DNA synthesis to 43% of that for scrambled siRNA-treated cells (Figure 1b). Therefore, the inhibitory effect of tamoxifen was enhanced by the siRNA knockdown of SK1 expression, consistent with the possibility that SK1 functions to reduce the effect of tamoxifen in breast cancer cell growth (ie, SK1 increases tamoxifen resistance). These data are consistent with the finding that enforced expression of SK1 in MCF-7 cells induces tamoxifen resistance.16
Clinicopathological Details
Our full cohort consisted of 304 breast cancer patients with ER-positive tumors, who received tamoxifen therapy for a median period of 5 years. Median age was 62 years (inter-quartile range 53–70). Thirty-eight percent of the cancer specimens were pathologically graded as grade 2 and 3, median size of the invasive cancer was 20 mm (inter-quartile range 15–30 mm). Forty-nine percent of the patients were axillary lymph node positive. Mean patient follow-up was 7.9 years (minimum follow-up was 0.1 years and the maximum follow-up was 25.9 years). During this period, 67 patients experienced disease recurrence while receiving tamoxifen therapy, 79 patients died of their cancer, and an additional 57 patients died of intercurrent disease. The association with clinical parameters, recurrence on tamoxifen and disease-specific survival, are provided in Tables 1 and 3, respectively, and correlations between the clinicopathological characteristics of this cohort are shown in Table 2.
Table 1.
The Patient Cohort’s Characteristics Correlated to Recurrence on Tamoxifen
| Patient cohort 304 | Numbers | Univariate P value | Multivariate P value | Hazard ratio | Inter-quartile range |
|---|---|---|---|---|---|
| Age (unknown/<50 yr/>50 yr) | 2/61/241 | 0.978 | |||
| Tumor type (unknown/duct/lob/tub/others) | 12/252/21/19 | 0.078 | |||
| Grade (unknown/G1/G2/G3) | 15/62/138/79 | 0.002 | NS | ||
| Size (mm) (unknown/<20, 20–50, >50) | 19/142/130/12 | <0.001 | 0.029 | 1.9 | 1.1–3.4 |
| Lymph node (unknown/positive/negative) | 32/138/134 | 0.003 | NS | ||
| PR status (unknown/positive/negative) | 15/171/118 | 0.016 | NS | ||
| HER2 status (unknown/positive/negative) | 8/20/276 | 0.042 | NS | ||
| SK1membrane (unknown/positive/negative) | 37/60/207 | 0.855 | |||
| SK1cytoplamic (unknown/positive/negative) | 37/20/247 | 0.022 | NS | ||
| SK1nuclear (unknown/positive/negative) | 37/95/172 | 0.016 | NS | ||
| S1P1membrane (unknown/positive/negative) | 22/45/236 | 0.008 | 0.02 | 2.1 | 1.1–4.2 |
| S1P1cytoplamic (unknown/positive/negative) | 22/35/246 | 0.351 | |||
| S1P1nuclear (unknown/positive/negative) | 22/55/226 | 0.775 | |||
| S1P2membrane (unknown/positive/negative) | 24/45/233 | 0.517 | |||
| S1P2cytoplamic (unknown/positive/negative) | 24/51/277 | 0.615 | |||
| S1P2nuclear (unknown/positive/negative) | 24/53/225 | 0.135 | |||
| S1P3membrane (unknown/positive/negative) | 20/37/247 | 0.920 | |||
| S1P3cytoplamic (unknown/positive/negative) | 20/109/175 | 0.183 | |||
| S1P3nuclear (unknown/positive/negative) | 20/63/221 | 0.911 |
Each clinical and pathological parameter was correlated to recurrence on tamoxifen, when both unilateral and bilateral recurrences are considered (P values). Grade, Bloom and Richardson grade. Histology: duct, ductal carcinoma; lob, lobular carcinoma; tub, tubular carcinoma; and others, mucinous, mucoid, and micropapillary carcinoma. NS, nonsignficant. Numbers in bold represent significant findings. Univariate analysis is performed for each parameter. However, only markers with P < 0.05 are included in the multivariate model.
Table 3.
