Abstract
IL-1β is a proinflammatory cytokine that plays a central role in the inflammatory process of the gut. IL-1β causes an increase in intestinal epithelial tight junction (TJ) permeability, but the intracellular pathways that mediate intestinal TJ permeability remain unclear. The major aims of this study were to delineate the protein kinases that regulate the IL-1β modulation of intestinal TJ barrier function and to determine the intracellular mechanisms involved, using filter-grown Caco-2 monolayers as the in vitro model system. Our results showed that IL-1β caused a rapid activation of MEKK-1 and NIK. The knockdown of MEKK-1, but not NIK, inhibited the IL-1β increase in Caco-2 TJ permeability. IL-1β caused an activation of both canonical and noncanonical NF-κB pathways; MEKK-1 regulated the activation of the canonical pathway, while NIK regulated the activation of the noncanonical pathway. Inhibition of MEKK-1 activation of the canonical pathway prevented the IL-1β increase in TJ permeability. Our data also indicated that inhibitory κB kinase was the catalytic subunit primarily involved in canonical pathway activation and TJ barrier opening. MEKK-1 also played an essential role in myosin light chain kinase gene activation. In conclusion, our data show for the first time that MEKK-1 plays an integral role in IL-1β modulation of Caco-2 TJ barrier function by regulating the activation of the canonical NF-κB pathway and the MLCK gene.
Defective intestinal epithelial tight junction (TJ) barrier has been implicated to be an important pathogenic factor in number of inflammatory conditions of the gut and systemic inflammatory conditions, including Crohn’s disease (CD), postinfectious irritable bowel syndrome, nonsteroidal anti-inflammatory drug associated enteritis, ulcerative colitis, heat stroke, alcoholic hepatitis, and various infectious diarrheal syndromes.1,2,3,4,5 It has been postulated that the defective intestinal TJ barrier allows paracellular permeation of noxious luminal antigens that propagate and contribute to the inflammatory response.5,6,7 It is well-established that patients with CD have a defective intestinal TJ barrier manifested by an increase in intestinal permeability.1,2,5,8 Intestinal permeability studies in healthy first degree relatives of CD patients (an at risk population to develop CD) showed that the healthy relatives also had an abnormal increase in intestinal permeability, prompting the investigators to conclude that the increase in intestinal permeability “is a primary defect that may be an etiological factor in this disease.”1,8
Interleukin-1β (IL-1β) is one of the first cytokines to be discovered and has been shown to play a central role in intestinal inflammation in CD.9,10,11,12 A direct correlation exists between elevated levels of IL-1β and severity of intestinal inflammation in CD.11,12,13,14 Patients with CD also have an imbalance between the level of IL-1β and its naturally occurring antagonist IL-1 receptor antagonist (IL-1ra) such that they have deficiency of anti-inflammatory form of IL-1 and excess production of IL-1β.15,16 In addition, CD patients have increased incidence of IL-1β gene polymorphism that determines the severity of intestinal inflammation.17,18 IL-1β antagonists have been shown to be effective in the treatment of immune-mediated inflammation in mice and are currently being developed for clinical usage.19,20 Previous studies have shown that IL-1β causes an increase in intestinal TJ permeability, and it has been postulated that the defect in intestinal TJ barrier contributes to the development of intestinal inflammation.21,22
Recent studies from several laboratories have shown that proinflammatory cytokines (including IL-1β, TNF-α, and IFN-γ) cause an increase in intestinal epithelial TJ permeability.22,23,24,25,26,27 The cytokine-induced increase in intestinal TJ permeability has been postulated to be an important factor in the development of intestinal inflammation.2,5,7,28 The role of cytokine-induced alteration in intestinal permeability as a pathogenic factor of intestinal inflammation has been supported by animal studies showing that the preservation of intestinal TJ barrier function prevents the development of intestinal inflammation.7,28,29,30,31 In IL-10–deficient mice (IL-10−/−), a commonly used murine model of inflammatory bowel disease, the development of intestinal inflammation was preceded by an initial increase in intestinal permeability,32 suggesting a possible cause-and-effect relationship. The inhibition of intestinal TJ barrier defect by oral administration of TJ barrier enhancing agent AT-1001 (a zonulin peptide inhibitor) prevented the development of enterocolitis in IL-10−/− mice, leading the authors to conclude that the “abnormal small intestinal permeability not only precedes the development of colitis but is etiologically important.”31 Similarly, other investigators have shown that the maintenance of intestinal TJ barrier function in various murine models of intestinal inflammation also prevents the development of intestinal inflammation and its clinical sequelae.29,30,33 Consistent with the above animal studies, clinical studies have also revealed that the therapeutic re-tightening of intestinal TJ barrier is associated with more rapid improvement and resolution of active CD and prolonged clinical remission.34,35,36 Conversely, persistent increase in intestinal permeability after medical therapy was predictive of poor clinical outcome and early exacerbation of the disease.35,36 Together, these studies suggested that the therapeutic preservation or re-tightening of intestinal TJ barrier has important potential clinical implications.
Myosin light chain kinase (MLCK) gene and protein play a central role in IL-1β modulation of intestinal epithelial TJ barrier.2,5,7,28 Previous studies have shown that the IL-1β–induced increase in intestinal epithelial TJ permeability was mediated by an increase in MLCK mRNA transcription and protein synthesis.22 The inhibition of MLCK transcription or protein synthesis completely inhibited the IL-1β–induced increase in Caco-2 TJ permeability.22 The intracellular processes or protein kinase pathways that mediate the IL-1β alteration in MLCK gene activity or increase in intestinal epithelial TJ permeability are unknown. Mitogen activated protein kinase kinase kinases (MAP3 kinase) are recruited by IL-1β receptor complex and play a crucial role in the regulation of variety of biological activities in intestinal epithelial cells.37,38,39 However, their involvement in TJ barrier regulation remains unknown. IL-1β has been shown to activate MAP3 kinases including MEKK-1 and NIK in different cell types.40,41,42,43 The major aim of this study was to examine the regulatory role of MAP3 kinases MEKK-1 and NIK in IL-1β–induced increase in intestinal epithelial TJ permeability, using filter-grown Caco-2 intestinal epithelial monolayers as an in vitro model of intestinal epithelium. Our data show for the first time that the IL-1β–induced increase in intestinal epithelial TJ permeability is mediated by MEKK-1 activation. Our data also suggest that the IL-1β effect on Caco-2 TJ barrier is due to MEKK-1 regulation of canonical NF-κB pathways and MLCK gene activity.
