Abstract
The aim of the present study was to investigate whether biomarkers improve the prediction of recurrence-free, disease-specific, and overall survival in patients with clinically localized prostate cancer. A tissue microarray was constructed from prostate specimens of 278 patients who underwent open radical retropubic prostatectomy for clinically localized prostate cancer. For immunohistochemical studies, antibodies were used against matrix metalloproteinase (MMP)−2, MMP-3, MMP-7, MMP-9, MMP-13, and MMP-19, as well as against vascular endothelial growth factor, hypoxia-induced factor 1α, basic fibroblast growth factor, and cluster of differentiation 31. Univariate and multivariable analyses were performed to evaluate the potential predictors of overall, disease-specific, and recurrence-free survival. In univariate analysis of patients with clinically organ-confined prostate cancer, only higher expression levels of MMP-9 (hazard ratio [0.6], 95% CI 0.45−0.8) had a protective effect in terms of overall survival. This positive effect of high MMP-9 expression was also observed for recurrence-free (HR 0.88, 95% CI 0.78−0.99) and disease-specific survival (HR 0.5, 95% CI 0.36−0.73). In multivariable analysis, none of these potential markers was found to be an independent prognostic factor of survival. Of all MMPs and angiogenic factors tested, MMP-9 expression has the potential as a prognostic marker in patients undergoing radical prostatectomy for clinically organ-confined cases of prostate cancer.
Prostate cancer (PC) is one of the leading causes of cancer death in men and represents a major burden for any health care system.1 Radical prostatectomy is an accepted treatment option for patients with organ-confined disease and a life expectancy of >10 years. Radical prostatectomy has been shown to reduce overall and disease-specific mortality, and the risks of metastasis and local progression.2 Although radical prostatectomy is one of the most common major surgical procedures in many Western countries, it remains unclear who would most likely benefit from this operation.
Prostate cancer progression depends on matrix metalloproteinases (MMPs) and angiogenic factors3.4 Screening has been shown to decrease mortality, but with a high risk of overdetection and overtreatment.5 Therefore there is a dire need for new prognostic markers. Matrix metalloproteinases are involved in many physiological and pathological processes. In cancer, MMPs play an important role by extracellular remodeling in tumor progression and metastases and have gained in interest.6 MMPs are also important for tumor angiogenesis, which is a key factor for tumor cell survival. Thus, we investigated selected MMPs (MMP-2, −3, −7, −9, −13, −19) and angiogenic factors (vascular endothelial growth factor [VEGF], hypoxia-induced factor 1α [HIF 1α], basic fibroblast growth factor[bFGF ]) as well as the intratumoral microvessel density (IMD) in prostate cancer as predictors for survival and recurrence in men with clinically organ confined prostate cancer using a tumor tissue micro array (TMA) derived from patients undergoing bilateral pelvic lymphadenectomy and radical retropubic prostatectomy (RRP).
Materials and Methods
Patients
All patients underwent open RRP with extended pelvic lymph node dissection as described previously7 at the Department of Urology, University of Bern, Bern, Switzerland between 1990 and 1999. All patients (n = 278) were clinically and radiologically staged before surgery as cT1-2 cN0 cM0, had at least 12 months of clinical follow-up and contributed representative tumor tissue in sufficient quality and quantity for sequential and repetitive immunohistochemical analysis. No patient received neoadjuvant therapy. Lymph node status data were available for 261 of the 278 patients analyzed. Follow up with prostate-specific antigen (PSA) measurement was performed initially every 3 months for 12 months and then in yearly intervals by the general practitioner and family doctor. In case of elevated PSA, the patient was referred for re-staging and evaluation. Patients without events (ie, still alive, no recurrence) were censored at the last known follow up.
Pathological Processing of Specimen
The external surface of the prostate was marked with ink and the specimen fixed in neutral buffered formalin (4%) overnight. Weight and dimensions of the specimen were recorded. Apex and base were transversally removed and these slices cut parasagittally and completely embedded as perpendicular margins. The junction of the prostate and the seminal vesicles as well as the resection margins of the vasa deferentia were resected. The prostate was sliced serially in 3- to 4-mm thick sections perpendicular to the longitudinal axis and totally embedded. The cut surfaces were evaluated macroscopically for tumor localization within the lobes and tumor extension (seminal vesicles, extraprostatic extension). In addition, non-neoplastic conditions (eg, nodular hyperplasia) were described. The following microscopic tumor characteristics were noted: type, Gleason score, extraprostatic extension, and percentage of prostate tissue area on the sections occupied by the tumor. Tumor volume was estimated by multiplying the percentage of the specimen involved by cancer by the prostate volume. Other pathological conditions were also assessed (eg, vascular invasion, prostatic intraepithelial neoplasia, hyperplasia, inflammation).
Pathological Evaluation
Radical prostatectomy specimens were staged and graded by an experienced urological pathologist (R.M.) who selected the slide with the least differentiated tumor area of the primary and secondary Gleason pattern in the pathological specimen according to the initial Gleason scoring system and marked the most appropriate and representative tumor target area on this slide for subsequent tissue core retrieval.
TMA Construction
Donor tissue blocks of 278 patients with clinically and radiologically staged cT1-2 cN0 cM0 prostate cancer which had at least 12 months of clinical follow-up, representative tumor tissue in sufficient quality and quantity were available for TMA construction. A prostate TMA was constructed from formalin-fixed paraffin-embedded tissue specimens as described previously.8 Briefly, one core tissue-biopsy (diameter 0.6 mm) was taken from the marked region of individual paraffin-embedded prostate tumors (donor blocks) and precisely arrayed into a new recipient paraffin block (35 mm × 20 mm) with a custom built precision instrument (Beecher Instruments, Silver Springs, MD). Three TMAs containing the identical set of tumors were constructed. After the block construction was completed, 2- to 3-μm sections were cut with a microtome and the presence of tumor tissue on the arrayed samples was verified on a H&E-stained section.