The Full Patient Cohort’s Characteristics Correlated to Disease-Specific Survival
| Patient cohort 304 | Numbers | Univariate P value | Multivariate P value | HR | Inter-quartile range |
|---|---|---|---|---|---|
| Age (unknown/<50 yr/>50 yr) | 2/61/241 | 0.18 | |||
| Tumour type (unknown/duct/lob/tub/others) | 12/252/21/19 | 0.05 | NS | ||
| Grade (unknown/G1/G2/G3) | 15/62/138/79 | 0.006 | NS | ||
| Size (mm) (unknown/<20, 20–50, >50) | 19/142/130/12 | <0.001 | 0.004 | 2.2 | 1.2–3.9 |
| Lymph node (unknown/positive/negative) | 32/138/134 | <0.001 | 0.014 | 2.2 | 1.1–4.2 |
| PR status (unknown/positive/negative) | 15/171/118 | 0.198 | |||
| HER2 status (unknown/positive/negative) | 8/20/276 | 0.023 | NS | ||
| SK1membrane (unknown/positive/negative) | 37/60/207 | 0.625 | |||
| SK1cytoplamic (unknown/positive/negative) | 37/20/247 | 0.026 | NS | ||
| SK1nuclear (unknown/positive/negative) | 37/95/172 | 0.044 | NS | ||
| S1P1membrane (unknown/positive/negative) | 22/45/236 | 0.149 | |||
| S1P1cytoplamic (unknown/positive/negative) | 22/35/246 | 0.036 | NS | ||
| S1P1nuclear (unknown/positive/negative) | 22/55/226 | 0.280 | |||
| S1P2membrane (unknown/positive/negative) | 24/45/233 | 0.230 | |||
| S1P2cytoplamic (unknown/positive/negative) | 24/51/277 | 0.683 | |||
| S1P2nuclear (unknown/positive/negative) | 24/53/225 | 0.775 | |||
| S1P3membrane (unknown/positive/negative) | 20/37/247 | 0.511 | |||
| S1P3cytoplamic (unknown/positive/negative) | 20/109/175 | 0.019 | 0.015 | 2.0 | 1.4–3.5 |
| S1P3nuclear (unknown/positive/negative) | 20/63/221 | 0.316 |
Each clinical and pathological parameter was correlated to disease-specific survival (P values). Grade: Bloom and Richardson grade. Histology: duct, ductal carcinoma; lob, lobular carcinoma; tub, tubular carcinoma; and others, mucinous, mucoid, and micropapillary carcinoma. Numbers in bold represent the significant findings. Univariate analysis is performed for each parameter. However, only markers with P < 0.05 are included in the multivariate model.
Table 2.
Correlation between SK1, S1P1, S1P2, and S1P3 Expression and the Clinicopathological Characteristics of the Cohort
| Variables | Tumor type | Grade | Size | LN status | PR status | HER status | SK1 mem | SK1 cyto | SK1 nuc | S1P1 mem | S1P1 cyto | S1P1 nuc | S1P2 mem | S1P2 cyto | S1P2 nuc | S1P3 mem | S1P3 cyto | S1P3 nuc |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (<50 yr/>50 yr) | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Tumor type (duct/lob/tub/others) | NS | NS | <0.01 | NS | NS | NS | <0.05 | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | |
| Grade (G1/G2/G3) | <0.01 | NS | <0.05 | <0.01 | NS | NS | <0.05 | NS | NS | NS | NS | NS | >0.05 | NS | NS | NS | ||
| Size (mm) (<20, 20–50, >50) | <0.01 | NS | NS | NS | NS | NS | <0.01 | NS | NS | NS | NS | NS | NS | NS | <0.05 | |||
| Lymph node (positive/negative) | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | ||||
| PR status (positive/negative) | <0.05 | NS | NS | NS | <0.001 | NS | NS | NS | NS | NS | <0.01 | <0.05 | NS | |||||
| HER2 status (positive/negative) | NS | NS | NS | <0.05 | <0.001 | <0.05 | NS | NS | NS | NS | NS | NS | ||||||
| SK1membrane (positive/negative) | <0.005 | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | |||||||
| SK1cytoplamic (positive/negative) | <0.05 | <0.05 | NS | NS | NS | <0.05 | NS | NS | NS | NS | ||||||||
| SK1nuclear (positive/negative) | <.005 | NS | NS | NS | NS | NS | <0.01 | NS | NS | |||||||||
| S1P1membrane (positive/negative) | NS | NS | <0.005 | NS | NS | <0.05 | NS | NS | ||||||||||
| S1P1cytoplamic (positive/negative) | <0.001 | NS | <0.001 | NS | <0.001 | NS | NS | |||||||||||
| S1P1nuclear (positive/negative) | NS | <0.001 | NS | NS | <0.001 | <0.001 | ||||||||||||
| S1P2membrane (positive/negative) | NS | <0.001 | NS | NS | NS | |||||||||||||
| S1P2cytoplamic (positive/negative) | NS | <0.005 | NS | NS | ||||||||||||||
| S1P2nuclear (positive/negative) | NS | <0.001 | <0.05 | |||||||||||||||
| S1P3membrane (positive/negative) | <0.001 | NS | ||||||||||||||||
| S1P3cytoplamic (positive/negative) | <0.001 | |||||||||||||||||
| S1P3nuclear (positive/negative) |
Tumor type: duct, ductal carcinoma; lob, lobular carcinoma; tub, tubular carcinoma; and others, mucinous, mucoid, and micropapillary carcinoma. Grade: Bloom and Richardson grade. χ2 test: NS nonsignificant; P > 0.05.