Materials and Methods
Chemicals
Cell culture media (DMEM), trypsin, FBS, glutamine, penicillin, streptomycin, and PBS were purchased from GIBCO-BRL (Grand Island, NY). Anti–MEKK-1, NIK, IKK-α, IKK-β, IκB-α, MLCK, and anti–β-actin antibodies were obtained from Sigma (St. Louis, MO). Anti–phospho-MEKK1, phospho-NIK, phospho-IKKα/β antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Anti–NF-κB p65 and p100/p52 antibodies were purchased from Abcam (Cambridge, MA). Horseradish peroxidase–conjugated secondary antibodies for Western blot analysis were purchased from Invitrogen (San Francisco, CA). siRNA of MEKK-1, NIK, IKKα, IKKβ, p65, and p100 and transfection reagents were obtained from Dharmacon (Lafayette, CO). All other chemicals were purchased from Sigma, VWR (West Chester, PA) or Fisher Scientific (Pittsburgh, PA).
Cell Cultures
Caco-2 cells (passage 20) were purchased from the American Type Culture Collection (Rockville, MD) and maintained at 37°C in a culture medium composed of DMEM with 4.5 mg/ml glucose, 50 U/ml penicillin, 50 U/ml streptomycin, 4 mmol/L glutamine, 25 mmol/L HEPES, and 10% FBS. The cells were kept at 37°C in a 5% CO2 environment. Culture medium was changed every 2 days. Caco-2 cells were subcultured after partial digestion with 0.25% trypsin and 0.9 mmol/L EDTA in Ca2+- and Mg2+-free PBS.21,22
Determination of Epithelial Monolayer Resistance and Paracellular Permeability
An epithelial voltohmeter (World Precision Instruments, Sarasota, FL) was used for measurements of the transepithelial electrical resistance (TER) of the filter-grown Caco-2 intestinal monolayers as previously reported. The effect of IL-1β on Caco-2 paracellular permeability was determined using an established paracellular marker inulin (m.w. = 5000 g/mol).2 For determination of mucosal-to-serosal flux rates of inulin, Caco-2-plated filters having epithelial resistance of 400–500 Ω · cm2 were used. Known concentrations of inulin (2 μmol/L) and its radioactive tracer were added to the apical solution.
Assessment of Protein Expression by Western Blot Analysis
Caco-2 monolayers were treated with IL-1β (10 ng/ml) for varying time periods. At the end of the experimental period, Caco-2 monolayers were immediately rinsed with ice-cold PBS, and cells were lysed with lysis buffer (50 mmol/L Tris·HCl, pH 7.5, 150 mmol/L NaCl, 500 μmol/L NaF, 2 mmol/L EDTA, 100 μmol/L vanadate, 100 μmol/L PMSF, 1 μg/ml leupeptin, 1 μg/ml pepstatin A, 40 mmol/L paranitrophenyl phosphate, 1 μg/ml aprotinin, and 1% Triton X-100) and scraped, and the cell lysates were placed in Microfuge tubes. Cell lysates were centrifuged to yield a clear lysate. Supernatant was collected, and protein measurement was performed using Bio-Rad Protein Assay kit (Bio-Rad Laboratories). Laemmli gel loading buffer was added to the lysate containing 10–20 μg of protein and boiled for 7 minutes, after which proteins were separated on SDS-PAGE gel. Proteins from the gel were transferred to the membrane (Trans-Blot Transfer Medium, Nitrocellulose Membrane; Bio-Rad Laboratories) overnight. The membrane was incubated for 2 hours in blocking solution (5% dry milk in TBS-Tween 20 buffer). The membrane was incubated with appropriate primary antibodies in blocking solution. After being washed in TBS-1% Tween buffer, the membrane was incubated in appropriate secondary antibodies and developed using the Santa Cruz Western Blotting Luminol Reagents (Santa Cruz Biotechnology, Santa Cruz, CA) on the Kodak BioMax MS film (Fisher Scientific, Pittsburgh, PA).
SiRNA of MEKK-1, NIK, IKK-α, IKK-β, p65, and p100/p52
Targeted siRNAs were obtained from Dharmacon, Inc. (Chicago, IL). Caco-2 monolayers were transiently transfected using DharmaFect transfection reagent (Lafayette, Co).22 Briefly, 5 × 105cells per filter were seeded into a 12-well transwell plate and grown to confluency. Caco-2 monolayers were then washed with PBS twice and 1.0 ml Opti-MEM medium was added to the apical compartment of each filter and 1.5 ml were added to the basolateral compartment of each filter. siRNA of interest (5 nmol/L) and 2 μl of DharmaFect reagent were preincubated in Opti-MEM. After 5 minutes of incubation, two solutions were mixed and incubated for another 20 minutes, and the mixture was added to the apical compartment of each filter. The IL-1β experiments were carried out 96 hours after transfection. The efficiency of silencing was confirmed by Western blot analysis.
Nuclear Extracts and ELISA for Transcription Factor Activation
Filter-grown Caco-2 monolayers were treated with IL-1β (10 ng/ml) for 30 minutes. Caco-2 monolayers were washed with ice-cold PBS, scraped, collected, and centrifuged at 14,000 rpm for 30 seconds. The cell pellets were resuspended in 200 μl of buffer A (in millimoles: 10 HEPES-KOH, 1.5 MgCl2, 10 KCl, 0.5 DTT, and 0.2 PMSF [pH 7.9]), and incubated on ice for 15 minutes. After centrifugation at 14,000 rpm for 30 s, pelleted nuclei were resuspended in 30 μl of buffer C (in millimoles: 20 HEPES-KOH [25% glycerol], 420 NaCl, 1.5 MgCl2, 0.2 EDTA, 0.5 DTT, and 0.2 PMSF [pH 7.9]). After incubation on ice for 20 minutes, the lysates were centrifuged at 14,000 rpm for 20 minutes. Protein concentrations were determined using the Bradford method. The NF-κB p65 and p52 DNA-binding assay was performed using Trans-AM ELISA-based kits from Active Motif according to the manufacturer’s protocol. In brief, the binding reactions contained 1 pM biotinylated probe (Integrated DNA Technologies) and 5 μg of nuclear extract in complete binding buffer with a total volume of 50 μl. After 30 minutes of incubation, the solution was transferred to an individual well on 96-well plate and incubated for 1 hour. Appropriate antibody (2 μg/ml) was added to the well to bind the target protein in nuclear extract. After incubation for 1 hour, the antibody was removed, and 100 μl of horseradish peroxidase–conjugated secondary antibody was added to the well and incubated for 1 hour. Subsequently, 100 μl of developing solution was added for 2–10 minutes, and 100 μl of stop solution were added. The absorbance at 450 nm was determined using the SpectraMax 190 (Molecular Devices).