Immunohistochemistry
Paraffin sections (2- to 3-μm) were deparaffinized in three changes of xylol, rehydrated in ethanol, and rinsed twice in Tris-buffered saline [TBS; 50 mmol/L Tris/HCl (pH 7.4) containing 100 mmol/L sodium chloride]. Endogenous peroxidase activity was suppressed by treatment with 0.3% hydrogen peroxide for 10 minutes. Before incubation with antibodies, sections were bathed in 0.01 mol/L sodium citrate (pH 6.0) and/or heated in a microwave oven (180 W) for 15 minutes. In the case of mouse anti- cluster of differentiation 31 [CD31], this step is preceded by treatment with trypsin ([Difco Lab., Detroit, MI) 0.2 mg/ml in TBS/CaCl2 buffer] for 10 minutes at 37°C. After the blockage of nonspecific binding by immersion in TBS containing 1% casein (Sigma 8654) for 10 minutes, sections are incubated with the first antibody diluted in TBS: mouse anti-CD31, 1:20 (JC/70A, M-0823; Dako, Glostrup, Denmark); rabbit anti-VEGF, 1:200 (sc-152; Santa Cruz Biotechnology, Santa Cruz, CA); mouse anti-bFGF, 1: 50 (05–118; Upstate Biotechnology, Inc.Lake Placid NY 12946); mouse anti-MMP-2, -MMP-3, -MMP-9, and MMP-13 (ready for use: MS806-R7, MS810-R7 and MS820-R7; MS826-R7 Oncogene, San Diego, USA); mouse anti-MMP-7, 1:100 (PC 492; Oncogene San Diego); and rabbit anti-MMP-19 (kindly provided by Dr. R. Sedlacek), 1:100 as determined by antibody titration. Sections were exposed to an affinity-purified biotinylated second antibody [anti-mouse EO 433, anti-rabbit EO 353, Dako; Glostrup, Denmark diluted 1:200 in TBS)] for 45 minutes at ambient temperature, washed three times in TBS, then treated with the avidin-biotin-horseradish peroxidase complex (P355, Dako, Glostrup, Denmark) for a similar period at the same temperature. The reaction product is visualized by exposing sections to 3-amino-9-ethylcarbazole or 3.3-diaminobenzidine (Sigma Chemicals Company, St. Louis, Missouri) for 1 to 12 minutes. These were then counterstained with hematoxylin and mounted in Aquatex (Merck, Darmstadt, Germany). Negative controls were prepared using non-specific mouse and rabbit sera. For HIF 1α, the sections were deparaffinized in xylene (three changes), rehydrated in a graded series of decreasing ethanol concentration, and rinsed in TBS [50 mmol/L Tris/HCl (pH 7.4) containing 100 mmol/L sodium chloride]. Immunostaining was performed according to the Catalyzed Signal Amplification System (Dako, Carpinteria, CA), which uses a streptavidin-biotin-horseradish peroxidase complex. The slides were initially immersed in target retrieval solution (Dako) at 97°C for 15 minutes and then treated in accordance with the manufacturer’s instructions. They were exposed to a monoclonal antibody against HIF 1α (H1a67, used at a dilution of 1:10,000) for 30 minutes. The biotinyl tyramide amplification reagent is diluted 1:10 in protein blocking solution (Dako). The reaction product was visualized by exposing sections to 3,3-diaminobenzidine for 1 minute. Nuclei were lightly counterstained with hematoxylin. Sections were mounted in Aquatex (Merck, Darmstadt, Germany). All samples have been collected and fixed, and processed in the same way according to a standard procedure at the Insel hospital Bern. For quality control tumor material from previous studies was used for positive and negative controls.
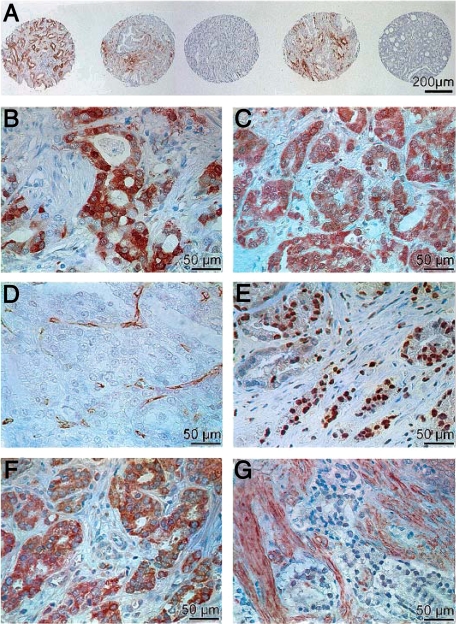
Tissue samples incubated with non-immune serum or with the antibody diluent (Dako) served as negative controls. Each core was evaluated for presence of tumor at analysis for immunohistochemical staining. Representative pictures of immunohistochemical staining of MMP-2 (a,b), MMP-9 (c), CD 31(d), HIF-1α (e), VEGF (f), and bFGF (g) are shown in Figure 1.
Figure 1.
Representative pictures of immunohistochemical staining of MMP-2 (A and B), MMP-9 (C), CD 31 (D), HIF-1α (E), VEGF (F), and bFGF (G).
Quantification
Quantification of MMPs, VEGF, HIF 1α, bFGF, and CD 31 staining was evaluated at a magnification of ×200 by two independent investigators (S.B., V.D.) blinded to the clinical data. For MMPs, VEGF, and HIF the intensity and the percentage of positive cells per area of 0.28 mm2 of the reaction was categorized into 5 groups (0 to 4) using the surrounding tissue as a base-level reference. For bFGF the intensity of the reaction of tumor and stromal cells was categorized into fve groups based on the literature. Categorization was as follows: Intensity: 0, no detectable immunoreactivity; 1, weak staining; 2, moderate staining; 3, moderate to intense staining; 4, very intense staining. Extent: 0, no detectable immunoreactivity; 1, <1% of cells registering positive; 2, 1 to 10% of cells positive; 3, 10 to 50% of cells positive; 4, >50% of cells positive. In addition, IMD was assessed by counting all of the CD 31 positive cells of the 0.28 mm2 area at a magnification of ×200. Any endothelial cell or endothelial cell cluster positive for CD31 and clearly separate from an adjacent cluster was considered as a single countable microvessel. IMD was categorized into four groups (1, IMD 0–9; 2, IMD 10–19; 3, IMD 20–39; 4, IMD >40). In case of a difference of ≥1 in evaluation of quantification the staining was re-evalutated by the two investigators.
Statistical Analysis
The dataset contained a small percentage of missing values in some of the potential predictor variables. To compensate for missing values we applied a single imputation method to keep the number of observations as large as possible.9 Variables were only imputed on micro array data and on angiogenetic factors, where “missingness” may be assumed to be at random. Kaplan-Meier plots, univariate and multivariable analysis applying Cox proportional hazard models were used to evaluate potential predictors for overall mortality (death due to any cause), disease-specific mortality (death due to prostate cancer), and recurrence in patients with clinically localized prostate cancer who underwent open RRP. Recurrence of prostate cancer was defined as a persistently detectable serum PSA (>0.1 μg/L) after radical prostatectomy, an increasing serum PSA in three serial determinations following a postoperative PSA nadir value in the case that PSA did not reach zero after surgery or histologically proven tumor recurrence. PSA did not return to zero postoperatively in 20 patients.