Antibody Specificity
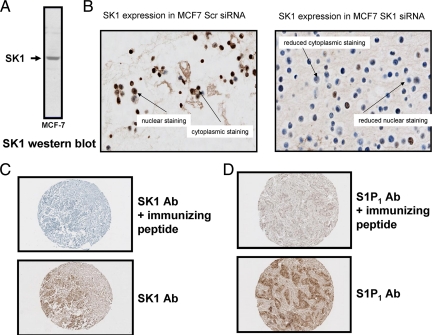
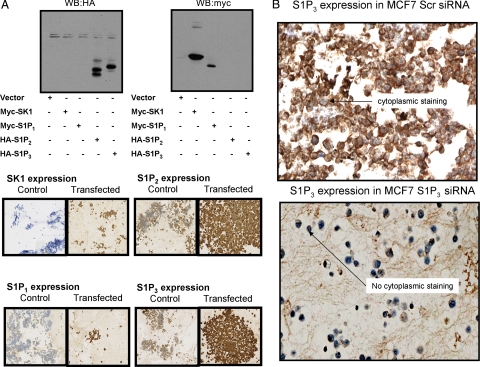
Before commencing tissue staining, all antibodies used in the current study were tested for specificity. Anti-SK1 antibody was demonstrated to immunostain a single protein band with Mr = 42,000, which is the correct size for native SK1, on a Western blot of MCF-7 cell lysates (Figure 2A). Moreover, siRNA knockdown of SK1 eliminated this protein band from MCF-7 cells (Figure 1A) and significantly decreased immunostaining in MCF-7 cell pellets (Figure 2B). When tissue was treated with SK1 immunizing peptide before IHC using anti-SK1 antibody, all tissue staining was abolished (Figure 2C). Similarly, when immunizing peptide was applied before anti-S1P1 antibody, all tissue staining was abolished (Figure 2D). However, there was no blocking peptide commercially available for the anti-S1P2 and anti-S1P3 antibody, so we were unable to determine antibody specificity in this manner. Therefore, HEK 293 cells were transfected with HA-tagged S1P2 or S1P3 plasmid constructs to separately overexpress these S1P receptors and these cells were then taken for IHC to confirm antibody specificity. Similar experiments were also performed using myc-tagged S1P1 and SK1 plasmid constructs. Western blotting of cell lysates was performed using anti-HA and-Myc tag antibodies to ensure successful transfection of the relevant S1P receptor or SK1 in HEK 293 cells (Figure 3A). Pellets from control and transfected HEK 293 cells were formalin fixed and paraffin embedded, and IHC was performed on pellets using separate anti-S1P1–3 and anti-SK1 antibodies. IHC demonstrated that the immunostaining of S1P1–3 and SK1 increased in transfected cells (Figure 3A), further confirming the specificity of the antibodies used for IHC of tumor samples. In addition, treatment of MCF-7 cells which express S1P39 with siRNA S1P3 also reduced immunostaining (Figure 3B).
Figure 2.
A: Western blot for SK1 demonstrating that antibodies detect a single band corresponding to 42 kDa, the recognized size of the SK1 protein. B: Cell pellets demonstrating that treatment with siRNA SK1 reduced SK1 expression as assessed by IHC versus scrambled siRNA-treated cells. C: Example of SK1 expression using antibody and antibody plus blocking peptide. The figure demonstrates that the addition of blocking peptide greatly diminishes the detection of SK1 by IHC. D: Example of S1P1 expression using antibody and antibody plus blocking peptide. The figure demonstrates that the addition of blocking peptide greatly diminishes the detection of S1P1 by IHC.
Figure 3.
A: Western blots for HA-tag and myc-tag using lysates derived from HEK 293 cells transfected with myc-tagged SK1 or S1P1 or with HA-tagged S1P2 or S1P3 plasmid constructs. The results show the successful expression of epitope-tagged SK1, S1P1, S1P2, and S1P3. In addition, cell pellets were produced from HEK 293 cells that were either not transfected or transfected with myc-tagged SK1 or S1P1 or with HA-tagged S1P2 or S1P3 plasmid constructs. Cell pellets were formalin fixed, paraffin embedded and processed for IHC using anti-SK1, -S1P1, S1P2-, or S1P3 antibodies, as indicated. The figure demonstrates the specificity of the antibodies under the conditions used. B: Cell pellets demonstrating that treatment with siRNA S1P3 reduced S1P3 expression as assessed by IHC versus scrambled siRNA-treated cells.
SK1 Expression Levels Are Associated with Clinicopathological Parameters
We previously reported7 that high SK1 cytoplasmic expression in tumors of ER+ breast cancer patients is associated with shorter time to recurrence on tamoxifen (low expression SK1 expression, 12.61 (11.3–13.92) years; high SK1 expression, 4.65 (3.44–5.87) years (P = 0.02 for low versus high SK1 expression) and shorter disease-specific survival (low SK1 expression, 18.1 (16.4–19.8) years; high SK1 expression 7.6 (5.9–9.2) years; P = 0.026 for low versus high SK1 expression when compared with patients with ER+ breast cancer with low cytoplasmic SK1 expression in their tumors).
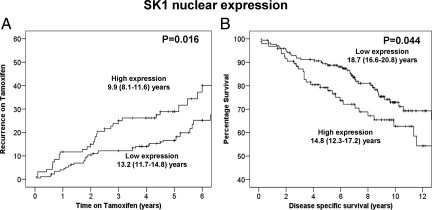
Here were investigate the association of SK1 (in all cellular locations) with clinical outcome measures. SK1 expression was successfully assessed in 88% (269 of 304) of the tumors analyzed. From this 88%, 32% of tumors exhibited membrane expression, 92% cytoplasmic, and 95% nuclear. Tumors were subdivided into those with high or low expression using the method described by Ruckhaberle et al.6 χ2 analysis demonstrated that cytoplasmic SK1 expression correlated with both membrane and nuclear SK1 expression (Table 2). On univariate analysis, membrane SK1 expression was not associated with recurrence on tamoxifen (Table 1) or disease-specific survival (Table 3). If both unilateral recurrences and bilateral recurrences were considered, nuclear SK1 expression was significantly associated with shorter time to recurrence on tamoxifen (P = 0.016) (Table 1, Figure 4A). This association was strengthened when only unilateral recurrences were considered (P = 0.008). In addition, nuclear SK1 expression was significantly associated with shorter disease-specific survival (P = 0.044) (Figure 4B). Those patients with high nuclear SK1 expression had a mean survival of 14.8 years (95% confidence interval (CI) 12.3–17.2) compared with those with low expression with mean survival of 18.7 years (95% CI 16.6–20.8). The nuclear localization of SK1 is consistent with the fact that SK1 has two functional nuclear export sequences and can transit through the nucleus.17 The expression levels of nuclear SK1 were not independent of known clinical parameters in multivariate analysis suggesting that nuclear SK1 is associated with clinical parameters. χ2 analysis demonstrated that high grade correlated with high nuclear SK1 expression (Table 2).