Immunostaining of NF-κB p65 and p52 Proteins
Cellular localization of NF-κB p65 and p52 was assessed by immunofluorescent antibody labeling.23 At the end of the experimental period, filter-grown Caco-2 monolayers were washed twice in cold PBS and were fixed with 2% paraformaldehyde for 20 minutes. After being permeabilized with 0.1% Triton X-100 in PBS at room temperature for 20 minutes, Caco-2 monolayers were then incubated in blocking solution composed of bovine serum albumin and normal donkey serum in PBS for 1 hour. Cells were then labeled with primary antibodies in blocking solution overnight at 4°C. After being washed with PBS, the cells were incubated in FITC and Cy-3-conjugated secondary antibodies for 1 hour at room temperature. ProLong Gold antifade reagent (Invitrogen, CA) was used to mount the filters onto the coverslips. Immunolocalizations of NF-κB p65 was visualized using a Confocal fluorescence microscope (LSM 510, University of New Mexico Imaging center) equipped with a Hamamatsu digital camera (Hamamatsu Photonics, Hamamatsu, Japan). Images were processed with LSM software (Zeiss, Germany).
RNA Isolation and Reverse Transcription
Caco-2 cells (5 × 105 per filter) were seeded into six-well transwell permeable inserts and grown to confluency. Filter-grown Caco-2 cells were then treated with appropriate experimental reagents for desired time periods. At the end of the experimental period, cells were washed twice with ice-cold PBS. Total RNA was isolated using Qiagen RNeasy Kit (Qiagen, ML) according to the manufacturer’s protocol. Total RNA concentration was determined by absorbance at 260/280 nm using SpectrraMax 190 (Molecular Devices). The reverse transcription (RT) was carried out using the GeneAmp Gold RNA PCR core kit (Applied Biosystems, Foster City, CA). Two micrograms of total RNA from each sample were reverse transcribed into cDNA in a 40-μl reaction containing 1× RT-PCR buffer, 2.5 mmol/L MgCl2, 250 μmol/L of each dNTP, 20 U RNase inhibitor, 10 mmol/L DTT, 1.25 μmol/L random hexamer, and 30 U multiscribe RT. The RT reactions were performed in a thermocycler (MyCycler, Bio-Rad, Hercules, CA) at 25°C for 10 minutes, 42°C for 30 minutes, and 95°C for 5 minutes.
Quantification of Gene Expression Using Real-Time PCR
The real-time PCRs were carried out using ABI prism 7900 sequence detection system and Taqman universal PCR master mix kit (Applied Biosystems, Branchburg, NJ) as previously described.44 Each real-time PCR reaction contained 10 μl RT reaction mix, 25 μl 2× Taqman universal PCR master mix, 0.2 μmol/L probe, and 0.6 μmol/L primers. Primer and probe design for the real-time PCR was made with Primer Express version 2 from Applied Biosystems. [The primers used in this study are as follows: MLCK specific primer pairs consisted of 5′-AGGAAGGCAGCATTGAGGTTT-3′ (forward), 5′-GCTTTCAGCAGGCAGAGGTAA-3′ (reverse); probe specific for MLCK consisted of FAM 5′-TGAAGATGCTGGCTCC-3′ TAMRA; the internal control GAPDH-specific primer pairs consisted of 5′ CCACCCATGGCAAATTCC-3′ (forward), 5′-TGGGATTTCCATTGATGACCAG-3′ (reverse); probe specific for GAPDH consisted of JOE 5′-TGGCACCGTCAAGGCTGAGAACG-3′ TAMRA]. All runs were performed according to the default PCR protocol (50°C for 2 minutes, 95°C for 10 minutes, 40 cycles of 95°C for 15 s, and 60°C for 1 minute). For each sample, real-time PCR reactions were performed in triplicate, and the average threshold cycle was calculated. A standard curve was generated to convert the threshold cycle to copy numbers. Expression of MLCK mRNA was normalized with GAPDH mRNA expression. The average copy number of MLCK mRNA expression in control samples was set to 1.0. The relative expression of MLCK mRNA in treated samples was determined as a fold increase compared with control samples.
Transfection of MLCK DNA and Measurement of Promoter Activity
The MLCK promoter region was cloned using GenomeWalker system (Clontech, CA). A 2091-bp DNA fragment (−2109 to −18) was amplified by PCR.44 The amplification condition was 1 cycle at 94°C for 2 minutes, followed by 43 cycles at 94°C for 1 minute, 50°C for 1 minute, and 72°C for 2 minutes and 1 cycle at 72°C for 5 minutes. The resultant PCR product was digested with HindIII and KpnI and inserted into pGL3-basic luciferase reporter vector (Promega). The sequence was confirmed by DNA services at the University of New Mexico. MLCK promoter was transiently transfected into Caco-2 cells using transfection reagent lipofectamine 2000 (Life Technologies). Renilla luciferase vector (pRL-TK, Promega) was cotransfected with each plasmid construct as an internal control. Cells (5 × 105 per filter) were seeded into a six-well transwell plate and grown to confluency. Caco-2 monolayers were then washed with PBS twice and 1.0 ml Opti-MEM medium was added to the apical compartment of each filter and 1.5 ml were added to the basolateral compartment of each filter. One microgram of each plasmid construct and 0.25 μg pRL-TK or 2 μl lipofectamine 2000 was preincubated in 250 μl Opti-MEM, respectively. After 5 minutes of incubation, two solutions were mixed and incubated for another 20 minutes, and the mixture was added to the apical compartment of each filter. After incubation for 3 hours at 37°C, 500 μl DMEM containing 10% FBS were added to both sides of the filter to reach a 2.5% final concentration of FBS. Subsequently, media were replaced with normal Caco-2 growth media 16 hours after transfection. Specific experiments were carried out 48 hours after transfection. At the completion of specific experimental treatments, Caco-2 cells were washed twice with 1 ml ice-cold PBS, followed by the addition of 400 μl 1× passive lysis buffer, incubated at room temperature for 15 minutes, scraped and transferred into an Eppendorf tube, and centrifuged for 15 seconds at 13,000 rpm in a microcentrifuge. Luciferase activity was determined using the dual luciferase assay kit (Promega). Twenty microliters of the supernatant were used for each assay. Luciferase values were determined by Lumat LB 9507 (EG&G Berthold, Oak Ridge, TN). The value of reporter luciferase activities were then divided by that of renilla luciferase activities to normalize for differences in transfection efficiencies. The average activity value of the control samples was set to 1.0. The luciferase activity of MLCK promoter in treated samples was determined relative to the control samples.
Statistical Analysis
Results are expressed as means ± SE. Statistical significance of differences between mean values was assessed with Student’s t-tests for unpaired data and analysis of variance analysis whenever was required. All reported significance levels represent two-tailed P values. A P value of <0.05 was used to indicate statistical significance. Each experiment was performed in triplicate or quadruplicates (n = 3 or 4) and all experiments were repeated at least three times to ensure reproducibility.