We performed a post hoc power calculation for the hazard ratio (HR) of overall survival depending on MMP-9 intensities. We assumed an effect size of 0.6; this is the estimated coefficient (univariately) of MMP-9 intensity for overall mortality. For the power calculation in Cox proportional hazards settings, we additionally needed to specify the SD of MMP-9 intensity (= 0.92), the percentage of failures (here P = 0.24), and a measure of correlation with co-variates (= 0.8). We found a power of 40%, which is acceptable in this kind of study setting.10
The selection of variables for the multiple model was based on the univariate comparisons of these variables (significant on a 5% level in pairwise comparisons and additionally adjusted for age) with overall mortality, disease specific mortality and recurrence. In these Cox proportional hazard models, the % staining and intensity entered in linear fashion. We repeated the analysis entering the variates for MMP-9% and intensity using indicator variates (dummies). This alternative approach showed similar results. Statistical analyses were performed using the Stata 9.2 statistics software package (StataCorp LP, College Station, TX) and R 2.10 (R Foundation for Statistical Computing; Vienna, Austria, 2009, http://www.R-project.org, last accessed on November 20, 2009).
Results
At surgery, median age of the 278 patients was 64 years (interquartile range [IQR] 59 to 68) and median follow up was 7.8 years (IQR = 6 to 10.3). The majority of tumors were pT2 (52%) or pT3 (45%), 28% had positive lymph nodes and more than 50% a Gleason score 5 to 7 (table 1).
Table 1.
Characteristics of the 278 Patients with Clinically Localized Prostate Cancer who Underwent Open Radical Retropubic Prostatectomy
| Median age in years (IQR) | 64 (59 to 68) |
| Median follow-up in years (IQR) | 7.8 (6 to 10.3) |
| Median preoperative PSA in μg/L (IQR)† | 12.3 (7.1 to 22) |
| Pathological tumor stage | |
| pT2 | 145 (52%) |
| pT3 | 126 (45%) |
| pT4* | 7 (3%) |
| Pathological lymph node status† | |
| pN0 | 189 (72%) |
| pN+ | 72 (28%) |
| Gleason score† | |
| 2 to 4 | 74 (27%) |
| 5 to 7 | 158 (57%) |
| 8 to 10 | 44 (16%) |
IQR: interquartile range.
Bladder neck invasion only.
n = 276 for preoperative PSA, n = 261 for pathological lymph node status, n = 276 for Gleason score.
In our cohort preoperative understaging occurred in 133 cases (48%). At the time of censoring 210 patients were alive, 228 patients had no event with regard to disease-specific mortality, and 87 patients were without biochemical recurrence.
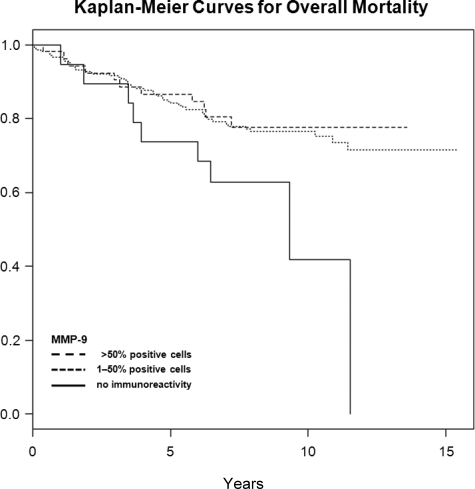
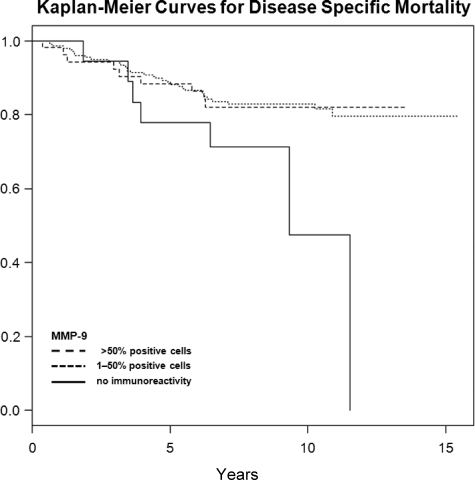
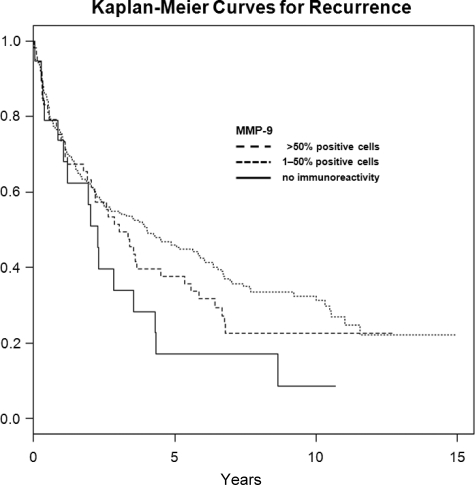
In univariate analysis (table 2), pathological tumor stage, pathological lymph node status, tumor grade, and Gleason score were significantly associated with overall, disease-specific, and recurrence-free survival after open RRP. Of the angiogenic and proteolytic factors, MMP-9 was also significantly associated with overall, disease-specific, and recurrence-free survival: No cells staining positive for MMP-9 was associated with lower overall (intensity 0, extent 0), disease-specific (intensity 0, extent 0), and recurrence-free (extent 0) survival (Figures 2–4). The other factors evaluated did not show a statistically significant association with neither survival nor recurrence (Table 2).
Table 2.