Figure 4.
A: High nuclear SK1 expression is associated with shorter time to recurrence on tamoxifen (P = 0.016). B: High nuclear SK1 expression is associated with shorter disease-specific survival (P = 0.044).
S1P1 Expression Levels Are Associated with Tumor Size, PR, and HER2 Status
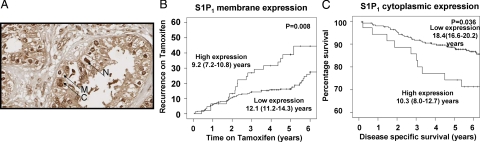
S1P1 expression was successfully assessed in 92% (281 of 304) of the tumors analyzed. From this 92%, 92% of tumors exhibited membrane expression, 99% cytoplasmic, and 90% nuclear (Figure 5A). Tumors were subdivided into those with high or low expression as described above. χ2 analysis demonstrated that membrane S1P1 expression correlated with both cytoplasmic and nuclear SK1 expression (Table 2). On univariate analysis, cytoplasmic and nuclear S1P1 expression was not associated with recurrence on tamoxifen. In contrast, high membrane S1P1 expression was significantly associated with shorter time to recurrence, when either unilateral or both unilateral and bilateral recurrences were considered (P = 0.001 unilateral, and P = 0.008 unilateral and bilateral) (Table 1, Figure 5B), and this was independent on multivariate analysis (P = 0.02) (Table 1). Those patients with high membrane S1P1 expression had a mean time to recurrence of 9.2 years (95% CI 7.2–10.8) compared with those with low expression whose mean time to recurrence was 12.1 years (95% CI 11.2–14.3). In addition, high cytoplasmic S1P1 expression was significantly associated with shorter disease-specific survival (P = 0.036) (Figure 5C). This was not independent in tumors multivariate analysis (Table 3). Those patients with high cytoplasmic S1P1 expression had a mean disease-specific survival of 10.3 years (95% CI 8.0–12.7) compared with those with low expression with mean time to recurrence of 18.4 years (95% CI 16.6–20.2). χ2 analysis demonstrated that membrane S1P1 expression correlated with tumor size and PR status, whereas membrane, cytoplasmic, and nuclear S1P1 correlated with HER2 status (Table 2).
Figure 5.
A: Example of membrane (M), cytoplasmic (C), and nuclear (N) S1P1 expression from tumors within the cohort. Magnification ×400. B: High membrane S1P1 expression is associated with shorter time to recurrence on tamoxifen (P = 0.008). C: High cytoplasmic S1P1 expression is associated with shorter disease-specific survival (P = 0.036).
Sphingosine 1-Phosphate Receptor 2
S1P2 expression was successfully assessed in 92% (280 of 304) of the tumors analyzed. From this 92%, 89% of tumors exhibited membrane expression, 99% cytoplasmic, and 95% nuclear. Tumors were subdivided into those with high or low expression as described above. χ2 analysis demonstrated that cytoplasmic S1P2 expression correlated with both cytoplasmic and nuclear S1P1 expression and also SK1 expression and membrane S1P2 expression correlated with nuclear S1P2 expression (Table 2). On univariate analysis S1P2 expression at any location was not associated with recurrence on tamoxifen or disease specific survival. χ2 analysis demonstrated that nuclear S1P2 expression correlated weakly with tumor grade (Table 2).
Sphingosine 1-Phosphate Receptor 3
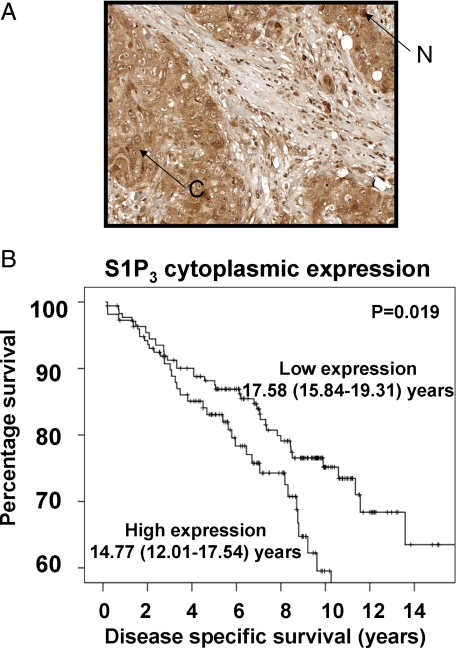
S1P3 expression was successfully assessed in 93% (284 of 304) of the tumors analyzed. From this 93%, 80% of tumors exhibited membrane expression, 93% cytoplasmic, and 71.5% nuclear (Figure 6A). Tumors were subdivided into those with high or low expression as described above. On univariate analysis, S1P3 expression was not associated with recurrence on tamoxifen, whereas high cytoplasmic S1P3 expression was significantly associated with shorter disease-specific survival (P = 0.019) (Figure 6B), and this was independent in multivariate analysis (P = 0.015) (Table 3). Those patients with high cytoplasmic S1P3 expression had a mean disease-specific survival of 14.7 years (95% CI 12.0–17.5) compared with those with low expression with mean time to recurrence of 17.5 years (95% CI 15.8–19.3). χ2 analysis demonstrated that membrane and cytoplasmic S1P3 expression correlated with PR status and nuclear S1P3 correlated with tumor size (Table 2).