Results
Role of MAP3 Kinases MEKK-1 and NIK in IL-1β–Induced Increase in Caco-2 TJ Permeability
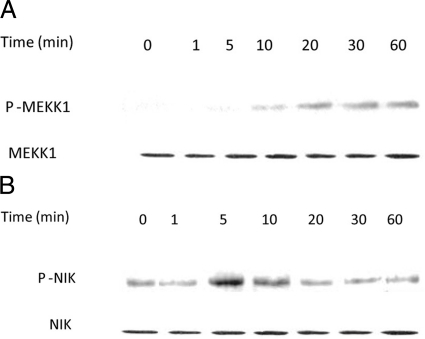
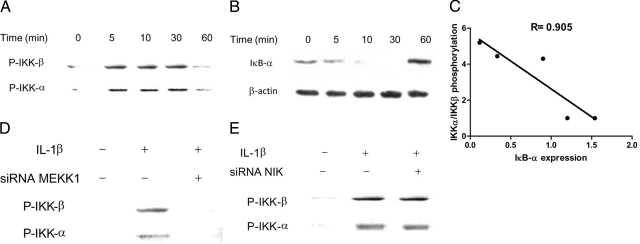
In the following studies, the effect of IL-1β on activation of MAP3 kinases MEKK-1 and NIK was determined in Caco-2 cells. The time-course effect of IL-1β (10 ng/ml) on MEKK-1 activation in filter-grown Caco-2 monolayers was assessed by MEKK-1 phosphorylation. IL-1β produced a time-dependent increase in MEKK-1 phosphorylation in Caco-2 cells, starting at about 10 minutes and continuing up to 60 minutes as determined by phospho-MEKK-1 immunoblotting (Figure 1A). IL-1β (10 ng/ml) also caused a rapid increase in NIK phosphorylation (peaking at 5 minutes) as assessed by phospho-NIK immunoblotting (Figure 1B). IL-1β did not affect the total MEKK-1 or NIK level (Figure 1). These results indicated that IL-1β induces a rapid phosphorylation of both MEKK-1 and NIK in Caco-2 cells.
Figure 1.
Time course effect of IL-1β on Caco-2 MEKK-1 and NIK activation. A: Time course effect of IL-1β (10 ng/ml) on Caco-2 MEKK-1 phosphorylation (total MEKK-1 was used for equal protein loading). B: Time course effect of IL-1β on NIK phosphorylation (total NIK was used for equal protein loading). IL-1β caused a time-dependent increase in Caco-2 MEKK-1 and NIK activation.
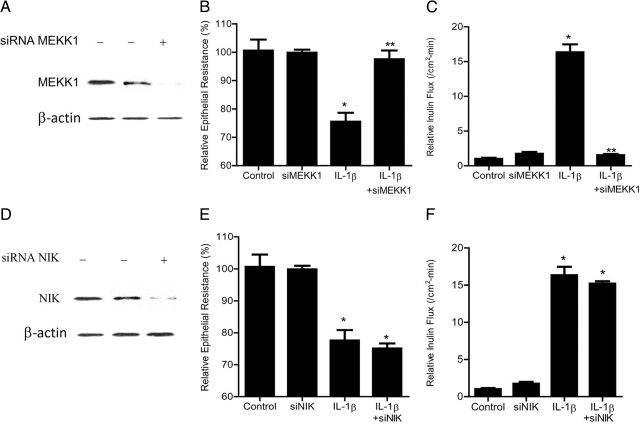
Next, to assess the involvement of MEKK-1 and/or NIK in IL-1β–induced increase in Caco-2 TJ permeability, MEKK-1 and NIK expression was selectively knocked-down via siRNA transfection of filter-grown Caco-2 cells. The MEKK-1 siRNA transfection resulted in a near-complete depletion of MEKK-1 expression in Caco-2 cells (Figure 2A); the siRNA induced knockdown of MEKK-1 inhibited the IL-1β–induced drop in Caco-2 TER and increase in mucosal-to-serosal flux of paracellular marker inulin (Figure 2, B and C). In contrast, the siRNA-induced knockdown of NIK (Figure 2D) did not affect the IL-1β–induced drop in Caco-2 TER (Figure 2E) or the increase in trans-epithelial flux of inulin (Figure 2F). These data suggested that MEKK-1 but not NIK was required for the IL-1β–induced increase in Caco-2 TJ permeability.
Figure 2.
Effect of siRNA-induced MEKK-1 and NIK knockdown of IL-1β–induced increase in Caco-2 TJ permeability. A: MEKK-1 siRNA transfection resulted in a near complete depletion in MEKK-1 protein expression as determined by Western blot analysis. B: MEKK-1 siRNA transfection prevented the IL-1β–induced drop in Caco-2 TER (means ± SE, n = 4). *P < 0.01 versus control; **P < 0.01 versus IL-1β treatment. C: MEKK-1 siRNA transfection prevented the IL-1β–induced increase in inulin flux (means ± SE, n = 4). *P < 0.001 versus control; **P < 0.001 versus IL-1β treatment. D: NIK siRNA transfection resulted in a near complete depletion in NIK protein expression. E: NIK siRNA transfection did not prevent the IL-1β–induced drop in Caco-2 TER (means ± SE, n = 4). *P < 0.01 versus control. F: NIK siRNA transfection did not prevent the IL-1β–induced increase in inulin flux (means ± SE, n = 4). *P < 0.001 versus control.
MAP3 Kinase Regulation of Canonical and Noncanonical NF-κB Pathways
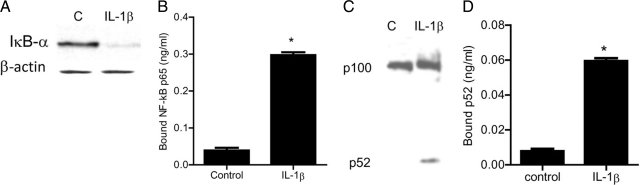
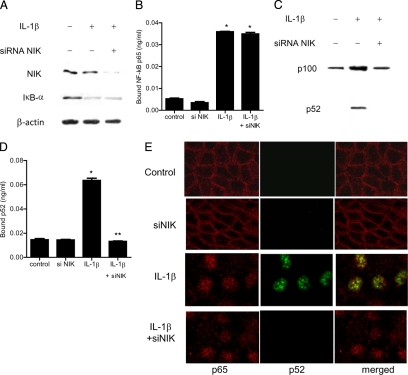
To examine the downstream processes involved in MAP3 kinase modulation of Caco-2 TJ permeability, the regulatory role of MEKK1 and NIK on NF-κB pathway activation was investigated. MAP3 kinases have been shown to regulate the activation of both canonical and noncanonical NF-κB pathways.45 The activation of canonical pathway involves the degradation of inhibitory κB (IκB) proteins, which results in the activation and nuclear translocation of NF-κB heterodimer p65/p50.45,46 The activation of noncanonical pathway results in the phosphorylation and processing of p100 into p52 subunit and activation and nuclear translocation of p52/Rel B dimer.45 The activation of canonical and noncanonical pathways is distinguished by the differential activation of dimers p65/p50 and p52/Rel B, respectively.45,46 In the following studies, IL-1β effect on canonical and/or noncanonical pathway activation was examined in filter-grown Caco-2 cells. The IL-1β effect on canonical pathway was determined by degradation of IκB-α and activation of NF-κB p65 subunit, while the effect on noncanonical pathway was determined by generation and activation of p52 subunit. IL-1β caused a rapid degradation of IκB-α and a cytoplasmic-to-nuclear translocation of p65 subunit in Caco-2 cells (Figures 3A and 4E). The IL-1β–induced nuclear translocation of p65 was accompanied by an increase in binding of activated p65 to the κB binding site on the oligonucleotide probe as determined by ELISA binding assay (3B). IL-1β also caused an increase in p52 generation (Figure 3C), nuclear translocation of p52 (Figure 4E), and an increase in binding of the activated p52 subunit to the κB binding site on the oligonucleotide probe (Figure 3D). These results suggested that IL-1β causes activation of both canonical and noncanonical NF-κB pathways in Caco-2 cells.