Univariate Analysis Evaluating Potential Predictors for Mortality in the 278 Patients with Clinically Localized Prostate Cancer who Underwent Open RRP
| Variables | No. | Overall mortality
|
Disease-specific mortality
|
Recurrence
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | ||
| Age* | 278 | 1.01 | (0.967; 1.05) | 0.74 | 0.98 | (0.934; 1.02) | 0.31 | 1.03 | (1.01; 1.05) | 0.01 |
| Pathological tumor stage | 278 | 3.48 | (2.28; 5.3) | <0.001 | 4.52 | (2.75; 7.4) | <0.001 | 1.96 | (1.51; 2.55) | <0.001 |
| Pathological lymph node status | 261 | 3.58 | (2.17; 5.91) | <0.001 | 5.48 | (2.96; 10.2) | <0.001 | 2.42 | (1.77; 3.32) | <0.001 |
| Tumor grade | 263 | 2.63 | (1.71; 4.05) | <0.001 | 3.21 | (1.92; 5.36) | <0.001 | 1.75 | (1.37; 2.23) | <0.001 |
| Gleason score† | 276 | 2.77 | (1.88; 4.08) | <0.001 | 3.3 | (2.09; 5.23) | <0.001 | 1.66 | (1.34; 2.06) | <0.001 |
| MMP-2% | 278 | 1.05 | (0.871; 1.26) | 0.63 | 1.08 | (0.87; 1.35) | 0.47 | 1.08 | (0.968; 1.20) | 0.17 |
| MMP-2 intensity | 278 | 1.03 | (0.796; 1.32) | 0.84 | 0.947 | (0.703; 1.27) | 0.72 | 1.03 | (0.89; 1.20) | 0.69 |
| MMP-3% | 278 | 0.917 | (0.725; 1.16) | 0.47 | 0.858 | (0.637; 1.16) | 0.31 | 0.953 | (0.836; 1.09) | 0.47 |
| MMP-3 intensity | 278 | 0.891 | (0.588; 1.35) | 0.59 | 0.756 | (0.43; 1.33) | 0.33 | 0.995 | (0.79; 1.25) | 0.96 |
| MMP-7% | 278 | 0.893 | (0.773; 1.03) | 0.13 | 0.87 | (0.738; 1.03) | 0.1 | 0.933 | (0.85; 1.02) | 0.15 |
| MMP-7 intensity | 278 | 1.01 | (0.695; 1.46) | 0.97 | 1.08 | (0.704; 1.64) | 0.73 | 0.993 | (0.775; 1.27) | 0.96 |
| MMP-9% | 278 | 0.832 | (0.693; 0.99) | 0.048 | 0.813 | (0.661; 0.999) | 0.049 | 0.875 | (0.775; 0.99) | 0.03 |
| MMP-9 intensity | 278 | 0.602 | (0.452; 0.8) | <0.001 | 0.511 | (0.359; 0.727) | <0.001 | 0.874 | (0.746; 1.02) | 0.096 |
| MMP-13% | 278 | 0.922 | (0.735; 1.16) | 0.48 | 0.925 | (0.71; 1.21) | 0.56 | 0.948 | (0.821;1.10) | 0.47 |
| MMP-13 intensity | 278 | 0.814 | (0.55; 1.21) | 0.31 | 0.765 | (0.481; 1.22) | 0.26 | 0.833 | (0.663; 1.05) | 0.12 |
| MMP-19% | 278 | 0.997 | (0.868; 0.15) | 0.97 | 0.983 | (0.835; 1.16) | 0.84 | 0.948 | (0.87; 1.03) | 0.22 |
| MMP-19 intensity | 278 | 0.727 | (0.476; 1.11) | 0.14 | 0.794 | (0.486; 1.30) | 0.36 | 0.83 | (0.649; 1.06) | 0.14 |
| CD 31 count | 278 | 1.19 | (0.965; 1.47) | 0.1 | 1.24 | (0.97; 1.58) | 0.088 | 1.03 | (0.903; 1.17) | 0.69 |
| VEGF % | 278 | 1.11 | (0.934; 1.33) | 0.23 | 1.05 | (0.864; 1.28) | 0.61 | 1.05 | (0.944; 1.16) | 0.38 |
| VEGF intensity | 278 | 1.24 | (0.954; 1.61) | 0.11 | 1.09 | (0.794; 1.49) | 0.6 | 1.08 | (0.917; 1.27) | 0.36 |
| BFGF tumor | 278 | 0.952 | (0.679; 1.34) | 0.78 | 0.918 | (0.614; 1.37) | 0.67 | 0.93 | (0.755; 1.15) | 0.5 |
| BFGF stroma | 278 | 0.952 | (0.673; 1.35) | 0.78 | 1.24 | (0.851; 1.81) | 0.26 | 1.01 | (0.816; 1.25) | 0.93 |
| HIF 1α % | 278 | 0.992 | (0.84; 1.17) | 0.92 | 1.01 | (0.828; 1.22) | 0.95 | 1.04 | (0.942; 1.15) | 0.42 |
| HIF 1α intensity | 278 | 1.01 | (0.72; 1.46) | 0.95 | 1.07 | (0.699; 1.63) | 0.77 | 1.11 | (0.886; 1.39) | 0.37 |
HR: hazard ratio.
95% CI: confidence interval.
Age: per additional year.
Gleason score: low- (2 to 4), intermediate- (5 to 7), high- (8 to 10) risk disease.
Bold indicates P < 0.05, significant.
Figure 2.
Kaplan-Meier curves for overall mortality in categories of MMP-9 (%), censored times unmarked.
Figure 3.
Kaplan-Meier curves for disease specific mortality in categories of MMP-9 (%), censored times unmarked.
Figure 4.
Kaplan-Meier curves for recurrence in categories of MMP-9 (%), censored times unmarked.
In multivariable analysis (Table 3), as expected pathological tumor stage was the most important predictor for overall, disease-specific and recurrence-free survival as was pathological lymph node status for disease-specific survival and recurrence-free survival. Age was significantly associated with recurrence-free survival. All other factors were not statistically significant predictors in multivariable analysis.
Table 3.
Multivariable Analysis of 259 Patients with Nonmissing Values in Any of the Co-Variates using Cox Proportional Hazards Regression Including All Significant Predictors of Univariate Analysis
| Variables | Overall mortality
|
Disease-specific mortality
|
Recurrence
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | |
| Age | 1.016 | (0.971; 1.06) | 1.490 | 0.981 | (0.930; 1.03) | 0.480 | 1.03 | (1.003; 1.05) | 0.031 |
| Pathological tumor stage | 2.377 | (1.464; 3.86) | <0.001 | 2.905 | (1.602; 5.27) | <0.001 | 1.65 | (1.228; 2.22) | 0.001 |
| Pathological lymph node status | 1.657 | (0.942; 2.92) | 0.080 | 2.377 | (1.192; 4.74) | 0.014 | 1.75 | (1.219; 2.5) | 0.002 |
| Tumor grade | 1.512 | (0.870; 2.63) | 0.140 | 1.690 | (0.857; 3.33) | 0.130 | 1.29 | (0.959; 1.74) | 0.09 |
| Gleason score | 1.577 | (0.929; 2.65) | 0.085 | 1.526 | (0.806; 2.89) | 0.190 | 1.15 | (0.875; 1.51) | 0.317 |
| MMP-9 intensity | 1.793 | (0.599; 1.05) | 0.100 | 0.764 | (0.542; 1.08) | 0.120 | 0.995 | (0.846; 1.17) | 0.95 |
HR: hazard ratio.
95% CI: confidence interval.
The factors were additionally adjusted for age.
Bold indicates P < 0.05, significant.
The morphological distribution of expression of the factors was as follows. The MMPs were mainly expressed by the tumor cells with staining of the cytoplasm. Neither strong nuclear nor stromal staining was observed. Rarely stained stromal cells were not taken into account. HIF 1α on the other hand, was mainly expressed nuclearly. As mentioned before for bFGF, the staining of the stroma and the tumor cells was evaluated separately. Both compartments showed different levels of staining with no correlation.