Figure 6.
A: Example of cytoplasmic (C) and nuclear (N) S1P3 expression from tumors within the cohort. Magnification ×400. B: High cytoplasmic S1P3 expression is associated with shorter disease-specific survival (P = 0.019).
Interaction Between SK1, S1P1–3, and ERK-1/2 Signaling Cascade and Recurrence on Tamoxifen
The literature regarding breast cancer cell lines suggests that there may be an E2-dependent regulatory loop between SK1 and ERK-1/2.10 We have also demonstrated that the siRNA knockdown of SK1 reduces S1P3 expression in MCF-7 cells, resulting in a decrease in the S1P/S1P3-stimulated activation of ERK-1/2 and formation of the migratory phenotype.7
Therefore to test whether SK1 and ERK-1/2 have a combined effect on the time to recurrence while on tamoxifen in our clinical cohort, we constructed Kaplan-Meier plots to compare patients that have both high expression of SK1 and ERK-1/2 compared with those that had low expression of SK1 and ERK-1/2. On univariate analysis, when only unilateral recurrences were considered, patient whose tumors expressed high levels of cytoplasmic SK1 and cytoplasmic ERK-1/2 had a significantly shorter time to recurrence on tamoxifen than those who expressed low levels of cytoplasmic SK1 and cytoplasmic ERK-1/2 (P = 0.00008). Those patients with high SK1 and ERK-1/2 expression had a mean time to recurrence of 3.6 years (95% CI 1.7–5.5) compared with those with low expression of SK1 and ERK-1/2 having a mean time to recurrence of 14.1 years (95% CI 12.6–15.5). The difference in mean time to recurrence was 10.5 years, and this was independent in multivariate analysis when combined with age, grade, tumor size, and PR status (P = 0.0002, hazard ratio 2.5 (95% CI 1.6–4.2)). This association was also observed when unilateral and bilateral recurrences were considered (P = 0.00009) and this was also independent in multivariate analysis when combined with age, grade, tumor size, PR status, and HER2 status (P = 0.0001, hazard ratio 2.6 (95% CI 1.6–4.1)). However, this was not independent of treatment received, as the association was lost when patients received chemotherapy (unilateral recurrences, P = 0.0001, no chemotherapy versus P = 0.215, receiving chemotherapy, uni- and bilateral recurrences, P = 0.0003, no chemotherapy versus P = 0.186, receiving chemotherapy), suggesting that this patient group should be receiving chemotherapy.
When patients with high expression of cytoplasmic S1P1 or S1P3 and cytoplasmic ERK-1/2 were compared with those that had low expression of S1P1 or S1P3 and ERK-1/2, a similar observation was made as to that with SK1 and ERK-1/2. On univariate analysis, when only unilateral recurrences were considered, patient whose tumors expressed high levels of cytoplasmic S1P1 and cytoplasmic ERK-1/2 had a significantly shorter time to recurrence on tamoxifen than those who expressed low levels of cytoplasmic S1P1 and cytoplasmic ERK-1/2 (P = 0.004), and this was independent in multi variate analysis when combined with age, grade, tumor size, PR status, and HER2 status (P = 0.003, hazard ratio 1.8 (95% CI 1.2–2.7)). The difference in time to recurrence was 9.8 years (14.5 versus 4.7 years). A similar observation was also made with membrane S1P1 and cytoplasmic ERK-1/2 (univaritate analysis P = 0.00003, multivariate 0.00006, hazard ratio 2.3(95% CI 1.5–3.5)). The difference in time to recurrence was 10.1 years (14.7 versus 4.6 years). On univariate analysis, when only unilateral recurrences were considered, patient whose tumors expressed high levels of cytoplasmic S1P3 and cytoplasmic ERK-1/2 had a significantly shorter time to recurrence on tamoxifen than those who expressed low levels of cytoplasmic S1P3 and cytoplasmic ERK-1/2 (P = 0.004), and this was independent in multivariate analysis when combined with age, grade, tumor size, and PR status (P = 0.001, hazard ratio 1.9 (95% CI 1.3–2.8)). The difference in time to recurrence was 5.5 years (12.9 versus 7.4 years). This observation was also seen with membrane S1P3 and cytoplasmic ERK-1/2 (univariate analysis P = 0.002, multivariate 0.005, hazard ratio 1.7 (95% CI 1.2–2.4)). The difference in time to recurrence was 7.0 years (14.1 versus 7.1 years).