Figure 3.
Effect of IL-1β (10 ng/ml) on Caco-2 NF-κB pathways (p65 and p52) activation. A: IL-1β caused the degradation of IκB-α expression (30-minute experimental period) as assessed by Western blot analysis. B: ELISA-based DNA binding assay of NF-κB p65. IL-1β treatment caused a significant increase in Caco-2-NF-κB p65 binding to the DNA probe. *P < 0.001 versus control. C: IL-1β caused activation of p100 and generation of p52 (30 minutes experimental period) as assessed by Western blot analysis. D: ELISA-based DNA binding assay of NF-κB p52. IL-1β treatment caused a significant increase in Caco-2-NF-κB p52 binding to the DNA probe. *P < 0.001 versus control.
Figure 4.
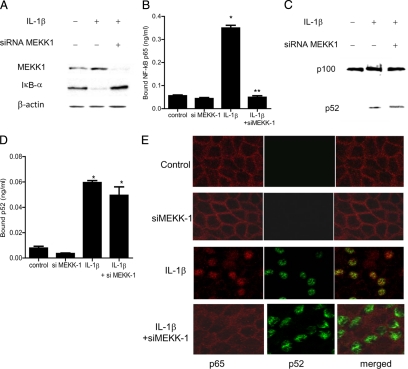
Effect of siRNA-induced MEKK-1 knockdown of IL-1β-activation of NF-κB p65 and p52. A: MEKK-1 siRNA transfection prevented the IL-1β–induced degradation of IκB-α as assessed by Western blot analysis. B: MEKK-1 silencing inhibited the IL-1β–induced binding of p65 to its binding site on the DNA probe as measured by DNA ELISA-binding assay. *P < 0.001 versus control; **P < 0.001 versus IL-1β treatment. C: MEKK-1 siRNA transfection did not prevent the IL-1β generation of p52. D: MEKK-1 silencing did not inhibit the IL-1β–induced binding of p52 to its binding site on DNA probe. *P < 0.001 versus control. E: Effect of MEKK−1 siRNA transfection on IL-1β–induced nuclear translocation of p65 and p52 as viewed by confocal microscopy. Magnification, ×40.
Next, the regulatory role of MEKK-1 or NIK on IL-1β induced activation of p65 or p52 subunits was determined by selective silencing of MEKK-1 or NIK. The siRNA induced knockdown of MEKK-1 prevented the IL-1β–induced degradation of IκB-α and activation of p65 in Caco-2 cells but did not affect the generation or activation of p52 (Figure 4, A–E). In contrast, the silencing of NIK did not affect the activation of p65 but inhibited the generation and activation of p52 subunit (Figure 5, A–E). Together, these data suggested that MEKK-1 was responsible for the activation of canonical pathway and that NIK was involved in the activation of noncanonical pathway. To determine the role of canonical or noncanonical pathway in IL-1β–induced increase in Caco-2 TJ permeability, the expression p65 or p100 subunit was selectively knocked-down by siRNA transfection. In the noncanonical pathway, p100 is phophorylated and processed to generate the p52 subunit. The siRNA induced knockdown of p100 subunit (see Supplemental Figure S1A at http://ajp.amjpathol.org) or p52 subunit (data not shown) did not affect the IL-1β–induced increase in paracellular permeability or drop in TER (see Supplemental Figure S1B at http://ajp.amjpathol.org) or increase in inulin flux (see Supplemental Figure S1C at http://ajp.amjpathol.org). In contrast, siRNA-induced knockdown of p65 completely inhibited the IL-1β–induced drop in TER and increase in paracellular permeability, as previously reported by us,22 suggesting that the canonical pathway activation of p65 was required for the IL-1β increase in Caco-2 TJ permeability.
Figure 5.
Effect of siRNA-induced NIK knockdown of IL-1β activation of NF-κB p65 and p52. A: NIK siRNA transfection did not prevent the IL-1β–induced degradation of IκB-α as assessed by Western blot analysis. B: NIK silencing did not inhibit the IL-1β–induced binding of p65 to its binding site on DNA probe as measured by DNA ELISA-binding assay. *P < 0.001 versus control. C: NIK siRNA transfection prevented the IL-1β generation of p52. D: NIK silencing inhibited the IL-1β–induced binding of p52 to its binding site on the DNA probe. *P < 0.001 versus control; **P < 0.001 versus IL-1β treatment. E: Effect of NIK siRNA transfection on IL-1β–induced nuclear translocation of p65 and p52 as viewed by confocal microscopy. Magnification, ×40.