Discussion
In PC patients current predictors of disease extent and outcome after open RRP are tumor stage, Gleason score, and serum PSA levels at diagnosis. Yet, when making the decision for (adjuvant) treatment for organ-confined or locally advanced disease biological behavior of the tumor is often difficult to estimate accurately. Hence also the discussion about the significance of a tertiary Gleason grade and the reported difference between the score groups 3 + 4 and 4 + 3.11 The literature reports 24%12 up to 60% of cases clinically classified as T2 to be understaged, showing extracapsular disease in pathological evaluation after RRP.13,14,15,16,17,18 In our cohort preoperative understaging occurred in 48% of the cases. Therefore it is crucial to establish and compare new markers for tumor behavior and patient outcome.
In the present study, expression of the gelatinase MMP-9 improved overall, disease-specific, and recurrence free survival. This stands in contrast to other studies, showing an association of MMP-9 expression with tumor progression in PC19,20,21 and in other malignancies.22 MMP-9 has been discussed as a major contributor in the tumor induced angiogenic switch essential for tumor survival and progression.23 In addition, down-regulation of MMP-9 in a prostate carcinoma xenograft model decreased tumor growth and caused tumor regression.24 However, to the best of our knowledge no study investigated the prognostic value of MMP-9 expression in PC in such a large population using TMAs. Our findings are in line with the study of Pellikainen et al25 reporting that in breast carcinoma a high expression of MMP-9 in tumor cells favored survival and with a immunohistochemical study of Vasala et al26 who found a high MMP-9 expression in bladder cancer to be favorable looking at overall and disease-free survival.
MMP-9 has been reported to have an anti-angiogenic role through the generation of endogenous angiogenesis inhibitors and by modulating cell receptor signaling by cleaving off their ligand-binding domains.27 In the knockout mouse model, the protective role of host MMP-9 appears to be mediated by involving natural angiogenic inhibitors such as angiostatin and tumstatin.28,29 MMPs, and especially MMP-9, appear to be part of a complex balance of angiogenesis rather than being exclusively pro- or anti-angiogenic. Similar mechanisms may be involved in PC supporting the protective effect of MMP-9 in the present study. Using enzyme-linked immunosorbent assay and zymography Castallano and colleagues30 demonstrated that MMP-9 and osteopontin31 plasma levels are elevated in patients with prostate cancer. These levels normalize after radical surgery.
With regard to MMPs and PC there is broad evidence in the literature for MMP-2 to play an important role in the pathogenesis of prostate cancer.32 It has been shown that MMP-2 expression is correlated with disease stage and the Gleason score.33,34,35 Wood et al36 reported MMP-2 to be a possible independent prognostic factor using in situ hybridization to quantify expression. The mRNA processing could explain possible differences to our findings. Furthermore positive staining of the stromal compartment was observed and counted, which was not the case in our study.
The rationale for testing MMP-3, −7, −13, and −19 were the complexity and interaction of different MMPs in networks. The role of MMP-3 and MMP-19 expression in PC has to our knowledge not been investigated. Besides digesting ECM components, the stromelysin 1 MMP-3 activates a number of proMMPs such as proMMP-1. In transgenic mice MMP-3 is a promoter of mammary carcinogenesis37,38 and depletion of MMP-3 protective in skin carcinogenesis39 in knockout mice, again suggestive of a more complex function of MMPs in tumor progression. The role of MMP-19 has not yet been clarified.40 In breast carcinomas, Djonov et al41,42 found that higher expression of MMP-19 could be beneficiary whereas in oropharyngeal squamous cell carcinoma no correlation existed between MMP-19 expression and clinicopathological features or a patient’s outcome and prognosis.
MMP-7 expression has been linked to prostate cancer pathological stage and incidence of metastasis.43 In our collective, no significant correlation with pathological stage or survival was observed. MMP 13 is a collagenase,40 which is expressed at a higher level in more invasive prostate cancer cell lines.44 In our study we found a potential interaction for disease specific mortality with high MMP-9 and MMP-13 intensity.
In contrast to our findings others have found that angiogenic factors may be prognostic in PC. Increased expression of HIF-1α protein has been shown in multiple types of human cancer and in regional and distant metastases including lung, breast, colon, and prostate carcinoma.45 Up-regulation of HIF-1α observed in high-grade prostate intraepithelial neoplasia lesions suggests this to be an early event in the development of prostate cancer, which might explain why we did not find a correlation in our study.46 Gravdal et al47 found in patients with localized cancers that high vascular proliferation was significantly related to adverse clinicopathologic features and was a strong and independent predictor of biochemical failure, clinical recurrence, and skeletal metastasis in multivariate analysis. In our larger series including factors such as tumor stage, Gleason score and lymph node status this could not be confirmed.
Mazzucchelli et al48 reported that the expression of VEGF is significantly increased in PC and correlates with Gleason score. In their study the specimens of 35 patients were compared to normal sections. Strong immunohistochemical staining was observed at the periphery of tumor nodules. In our tissue microarray this area might not always be included which could explain this difference to our findings. The discussion on the prognostic value of VEGF is still open as there have been other contradictory results in clinical specimens.49,50 In the same line the role and expression pattern of bFGF in the development and progression of PC in the literature is controversial. Cronauer et al51 reported that bFGF is mainly expressed in carcinomatous areas whereas Trojan et al52 found a significantly stronger expression in non-tumerous tissue compared with carcinoma. Another study found an association between strong stromal bFGF expression with well-differentiated tumors, low preoperative PSA and low tumor proliferation.53 Strohmeyer et al54 observed higher bFGF expression in the tumor tissue with increased tumor stage.
In our study we evaluated the stromal and the tumor epithelial expression of bFGF independently. Both areas showed differential expression without correlation. Furthermore neither staining of the stroma nor of the tumor showed an association with survival.
Microvessel density or IMD has been reported as an estimate of tumor-associated angiogenesis and to provide strong and independent information in multiple tumors.55 In prostate cancer, high microvessel density has been linked to tumor aggressiveness and has been correlated to histological grade, tumor stage, preoperative PSA and time to recurrence.56,57,58,59,60 However, there has been critical concern on the use of IMD as a prognostic variable. There exists a wide range of results due to the use of different antibodies (eg, CD31 or CD34) and different areas of the tumor evaluated.55 In our study no correlation with patient outcome was observed.
We consider this study to be observational, meaning that we examined a large set of potential predictors for mortality after prostatectomy. Given the large number of markers evaluated we checked for potential interaction factors with MMP-9 intensity. The only one we found was for disease specific mortality with high MMP-13 intensity (P = 0.021). We are aware of the fact that the results of such observational studies tend to be opportunistic, and need further confirmation in future studies in a more experimental setting. As to be expected in univariate and multivariable analysis established parameters such as pathological tumor stage, pathological lymph node status, tumor grade, and Gleason score were found to be predictors for overall, disease-specific and recurrence free survival after open RRP in the present study. The effect of these strong prognostic factors may have masked relevant effects of other biomarkers due to co-linearity and could explain that also MMP-9 was no longer statistically significant in multivariate analysis. Although we included more than 250 patients in our analysis, a larger sample size may be necessary to detect an influence of other factors due to the large number of biomarkers investigated.