This association, for both S1P1 and S1P3 was also observed when unilateral and bilateral recurrences were considered (P = 0.004 and P = 0.002, respectively), and this was also independent in multivariate analysis for both S1P1 and S1P3 when combined with age, grade, tumor size, PR status and HER2 status (P = 0.001, hazard ratio 2.1 (95% CI 1.4–3.3) and P = 0.0004, hazard ratio 1.9 (95% CI 1.3–2.7)).
When patients with high expression of cytoplasmic SK1, S1P1, S1P3, and cytoplasmic ERK-1/2 were compared with those with low expression of all four, a significant difference in unilateral recurrence was observed (P = 0.0003) (the difference in time to unilateral recurrence was 9.1 years). When patients with high expression of cytoplasmic SK1, membrane S1P1, membrane S1P3 and cytoplasmic ERK-1/2 were compared with low expression of all four, a significant difference in unilateral recurrence was observed. (P = 0.002) (the difference in time to unilateral recurrence 9.9 years). However, the combination of all four proteins, whether using cytoplasmic S1P1 and S1P3 or membrane S1P1 and S1P3, does not offer any additional power over SK1 and cytoplasmic ERK-1/2 expression only (P = 0.0003 cytoplasmic or P = 0.002 membrane versus P = 0.00008), indicating that SK1 and ERK-1/2 is the strongest combination. In addition, when the differences in time to unilateral recurrence are considered, a 10.5-year difference was observed with SK1 and ERK-1/2, compared with 9.1 years when cytoplasmic SK1, S1P1, S1P3, and cytoplasmic ERK-1/2 or 9.9 years when cytoplasmic SK1 and membrane S1P1 or S1P3 and cytoplasmic ERK-1/2 are combined. This indicates that combining all four proteins does not offer any additional power over SK1 and ERK-1/2 only.
Discussion
In vitro cell line work reports that E2 elicits SK1 activation via interaction with the ERα and possibly GPR30.10 These studies suggest that a rapid response to E2 is mediated via nongenomic functions of ER, whereas a delayed prolonged effect requires the classical ER-mediated nuclear transcription activity.10,16 Both these methods of activating SK1 are linked with ERK-1/2 activation. We have previously reported in a cohort of ER-positive tamoxifen-treated breast cancer patients that activation of the ERK-1/2 cascade results in a reduction of patient survival.12 However, to our knowledge, no one has investigated the relationship between SK1, S1P receptors, and ERK-1/2 in clinical breast cancer samples. Because of the associations with E2, it was deemed that the most appropriate patient cohort in which to investigate this would be ER-positive breast cancer patients. A cohort of 304 ER-positive tamoxifen-treated breast cancer patients were therefore used to investigate if SK1 and S1P receptors were expressed in ER-positive breast cancer and whether association with ERK-1/2 expression and/or clinicopathological parameters. We observed that SK1 and S1P receptors were expressed in the majority of breast cancer tumor cells investigated. The expression of SK1 and S1P receptors was markedly greater in tumor cells compared with the surrounding stroma and inflammatory infiltrate, supporting the hypothesis that this pathway functions to influence signal transduction in breast cancer cells. Ruckhaberle et al6 previously observed expression of SK1 in human breast cancer tissue and demonstrated an association with reduced patient survival. We also demonstrated that high cytoplasmic expression of SK1 was associated with reduced time to recurrence on tamoxifen and cumulative disease-specific survival.7 However, neither study investigated S1P receptor expression nor correlations with ERK-1/2.
One of the major findings of the current study is that high expression of cytoplasmic S1P1 and S1P3 are associated with reduced disease-specific survival in patients with ER-positive breast cancer. These findings demonstrate that S1P1 and S1P3 are not only located in the cell membrane but may also be found in the cytoplasm and it is in this location that they are associated with disease-specific survival. Cell line studies have previously demonstrated that postactivation of S1P receptors results in their endocytosis following S1P stimulation.18 We therefore hypothesized that the cytoplasmic location of S1P1 and/or S1P3 is a surrogate marker of activation for these receptors in ER-positive breast cancer. The S1P3 receptor has previously been demonstrated to induce cellular proliferation and have a promigratory effect in a number of cell types,19,20 including MCF-7 breast cancer cells.7 This is in line with observations made in the current study, as it is associated with increased tumor size and reduced disease-specific survival. The results from the current study, combined with those in the literature, add weight to the suggestion that SK1 and S1P receptors could be used as negative prognostic markers in ER-positive breast cancer and also suggest that they could be possible therapeutic targets. In addition, progesterone, which is also known to correlate with breast cancer-specific survival, has been demonstrated to regulate SK1 expression.21 Although in the current study we do not have access to levels of circulating progesterone to correlate with SK1 expression levels, we do have PR status available for each tumor in the cohort. As activation of the ER can result in increased expression of PR, PR status can be used to determine whether ER is functionally active and therefore provide information on the transcriptional activity of the ER in breast tumors.22 It is therefore interesting to note that a strong correlation is observed between expression of PR, S1P1, and S1P3, suggesting that the negative prognostic effect we observed with the S1P receptors is in fact, as the literature suggests, ER dependent and is only observed when the ER is functioning as a transcription factor.22,23
The S1P3 5′-untranslated region (using TRANSFAC at Biobase) contains predicted ERα binding sites and Sp1 and c-Jun binding sites (which are regulated by ERK-1/2). The identification of the ERα as a potential regulator of S1P3 expression suggests that E2 might up-regulate S1P3 expression, thereby favoring this receptor system for use in ER-positive breast cancer cells. The S1P1 promoter also contains a predicted ERα binding site. Therefore, S1P1 and S1P3 might be suitable targets for therapeutic intervention (using S1P receptor antagonists) in ER-positive breast cancer.