Role of IKK Catalytic Subunits in IL-1β–Induced Increase in Caco-2 TJ Permeability
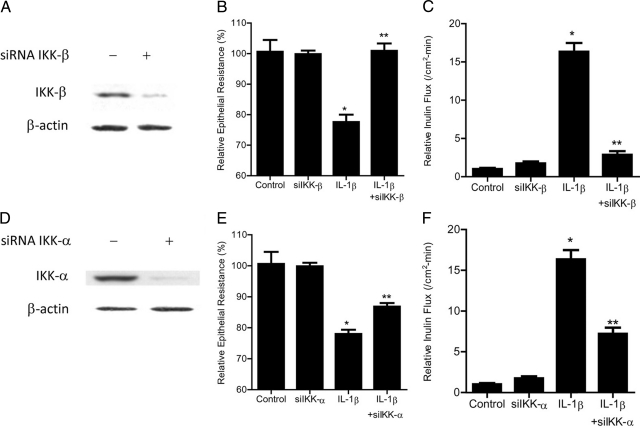
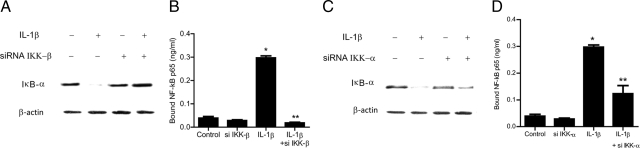
The inhibitory κB kinases (IKK) are important target of MAP kinases and play a crucial role in NF-κB pathway activation; however, the regulatory role of IKK in TJ barrier function remains unknown. In the following studies, the involvement of IKK catalytic subunits—IKK-α and IKK-β—in IL-1β–induced increase in Caco-2 TJ permeability was examined. The IL-1β effect on IKK-α and IKK-β activation was determined by assessing their phosphorylation by immunoblot analysis. IL-1β (10 ng/ml) treatment resulted in a rapid phosphorylation of both IKK-α and IKK-β (Figure 6A). The phosphorylation of IKK catalytic subunits reached the peak levels between 5–30 minutes and decreased thereafter to the baseline levels (Figure 6A). The time-course of IKK-α and IKK-β phosphorylation correlated closely with IκB-α degradation (relative correlation coefficient r = 0.905) (Figure 6, B and C). In the following studies, the role of MEKK-1 and NIK in IKK catalytic subunit activation was examined. The silencing of MEKK-1 inhibited the IL-1β–induced activation of both IKK-α and IKK-β (Figure 6D). In contrast, NIK silencing did not affect the activation of either IKK-α or IKK-β (Figure 6E), confirming that MEKK-1 but not NIK was involved in the IL-1β–induced activation of IKK catalytic subunits. To determine the involvement of IKK catalytic subunits in IL-1β modulation of Caco-2 TJ permeability, the expression of IKK-α or IKK-β was selectively silenced via siRNA transfection. IKK-β siRNA transfection of Caco-2 cells resulted in a near-complete knockdown of IKK-β (Figure 7A). The IKK-β knockdown almost completely inhibited the IL-1β–induced drop in Caco-2 TER (Figure 7B) and increase in paracellular permeability (Figure 7C), indicating that IKK-β was required for the increase in Caco-2 TJ permeability. The siRNA-induced depletion of IKK-α (Figure 7D) only partially inhibited the IL-1β induced drop in Caco-2 TER and increase in inulin flux (Figure 7, E and F), suggesting a minor role for IKK-α. Next, the involvement of IKK catalytic subunits in the activation of canonical NF-κB pathway was determined. The knockdown of IKK-β completely prevented the IL-1β–induced degradation of IκB-α (Figure 8A) and activation of p65 subunit (Figure 8B), whereas IKK-α silencing only partially inhibited the IL-1β–induced IκB-α degradation (Figure 8C) and p65 activation (Figure 8D). Together with the above data, these results suggested that IKK catalytic subunit regulation of Caco-2 TJ permeability was dependent on canonical pathway activation.
Figure 6.
Time-course effect of IL-1β on Caco-2 IKK catalytic subunit activation. A: Time-course effect of IL-1β (10 ng/ml) on Caco-2 IKK-α and IKK-β phosphorylation. IL-1β caused a time-dependent increase in Caco-2 IKK-α and IKK-β activation. B: Time-course effect of IL-1β on IκB-α degradation (β-actin was used for equal protein loading). C: Graph of IKK-α/IKK-β activation versus IκB-α degradation (r = 0.905). D: MEKK-1 siRNA transfection prevented the IL-1β–induced phosphorylation of IKK-α and IKK-β as assessed by Western blot analysis. E: NIK siRNA transfection did not prevent the IL-1β–induced phosphorylation of IKK-α and IKK-β.
Figure 7.
Effect of siRNA-induced IKK-β or IKK-α knockdown of IL-1β–induced increase in Caco-2 TJ permeability. A: IKK-β siRNA transfection resulted in a near complete depletion in IKK-β protein expression as determined by Western blot analysis. B: IKK-β siRNA transfection completely prevented the IL-1β–induced drop in Caco-2 TER (means ± SE, n = 4). *P < 0.01 versus control; **P < 0.01 versus IL-1β treatment. C: IKK-β siRNA transfection prevented the IL-1β–induced increase in inulin flux (means ± SE, n = 4). *P < 0.001 versus control; **P < 0.001 versus IL-1β treatment. D: IKK-α siRNA transfection resulted in a near complete depletion in IKK-α protein expression as determined by Western blot analysis. E: IKK-α siRNA transfection partially prevented the IL-1β–induced drop in Caco-2 TER. *P < 0.01 versus control; **P < 0.01 versus IL-1β treatment. F: IKK-α siRNA transfection partially prevented the IL-1β–induced increase in inulin flux (means ± SE, n = 4). *P < 0.001 versus control; **P < 0.001 versus IL-1β treatment.
Figure 8.
Effect of siRNA IKK subunit knockdown of IL-1β–induced activation of NF-κB p65. A: IKK-β siRNA transfection completely prevented the IL-1β–induced degradation of IκB-α as assessed by Western blot analysis. B: IKK-β siRNA transfection inhibited the IL-1β–induced binding of p65 to its binding site on the DNA probe as measured by DNA ELISA-binding assay. *P < 0.001 versus control; **P < 0.001 versus IL-1β treatment. C: IKK-α siRNA transfection partially prevented the IL-1β–induced degradation of IκB-α as assessed by Western blot analysis. D: IKK-α siRNA transfection partially inhibited the IL-1β–induced binding of p65 to its binding site on the DNA probe as measured by DNA ELISA-binding assay. *P < 0.001 versus control; **P < 0.01 versus IL-1β treatment.
Role of MEKK-1 in the Regulation of MLCK Gene Expression
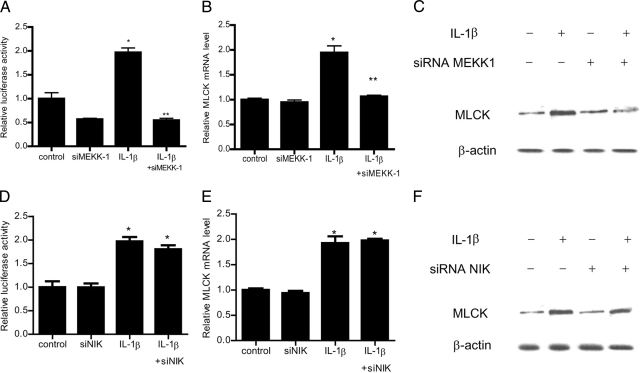
The above studies suggested that MEKK-1 plays a critical role in IL-1β modulation of Caco-2 TJ barrier; however, the downstream target of MEKK-1 regulatory action remains unclear. Previous studies from our laboratory indicated that the IL-1β–induced increase in Caco-2 TJ permeability was mediated by an increase in gene and protein expression of downstream effector protein MLCK.22 In the following studies, we examined the possibility that MEKK-1 signaling cascade was involved in the regulation of MLCK gene activity. The IL-1β effect on MLCK gene activity was assessed by transfection of Caco-2 cells with plasmid vector encoding the MLCK promoter region and luciferase reporter gene. The IL-1β treatment of filter-grown Caco-2 monolayers transfected with the plasmid vector encoding the MLCK promoter region resulted in an increase in MLCK promoter activity (Figure 9A). IL-1β also caused an increase in MLCK mRNA expression and MLCK protein expression (Figure 9, B and C). The siRNA knockdown of MEKK-1 inhibited the IL-1β–induced increase in MLCK promoter activity, MLCK mRNA expression, and MLCK protein expression (Figure 9, A–C). The NIK silencing did not affect the increase in MLCK promoter activity or MLCK expression (Figure 9, D–F). These results suggested that MEKK-1 regulation of Caco-2 TJ permeability was mediated in part by up-regulation of MLCK gene activity and increase in MLCK protein expression.