Gleason grading has changed over time and we analyzed according to classification at time of surgery. In a study by Albertsen and colleagues,61 reclassification resulted in apparent improvement in clinical outcomes and this finding reflects a statistical artifact known as the Will Rogers phenomenon. Whether this would change the predictive potential of the markers evaluated may be doubted.
TMAs have proven to be useful tools to screen new prognostic markers as it allows high through-put-analysis. There are limitations to this approach, as there might be sampling errors or insufficient sampling. We sampled the higher or more representative Gleason grade area. Furthermore, the heterogeneity of PC may limit the interpretation or necessitate very large sample sizes.62,63
In addition analysis of co-localization of different markers might be possible, however, current statistical tools are limited in their ability to dissect out co-localization of markers relevant for prognosis. Nevertheless, in the light of recent findings from the ERSPC screening study new prognostic markers are dearly needed to distinguish between tumors that need aggressive treatment from those that do not.5
In addition to classical histopathological factors such as tumor stage, lymph node status, tumor grade and Gleason score, lack of MMP-9 expression was also significantly associated with poor survival and risk of recurrence after RRP in univariate analysis, but was not an independent prognostic factor.
Footnotes
Address reprint requests to George N. Thalmann, M.D., Professor of Urology, Department of Urology, University of Bern, 3010 Bern, Switzerland. E-mail: george.thalmann@insel.ch.
Supported by Oncosuisse grant #OCS-01752-08-2005 and Swiss National Science Foundation grant #31003A-116237.
S.B. and V.D. contributed equally.
References
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer Statistics, 2008. CA: a Cancer Journal for Clinicians. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- Aus G, Abbou CC, Bolla M, Heidenreich A, Schmid HP, van Poppel H, Wolff J, Zattoni F. EAU guidelines on prostate cancer. Eur Urol. 2005;48:546–551. doi: 10.1016/j.eururo.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- Fingleton B. Matrix metalloproteinases: roles in cancer and metastasis. Front Biosci. 2006;11:479–491. doi: 10.2741/1811. [DOI] [PubMed] [Google Scholar]
- Schroder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, Kwiatkowski M, Lujan M, Lilja H, Zappa M, Denis LJ, Recker F, Berenguer A, Maattanen L, Bangma CH, Aus G, Villers A, Rebillard X, van der Kwast T, Blijenberg BG, Moss SM, de Koning HJ, Auvinen A, Investigators E. Screening and prostate-cancer mortality in a randomized European study.[see comment]. N Engl J Med. 2009;360:1320–1328. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- Roy R, Yang J, Moses MA. Matrix metalloproteinases as novel biomarkers and potential therapeutic targets in human cancer. J Clin Oncol. 2009;27:5287–5297. doi: 10.1200/JCO.2009.23.5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler TM, Burkhard FC, Studer UE. Nerve-sparing open radical retropubic prostatectomy. Eur Urol. 2007;51:90–97. doi: 10.1016/j.eururo.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Prtilo A, Leach FS, Markwalder R, Kappeler A, Burkhard FC, Cecchini MG, Studer UE, Thalmann GN. Tissue microarray analysis of hMSH2 expression predicts outcome in men with prostate cancer. J Urol. 2005;174:1814–1818. doi: 10.1097/01.ju.0000176796.47988.64. discussion 1818. [DOI] [PubMed] [Google Scholar]
- Longfordt NT. Longfordt NT, editor. London: Springer; Single imputation and related methods. 2005:pp. 37–58. [Google Scholar]
- Hsieh FY, Lavori PW. Sample-size calculations for the Cox proportional hazards regression model with nonbinary covariates. Control Clin Trials. 2000;21:552–560. doi: 10.1016/s0197-2456(00)00104-5. [DOI] [PubMed] [Google Scholar]
- Harnden P, Shelley MD, Coles B, Staffurth J, Mason MD. Should the Gleason grading system for prostate cancer be modified to account for high-grade tertiary components? A systematic review and meta-analysis. Lancet Oncology. 2007;8:411–419. doi: 10.1016/S1470-2045(07)70136-5. [DOI] [PubMed] [Google Scholar]
- Grossfeld GD, Chang JJ, Broering JM, Li YP, Lubeck DP, Flanders SC, Carroll PR. Under staging and under grading in a contemporary series of patients undergoing radical prostatectomy: results from the Cancer of the Prostate Strategic Urologic Research Endeavor database. J Urol. 2001;165:851–856. [PubMed] [Google Scholar]
- Partin AW, Yoo J, Carter HB, Pearson JD, Chan DW, Epstein JI, Walsh PC. The use of prostate specific antigen, clinical stage and Gleason score to predict pathological stage in men with localized prostate cancer. J Urol. 1993;150:110–114. doi: 10.1016/s0022-5347(17)35410-1. [DOI] [PubMed] [Google Scholar]
- Epstein JI, Walsh PC, Carmichael M, Brendler CB. Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1c) prostate cancer. JAMA. 1994;271:368–374. [PubMed] [Google Scholar]
- Partin AW, Kattan MW, Subong EN, Walsh PC, Wojno KJ, Oesterling JE, Scardino PT, Pearson JD. Combination of prostate-specific antigen, clinical stage, and Gleason score to predict pathological stage of localized prostate cancer. A multi-institutional update. JAMA. 1997;277:1445–1451. [PubMed] [Google Scholar]
- Catalona WJ, Smith DS. 5-year tumor recurrence rates after anatomical radical retropubic prostatectomy for prostate cancer. J Urol. 1994;152:1837–1842. doi: 10.1016/s0022-5347(17)32397-2. [DOI] [PubMed] [Google Scholar]
- Zincke H, Oesterling JE, Blute ML, Bergstralh EJ, Myers RP, Barrett DM. Long-term (15 years) results after radical prostatectomy for clinically localized (stage T2c or lower) prostate cancer. J Urol. 1994;152:1850–1857. doi: 10.1016/s0022-5347(17)32399-6. [DOI] [PubMed] [Google Scholar]