Cell line work also demonstrates that S1P receptors are endocytosed on activation and before they are able to influence activation of ERK-1/2.18 This interaction between S1P receptors and ERK-1/2 may therefore form part of a positive feedback loop that exists between SK1 and ERK-1/2. Here we demonstrate that S1P receptors are only associated with decreased disease specific survival when in the cytoplasm. This combined with the fact that those patients with both high SK1 or S1P1 or S1P3 expression and high ERK-1/2 expression do significantly worse than those with low expression of either provides evidence that this feedback loop not only exists in the in vitro setting but may also function in clinical breast cancer cells. This observation is made for patients with unilateral recurrence, ie, acquired tamoxifen resistance and also when unilateral and bilateral occurrences are combined, ie, combining acquired and de novo tamoxifen resistance, as it is generally considered that a recurrence in the opposite breast is classed a new primary with de novo tamoxifen resistance. Therefore, this mechanism may also be responsible for explaining why a small percentage of ER positive breast tumors do not initially respond to tamoxifen therapy, suggesting that SK1 and S1P receptors in combination with ERK-1/2 may be used in combination with ER status as biomarkers to predict response to tamoxifen.
Those patients whose tumors expressed both SK1 and ERK-1/2 or both S1P1 and ERK-1/2 at high levels relapsed on average 10 years earlier than those with low expression of these proteins, and 5.5–7 years earlier if their tumors expressed both S1P3 and ERK-1/2. This is a synergistic effect and is simply not due to adding the detrimental effect of both these pathways together. However, it appears that it is only necessary to consider SK1 or the receptors as including multiple members of the SK1 pathway does not strength this observation. In summary, the observations from this study provide strong support for a model in which S1P1/3 and SK1 and ERK-1/2 interact to influence ER-positive breast cancer cell behavior and disruption of this pathway may provide a target for treatment of tamoxifen-resistant breast cancer.
Footnotes
Address reprint requests to Joanne Edwards, Ph.D., Section of Surgery, Division of Cancer Studies and Molecular Pathology, Faculty of Medicine, University of Glasgow, Glasgow G31 2ER, U.K. E-mail: je10b@clinmed.gla.ac.uk.
Supported by Glasgow Royal Infirmary endowment fund and Think Pink (J.E.) and Cancer Research UK grant (C23158/A7536; to S.P. and N.J.P.).
References
- Hall JM, Couse JF, Korach KS. The multifaceted mechanisms of estradiol and estrogen receptor signaling. J Biol Chem. 2001;276:36869–36872. doi: 10.1074/jbc.R100029200. [DOI] [PubMed] [Google Scholar]
- Pancholi S, Lykkesfeldt AE, Hilmi C, Banerjee S, Leary A, Drury S, Johnston S, Dowsett M, Martine LA. ERBB2 influences the subcellular localization of the estrogen receptor in tamoxifen-resistant MCF-7 cells leading to the activation of AKT and RPS6KA2. Endocr Relat Cancer. 2008;15:985–1002. doi: 10.1677/ERC-07-0240. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Angulo AM, Morales-Vasquez F, Hortobagyi GN. Overview of resistance to systemic therapy in patients with breast cancer. Adv Exp Med Biol. 2007;608:1–22. doi: 10.1007/978-0-387-74039-3_1. [DOI] [PubMed] [Google Scholar]
- Osborne CK, Shou J, Massarweh S, Schiff R. Crosstalk between estrogen receptor and growth factor receptor pathways as a cause for endocrine therapy resistance in breast cancer. Clin Cancer Res. 2005;11(2 Pt 2):865s–870s. [PubMed] [Google Scholar]
- Pyne NJ, Pyne S. Sphingosine 1-phosphate and cancer. Nat Rev Cancer. 2010;10:489–503. doi: 10.1038/nrc2875. [DOI] [PubMed] [Google Scholar]
- Ruckhaberle E, Rody A, Engels K, Gaetje R, von Minckwitz G, Schiffmann S, Grösch S, Geisslinger G, Holtrich U, Karn T, Kaufmann M. Microarray analysis of altered sphingolipid metabolism reveals prognostic significance of sphingosine kinase 1 in breast cancer. Breast Cancer Res Treat. 2008;112:41–52. doi: 10.1007/s10549-007-9836-9. [DOI] [PubMed] [Google Scholar]
- Long JS, Edwards J, Watson C, Tovey S, Mair K, Schiff R, Natarajan V, Pyne NJ, Pyne S. Sphingosine kinase 1 induces tolerance to human epidermal growth factor receptor 2 and prevents formation of a migratory phenotype in response to sphingosine 1-phosphate in estrogen receptor positive breast cancer cells. Mol Cell Biol. 2010;30:3827–3841. doi: 10.1128/MCB.01133-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukocheva OA, Wang L, Albanese N, Pitson SM, Vadas MA, Xia P. Sphingosine kinase transmits estrogen signaling in human breast cancer cells. Mol Endocrinol. 2003;17:2002–2012. doi: 10.1210/me.2003-0119. [DOI] [PubMed] [Google Scholar]