Figure 9.
Effect of siRNA induced knockdown of MEKK-1 on IL-1β–induced increase in MLCK gene activity and protein expression. A: MEKK-1 siRNA transfection resulted in a complete inhibition of IL-1β–induced increase in MLCK promoter activity. *P < 0.01 versus control; **P < 0.001 versus IL-1β treatment. B: siRNA MEKK-1 transfection prevented the IL-1β–induced increase in MLCK mRNA levels. *P < 0.01 versus control; **P < 0.01 versus IL-1β treatment. C: MEKK-1 siRNA transfection prevented the IL-1β–induced increase in MLCK protein expression. D: NIK siRNA transfection did not prevent the IL-1β–induced increase in MLCK promoter activity. *P < 0.01 versus control. E: siRNA NIK transfection did not prevent the IL-1β–induced increase in MLCK mRNA levels. *P < 0.01 versus control. F: NIK siRNA transfection did not affect the IL-1β–induced increase in MLCK protein expression.
Discussion
MAP3 kinases play a crucial role in the regulation of wide array of biological processes in the intestinal epithelial cells.47,48 However, their involvement in the regulation of intestinal epithelial TJ barrier remains unknown. The major aim of this study was to investigate the role of MAP3 kinases NIK and MEKK-1 in the regulation of intestinal epithelial TJ permeability, using filter-grown Caco-2 intestinal epithelial cells as an in vitro model of intestinal epithelium. Previous studies have shown IL-1β to cause an increase in intestinal epithelial TJ permeability in vitro and in vivo21,49,50; however, the specific regulatory pathways involved remain unknown. In this study, we examined the involvement of MAP3 kinases in the regulation of IL-1β induced increase in Caco-2 intestinal epithelial TJ permeability. IL-1β is a prototypical, proinflammatory cytokine that plays an integral role in the inflammatory process of the gut.11,12 The IL-1β stimulation of biological actions is initiated by IL-1β binding to the IL-1 receptor on the cell membrane surface, and subsequent IL-1 receptor complex recruitment of adaptor proteins including IL-1 RacP, MyD88, Tollip, and tumor-necrosis factor receptor–associated factors 2 and 6.9,10 The recruitment of adaptor proteins to the IL-1β receptor complex leads to the recruitment and activation of MAP3 kinases. MAP3 kinases play a crucial regulatory role in the activation of protein kinase pathways that lead to specific cellular responses.37,38,39 IL-1β has been shown to induce activation of MAP3 kinases NIK and MEKK-1 in various cell types.40,41,42,43 Our data show that IL-1β causes a rapid activation of both MEKK-1 and NIK in Caco-2 cells. The siRNA-induced silencing of MEKK1 expression abolishes the IL-1β–induced increase in Caco-2 TJ permeability while NIK silencing does not have any effect. Thus, our results suggested that the IL-1β–induced increase in Caco-2 TJ permeability was mediated in part by the activation of MEKK-1 pathway.
The MAP-3 kinases are important regulators of NF-κB pathways. Two distinct signaling pathways have been described that lead to the activation of specific NF-κB dimers: the canonical (or classical) and the noncanonical (or alternative) pathways.45,46 In the canonical pathway, ligand binding to the cell surface receptor leads to the ligand–receptor complex recruitment of membrane shuttle kinases that lead to the phosphorylation and degradation of inhibitory IκB protein and activation of NF-κB dimer p50/p65.45 The ligand-induced activation of noncanonical pathway results in the phosphorylation of p100 subunit, leading to the generation and activation of p52/Rel B dimer.45,46 Previous studies have shown NF-κB to play an important role in the cytokine (TNF-α, IL-1β, and IFN-γ)-induced increase in intestinal epithelial TJ permeability.21,23 In these studies, the inhibition of NF-κB activity prevented the cytokine-induced increase in Caco-2 TJ permeability.21,23 In the present study, the role of NF-κB pathways in MAP3 kinase regulation of Caco-2 TJ permeability was examined. Our data show that IL-1β induces activation of both canonical and noncanonical NF-κB pathways in Caco-2 cells. The IL-1β activation of canonical pathway appeared to be mediated by MEKK-1 pathway as MEKK-1 knockdown inhibited the activation of p65 in Caco-2 cells without affecting p52 activation. Conversely, the NIK knockdown prevented the IL-1β activation of p52 without having an effect on p65 activation. Together, these results suggested that MEKK-1 mediated the IL-1β–induced activation of canonical pathway in Caco-2 cells and that NIK regulated the activation of noncanonical pathway. Consistent with the regulatory role of MEKK-1 in Caco-2 TJ permeability, the knockdown of p65 prevented the IL-1β–induced increase in Caco-2 TJ permeability while p100 silencing did not have any effect. Thus, our data suggested that the IL-1β–induced increase in Caco-2 TJ permeability was mediated by MEKK-1–induced activation of canonical pathway. Although NIK is primarily involved in the activation of noncanonical pathway, in certain conditions NIK has been shown to promote canonical pathway activation.51 In our studies, NIK did not play a role in canonical pathway activation or in Caco-2 TJ barrier regulation.