- Walsh PC, Partin AW, Epstein JI. Cancer control and quality of life following anatomical radical retropubic prostatectomy: results at 10 years. J Urol. 1994;152:1831–1836. doi: 10.1016/s0022-5347(17)32396-0. [DOI] [PubMed] [Google Scholar]
- Sauer CG, Kappeler A, Spath M, Kaden JJ, Michel MS, Mayer D, Bleyl U, Grobholz R. Expression and activity of matrix metalloproteinases-2 and -9 in serum, core needle biopsies and tissue specimens of prostate cancer patients. Virchows Arch. 2004;444:518–526. doi: 10.1007/s00428-004-1016-2. [DOI] [PubMed] [Google Scholar]
- Festuccia C, Bologna M, Vicentini C, Tacconelli A, Miano R, Violini S, Mackay AR. Increased matrix metalloproteinase-9 secretion in short-term tissue cultures of prostatic tumor cells. Int J Cancer. 1996;69:386–393. doi: 10.1002/(SICI)1097-0215(19961021)69:5<386::AID-IJC6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Morgia G, Falsaperla M, Malaponte G, Madonia M, Indelicato M, Travali S, Mazzarino MC. Matrix metalloproteinases as diagnostic (MMP-13) and prognostic (MMP-2. MMP-9) markers of prostate cancer. Urol Res. 2005;33:44–50. doi: 10.1007/s00240-004-0440-8. [DOI] [PubMed] [Google Scholar]
- Zhang S, Li L, Lin JY, Lin H. Imbalance between expression of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 in invasiveness and metastasis of human gastric carcinoma. World J Gastroenterol. 2003;9:899–904. doi: 10.3748/wjg.v9.i5.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, Hanahan D. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London CA, Sekhon HS, Arora V, Stein DA, Iversen PL, Devi GR. A novel antisense inhibitor of MMP-9 attenuates angiogenesis, human prostate cancer cell invasion and tumorigenicity. Cancer Gene Ther. 2003;10:823–832. doi: 10.1038/sj.cgt.7700642. [DOI] [PubMed] [Google Scholar]
- Pellikainen JM, Ropponen KM, Kataja VV, Kellokoski JK, Eskelinen MJ, Kosma VM. Expression of matrix metalloproteinase (MMP)-2 and MMP-9 in breast cancer with a special reference to activator protein-2, HER2, and prognosis. Clin Cancer Res. 2004;10:7621–7628. doi: 10.1158/1078-0432.CCR-04-1061. [DOI] [PubMed] [Google Scholar]
- Vasala K, Paakko P, Turpeenniemi-Hujanen T. Matrix metalloproteinase-9 (MMP-9) immunoreactive protein in urinary bladder cancer: a marker of favorable prognosis. Anticancer Research. 2008;28:1757–1761. [PubMed] [Google Scholar]
- Rundhaug JE. Matrix metalloproteinases and angiogenesis. J Cell Mol Med. 2005;9:267–285. doi: 10.1111/j.1582-4934.2005.tb00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamano Y, Kalluri R. Tumstatin, the NC1 domain of alpha3 chain of type IV collagen, is an endogenous inhibitor of pathological angiogenesis and suppresses tumor growth. Biochem Biophys Res Commun. 2005;333:292–298. doi: 10.1016/j.bbrc.2005.05.130. [DOI] [PubMed] [Google Scholar]
- Cornelius LA, Nehring LC, Harding E, Bolanowski M, Welgus HG, Kobayashi DK, Pierce RA, Shapiro SD. Matrix metalloproteinases generate angiostatin: effects on neovascularization. J Immunol. 1998;161:6845–6852. [PubMed] [Google Scholar]
- Castellano G, Malaponte G, Mazzarino MC, Figini M, Marchese F, Gangemi P, Travali S, Stivala F, Canevari S, Libra M. Activation of the osteopontin/matrix metalloproteinase-9 pathway correlates with prostate cancer progression. Clinical Cancer Res. 2008;14:7470–7480. doi: 10.1158/1078-0432.CCR-08-0870. [DOI] [PubMed] [Google Scholar]
- Thalmann GN, Sikes RA, Devoll RE, Kiefer JA, Markwalder R, Klima I, Farach-Carson CM, Studer UE, Chung LW. Osteopontin: possible role in prostate cancer progression. Clinical Cancer Res. 1999;5:2271–2277. [PubMed] [Google Scholar]
- Still K, Robson CN, Autzen P, Robinson MC, Hamdy FC. Localization and quantification of mRNA for matrix metalloproteinase-2 (MMP-2) and tissue inhibitor of matrix metalloproteinase-2 (TIMP-2) in human benign and malignant prostatic tissue. Prostate. 2000;42:18–25. doi: 10.1002/(sici)1097-0045(20000101)42:1<18::aid-pros3>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Stearns ME, Stearns M. Immunohistochemical studies of activated matrix metalloproteinase-2 (MMP-2a)expression in human prostate cancer. Oncol Res. 1996;8:63–67. [PubMed] [Google Scholar]
- Kuniyasu H, Troncoso P, Johnston D, Bucana CD, Tahara E, Fidler IJ, Pettaway CA. Relative expression of type IV collagenase. E-cadherin, and vascular endothelial growth factor/vascular permeability factor in prostatectomy specimens distinguishes organ-confined from pathologically advanced prostate cancers. Clin Cancer Res. 2000;6:2295–2308. [PubMed] [Google Scholar]
- Ross JS, Kaur P, Sheehan CE, Fisher HA, Kaufman RA, Jr, Kallakury BV. Prognostic significance of matrix metalloproteinase 2 and tissue inhibitor of metalloproteinase 2 expression in prostate cancer. Mod Pathol. 2003;16:198–205. doi: 10.1097/01.MP.0000056984.62360.6C. [DOI] [PubMed] [Google Scholar]
- Wood M, Fudge K, Mohler JL, Frost AR, Garcia F, Wang M, Stearns ME. In situ hybridization studies of metalloproteinases 2 and 9 and TIMP-1 and TIMP-2 expression in human prostate cancer. Clin Exp Metastasis. 1997;15:246–258. doi: 10.1023/a:1018421431388. [DOI] [PubMed] [Google Scholar]
- Sternlicht MD, Bissell MJ, Werb Z. The matrix metalloproteinase stromelysin-1 acts as a natural mammary tumor promoter. Oncogene. 2000;19:1102–1113. doi: 10.1038/sj.onc.1203347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternlicht MD, Lochter A, Sympson CJ, Huey B, Rougier JP, Gray JW, Pinkel D, Bissell MJ, Werb Z. The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis. Cell. 1999;98:137–146. doi: 10.1016/s0092-8674(00)81009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCawley LJ, Crawford HC, King LE, Jr, Mudgett J, Matrisian LM. A protective role for matrix metalloproteinase-3 in squamous cell carcinoma. Cancer Res. 2004;64:6965–6972. doi: 10.1158/0008-5472.CAN-04-0910. [DOI] [PubMed] [Google Scholar]
- Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circulation Research. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- Djonov V, Hogger K, Sedlacek R, Laissue J, Draeger A. MMP-19: cellular localization of a novel metalloproteinase within normal breast tissue and mammary gland tumours. J Pathol. 2001;195:147–155. doi: 10.1002/path.927. [DOI] [PubMed] [Google Scholar]
- Velinov N, Aebersold D, Haeni N, Hlushchuk R, Weinstein F, Sedlacek R, Djonov V. Matrix metalloproteinase-19 is a predictive marker for tumor invasiveness in patients with oropharyngeal squamous cell carcinoma. Int J Biol Markers. 2007;22:265–273. doi: 10.5301/JBM.2008.2632. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Kihira Y, Matuo Y, Usui T. Expression of matrix metalloproteinase-7 and tissue inhibitor of metalloproteinase-1 in human prostate. J Urol. 1998;160:1872–1876. [PubMed] [Google Scholar]
- Daja MM, Niu X, Zhao Z, Brown JM, Russell PJ. Characterization of expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in prostate cancer cell lines. Prostate Cancer Prostatic Dis. 2003;6:15–26. doi: 10.1038/sj.pcan.4500609. [DOI] [PubMed] [Google Scholar]
- Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL, Simons JW. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res. 1999;59:5830–5835. [PubMed] [Google Scholar]
- Zhong H, Semenza GL, Simons JW, De Marzo AM. Up-regulation of hypoxia-inducible factor 1alpha is an early event in prostate carcinogenesis. Cancer Detect Prev. 2004;28:88–93. doi: 10.1016/j.cdp.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Gravdal K, Halvorsen OJ, Haukaas SA, Akslen LA. Proliferation of immature tumor vessels is a novel marker of clinical progression in prostate cancer. Cancer Res. 2009;69:4708–4715. doi: 10.1158/0008-5472.CAN-08-4417. [DOI] [PubMed] [Google Scholar]
- Mazzucchelli R, Montironi R, Santinelli A, Lucarini G, Pugnaloni A, Biagini G. Vascular endothelial growth factor expression and capillary architecture in high-grade PIN and prostate cancer in untreated and androgen-ablated patients. Prostate. 2000;45:72–79. doi: 10.1002/1097-0045(20000915)45:1<72::aid-pros9>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Peyromaure M, Camparo P, Badoual C, Descazeaud A, Dinh-Xuan AT. The expression of vascular endothelial growth factor is associated with the risk of cancer progression after radical prostatectomy. BJU Int. 2007;99:1150–1153. doi: 10.1111/j.1464-410X.2007.06734.x. [DOI] [PubMed] [Google Scholar]
- Mao K, Badoual C, Camparo P, Delongchamps NB, Vieillefond A, Dinh-Xuan AT, Peyromaure M. The prognostic value of vascular endothelial growth factor (VEGF)-A and its receptor in clinically localized prostate cancer: a prospective evaluation in 100 patients undergoing radical prostatectomy. Canadian J Urol. 2008;15:4257–4262. [PubMed] [Google Scholar]
- Cronauer MV, Schulz WA, Seifert HH, Ackermann R, Burchardt M. Fibroblast growth factors and their receptors in urological cancers: basic research and clinical implications. Eur Urol. 2003;43:309–319. doi: 10.1016/s0302-2838(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Trojan L, Thomas D, Knoll T, Grobholz R, Alken P, Michel MS. Expression of pro-angiogenic growth factors VEGF, EGF, and bFGF and their topographical relation to neovascularisation in prostate cancer. Urol Res. 2004;32:97–103. doi: 10.1007/s00240-003-0383-5. [DOI] [PubMed] [Google Scholar]
- Gravdal K, Halvorsen OJ, Haukaas SA, Akslen LA. Expression of bFGF/FGFR-1 and vascular proliferation related to clinicopathologic features and tumor progress in localized prostate cancer. Virchows Arch. 2006;448:68–74. doi: 10.1007/s00428-005-0075-3. [DOI] [PubMed] [Google Scholar]
- Strohmeyer D, Strauss F, Rossing C, Roberts C, Kaufmann O, Bartsch G, Effert P. Expression of bFGF. VEGF and c-met and their correlation with microvessel density and progression in prostate carcinoma. Anticancer Res. 2004;24:1797–1804. [PubMed] [Google Scholar]
- Hlatky L, Hahnfeldt P, Folkman J. Clinical application of antiangiogenic therapy: microvessel density, what it does and doesn’t tell us. J Natl Cancer Inst. 2002;94:883–893. doi: 10.1093/jnci/94.12.883. [DOI] [PubMed] [Google Scholar]
- Halvorsen OJ, Haukaas S, Hoisaeter PA, Akslen LA. Independent prognostic importance of microvessel density in clinically localized prostate cancer. Anticancer Res. 2000;20:3791–3799. [PubMed] [Google Scholar]
- Strohmeyer D, Rossing C, Strauss F, Bauerfeind A, Kaufmann O, Loening S. Tumor angiogenesis is associated with progression after radical prostatectomy in pT2/pT3 prostate cancer. Prostate. 2000;42:26–33. doi: 10.1002/(sici)1097-0045(20000101)42:1<26::aid-pros4>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Bono AV, Celato N, Cova V, Salvadore M, Chinetti S, Novario R. Microvessel density in prostate carcinoma. Prostate Cancer Prostatic Dis. 2002;5:123–127. doi: 10.1038/sj.pcan.4500572. [DOI] [PubMed] [Google Scholar]
- Brawer MK, Deering RE, Brown M, Preston SD, Bigler SA. Predictors of pathologic stage in prostatic carcinoma. The role of neovascularity. Cancer. 1994;73:678–687. doi: 10.1002/1097-0142(19940201)73:3<678::aid-cncr2820730329>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Weidner N, Carroll PR, Flax J, Blumenfeld W, Folkman J. Tumor angiogenesis correlates with metastasis in invasive prostate carcinoma. Am J Pathol. 1993;143:401–409. [PMC free article] [PubMed] [Google Scholar]
- Albertsen PC, Hanley JA, Barrows GH, Penson DF, Kowalczyk PD, Sanders MM, Fine J. Prostate cancer and the Will Rogers phenomenon. J Natl Cancer Inst. 2005;97:1248–1253. doi: 10.1093/jnci/dji248. [DOI] [PubMed] [Google Scholar]
- Bubendorf L, Nocito A, Moch H, Sauter G. Tissue microarray (TMA) technology: miniaturized pathology archives for high-throughput in situ studies. J Pathol. 2001;195:72–79. doi: 10.1002/path.893. [DOI] [PubMed] [Google Scholar]
- Rubin MA, Dunn R, Strawderman M, Pienta KJ. Tissue microarray sampling strategy for prostate cancer biomarker analysis. Am J Surg Pathol. 2002;26:312–319. doi: 10.1097/00000478-200203000-00004. [DOI] [PubMed] [Google Scholar]