- Sukocheva O, Wadham C, Xia P. Role of sphingolipids in the cytoplasmic signaling of estrogens. Steroids. 2009;74:562–567. doi: 10.1016/j.steroids.2008.12.014. [DOI] [PubMed] [Google Scholar]
- Sukocheva O, Wadham C, Holmes A, Albanese N, Verrier E, Feng F, Bernal A, Derian CK, Ullrich A, Vadas MA, Xia P. Estrogen transactivates EGFR via the sphingosine 1-phosphate receptor Edg-3, the role of sphingosine kinase-1. J Cell Biol. 2006;173:301–310. doi: 10.1083/jcb.200506033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun J, Goetzl EJ, Hla T, Igarashi Y, Lynch KR, Moolenaar W, Pyne S, Tigyi G. International Union of Pharmacology: lysolipid receptor nomenclature. Pharmacol Rev. 2002;54:265–269. doi: 10.1124/pr.54.2.265. [DOI] [PubMed] [Google Scholar]
- McGlynn LM, Kirkegaard T, Edwards J, Tovey S, Cameron D, Twelves C, Bartlett JM, Cooke TG. Ras/Raf-1/MAPK pathway mediates response to tamoxifen but not chemotherapy in breast cancer patients. Clin Cancer Res. 2009;15:1487–1495. doi: 10.1158/1078-0432.CCR-07-4967. [DOI] [PubMed] [Google Scholar]
- Alderton F, Rakhit S, Kong KC, Palmer T, Sambi B, Pyne S, Pyne NJ. Tethering of the platelet-derived growth factor beta receptor to G protein-coupled receptors: a novel platform for integrative signalling by these receptor classes in mammalian cells. J Biol Chem. 2001;276:28578–28585. doi: 10.1074/jbc.M102771200. [DOI] [PubMed] [Google Scholar]
- Tovey SM, Witton CJ, Bartlett JM, Stanton PD, Reeves JR, Cooke TG. Outcome and human epidermal growth factor receptor (HER) 1–4 status in invasive breast carcinomas with proliferation indices evaluated by bromodeoxyuridine labelling. Breast Cancer Res. 2004;6:R246–R51. doi: 10.1186/bcr783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkegaard T, Edwards J, Tovey S, McGlynn LM, Krishna SN, Mukherjee R, Tam L, Munro AF, Dunne B, Bartlett JM. Observer variation in immunohistochemical analysis of protein expression, time for a change? Histopathology. 2006;48:787–94. doi: 10.1111/j.1365-2559.2006.02412.x. [DOI] [PubMed] [Google Scholar]
- Sukocheva O, Wang L, Verrier E, Vadas MA, Xia P. Restoring endocrine response in breast cancer cells by inhibition of the sphingosine kinase-1 signaling pathway. Endocrinology. 2009;150:4484–4492. doi: 10.1210/en.2009-0391. [DOI] [PubMed] [Google Scholar]
- Inagaki Y, Li PY, Wada A, Mitsutake S, Igarashi Y. Identification of functional nuclear export sequences in human sphingosine kinase 1. Biochem Biophys Res Comm. 2003;311:168–173. doi: 10.1016/j.bbrc.2003.09.194. [DOI] [PubMed] [Google Scholar]
- Pyne NJ, Pyne S. Sphingosine 1-phosphate, lysophosphatidic acid and growth factor signaling and termination. Biochim Biophys Acta. 2008;1781:467–476. doi: 10.1016/j.bbalip.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Takuwa Y, Takuwa N, Sugimoto N. The Edg family G protein-coupled receptors for lysophospholipids: their signaling properties and biological activities. J Biochem. 2002;131:767–771. doi: 10.1093/oxfordjournals.jbchem.a003163. [DOI] [PubMed] [Google Scholar]
- Yamashita H, Kitayama J, Shida D, Yamaguchi H, Mori K, Osada M, Aoki S, Yatomi Y, Takuwa Y, Nagawa H. Sphingosine 1-phosphate receptor expression profile in human gastric cancer cells, differential regulation on the migration and proliferation. J Surg Res. 2006;130:80–87. doi: 10.1016/j.jss.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Sukocheva OA, Yang Y, Gierthy JF. Estrogen and progesterone interactive effects in postconfluent MCF-7 cell culture. Steroids. 2009;74:410–418. doi: 10.1016/j.steroids.2008.12.010. [DOI] [PubMed] [Google Scholar]
- Hu X, Stern HM, Ge L, O'Brien C, Haydu L, Honchell CD, Haverty PM, Peters BA, Wu TD, Amler LC, Chant J, Stokoe D, Lackner MR, Cavet G. Genetic alterations and oncogenic pathways associated with breast cancer subtypes. Mol Cancer Res. 2009;7:511–522. doi: 10.1158/1541-7786.MCR-08-0107. [DOI] [PubMed] [Google Scholar]
- Liedtke C, Broglio K, Moulder S, Hsu L, Kau SW, Symmans WF, Albarracin C, Meric-Bernstam F, Woodward W, Theriault RL, Kiesel L, Hortobagyi GN, Pusztai L, Gonzalez-Angulo AM. Prognostic impact of discordance between triple-receptor measurements in primary and recurrent breast cancer. Ann Oncol. 2009;20:1953–1958. doi: 10.1093/annonc/mdp263. [DOI] [PMC free article] [PubMed] [Google Scholar]