The inhibitory κB kinase (IKK) complex plays an integral role in the regulation of NF-κB activity.45,52 IKK complex consists of two catalytic subunits IKK-α and IKK-β and a regulatory subunit IKKγ/NEMO. IKK-α and IKK-β are protein kinases and have enzymatic function.49 IKKγ is a scaffolding protein and does not have catalytic activity, but nevertheless plays a critical role in the formation of IKK complex.45,52 The activation of IKK complex leads to the phosphorylation of serine residues within the activation loop of IKK catalytic subunits. The activated IKK catalytic subunits phosphorylate IκB proteins at Ser32 and Ser36 in IκBα, leading to the eventual degradation of IκBα by 26S proteasome and activation and nuclear translocation of NF-κB p65/p50 dimer.49 The involvement of IKK catalytic subunits in the regulation of TJ barrier function has not been previously reported. In this study, the role of specific IKK catalytic subunits in IL-1β modulation of Caco-2 TJ barrier function was also examined. Our data indicated that both IKK-α and IKK-β were activated in response to IL-1β stimulation. IKK-β appeared to be the catalytic subunit primarily responsible for the IL-1β–induced increase in Caco-2 TJ permeability as IKK-β silencing completely inhibited, while IKK-α silencing only partially inhibited, the increase in Caco-2 TJ permeability. The involvement of IKK catalytic subunits in TJ barrier regulation correlated with their ability to induce IκBα degradation and induce activation of p65 subunit. These findings are consistent with the involvement of canonical pathways in the regulation of Caco-2 TJ barrier. Our results also confirmed that MEKK-1 was responsible for the IL-1β–induced activation of IKK catalytic subunits. Together, our results suggested that the IL-1β–induced increase in Caco-2 TJ permeability was regulated by MEKK-1–induced activation of IKK catalytic subunits; the activated IKK catalytic subunits presumably catalyzed the phosphorylation and subsequent degradation of IκBα and activation of NF-κB p65 subunits, leading to the opening of the Caco-2 TJ barrier (Figure 10).
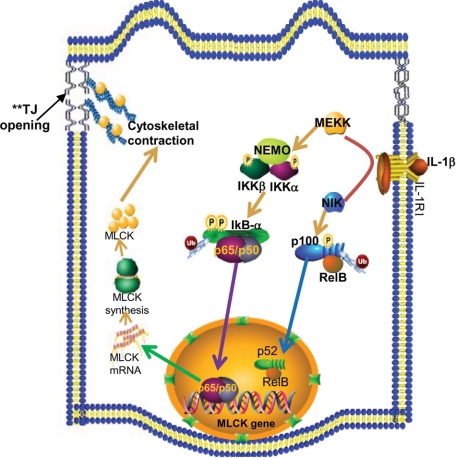
Figure 10.
Proposed scheme of the intracellular pathways involved in IL-1β–induced increase in intestinal epithelial tight junction (TJ) permeability. IL-1β treatment resulted in activation of the MEKK-1 and NIK signaling cascades. MEKK-1 activation resulted in a step-wise activation of IKK and the canonical NF-κB pathway and activation of the MLCK gene, culminating in the opening of the TJ barrier.
As for the downstream mechanisms involved, MLCK has been shown to be a key effector protein regulating the cytokine induced opening of the intestinal epithelial TJ barrier.2,22,28 Previous studies have shown that the IL-1β–induced increase in MLCK gene and protein expression was required for the increase in Caco-2 TJ permeability.22 Our present data show that MEKK-1 plays an important regulatory role in mediating the IL-1β–induced activation of MLCK gene and increase in MLCK protein expression. Our results suggested that the IL-1β–induced increase in MLCK gene and protein expression was due to MEKK-1 pathway up-regulation of MLCK gene activity (Figure 10). Presumably, MEKK-1–induced activation of IKK and NF-κB resulted in NF-κB–dependent activation of MLCK gene.44 As shown previously, the increase in MLCK protein expression leads to MLCK-dependent opening of the TJ barrier (Figure 10).21,53 It has been shown that MLCK catalyzes the phosphorylation of myosin light chain (MLC); which in turn activates Mg2+–myosin ATPase leading to the contraction of peri-junctional acto-myosin filaments and mechanical tension induced opening of the TJ barrier.54,55
In conclusion, our data show for the first time that MAP3 kinase MEKK-1 (but not NIK) plays an integral role in IL-1β modulation of Caco-2 intestinal epithelial TJ barrier. Our results indicated that the IL-1β–induced activation of MEKK-1 leads to the activation of IKK catalytic subunits IKK-β and IKK-α (Figure 10). The activated IKK catalytic subunits induce phosphorylation and degradation of IκB, leading to the activation of canonical NF-κB pathway and canonical pathway-dependent opening of the Caco-2 TJ barrier. Our data also indicate that MEKK-1 regulates the activation of MLCK gene activity, leading to the increase in MLCK protein expression. Together, these data provide important new insight into the role of MEKK-1 in regulating the IL-1β modulation of intestinal epithelial TJ barrier (Figure 10).
Acknowledgments
Images in this article were generated in the University of New Mexico & Cancer Center Fluorescence Microscopy Shared Resource, which receives separate funding.
Footnotes
Address reprint requests to Thomas Y. Ma, M.D., Ph.D., Internal Medicine-Gastroenterology, MSC10 5550, University of New Mexico, Albuquerque, NM 87131-0001. E-mail: tma@salud.unm.edu.
Supported by a Veterans Affairs (VA) Merit Review grant from the VA Research Service and National Institute of Diabetes and Digestive and Kidney Diseases grant RO 1-DK-64165 and RO 1-DK-81429.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Hollander D, Vadheim CM, Brettholz E, Petersen GM, Delahunty T, Rotter JI. Increased intestinal permeability in patients with Crohn’s disease and their relatives. A possible etiologic factor. Ann Intern Med. 1986;105:883–885. doi: 10.7326/0003-4819-105-6-883. [DOI] [PubMed] [Google Scholar]
- Ma TY, Anderson JM. Tight junctions and the intestinal barrier. Burlington, MA: Elsevier Academic Press,; Physiology of the Gastrointestinal Tract. 2006:pp1559–1594. [Google Scholar]
- DeMeo MT, Mutlu EA, Keshavarzian A, Tobin MC. Intestinal permeation and gastrointestinal disease. J Clin Gastroenterol. 2002;34:385–396. doi: 10.1097/00004836-200204000-00003. [DOI] [PubMed] [Google Scholar]
- Hecht G, Koutsouris A, Pothoulakis C, LaMont JT, Madara JL. Clostridium difficile toxin B disrupts the barrier function of T84 monolayers. Gastroenterology. 1992;102:416–423. doi: 10.1016/0016-5085(92)90085-d. [DOI] [PubMed] [Google Scholar]
- Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- Hollander D. Crohn’s disease–a permeability disorder of the tight junction? Gut. 1988;29:1621–1624. doi: 10.1136/gut.29.12.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaldo CT, Nusrat A. Cytokine regulation of tight junctions. Biochim Biophys Acta. 2009;1788:864–871. doi: 10.1016/j.bbamem.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May GR, Sutherland LR, Meddings JB. Is small intestinal permeability really increased in relatives of patients with Crohn’s disease? Gastroenterology. 1993;104:1627–1632. doi: 10.1016/0016-5085(93)90638-s. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. The interleukin-1 family: 10 years of discovery. FASEB J. 1994;8:1314–1325. [PubMed] [Google Scholar]
- Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- Ligumsky M, Simon PL, Karmeli F, Rachmilewitz D. Role of interleukin 1 in inflammatory bowel disease–enhanced production during active disease. Gut. 1990;31:686–689. doi: 10.1136/gut.31.6.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Saito H, Kasanuki J, Tamura Y, Yoshida S. Cytokine production in patients with inflammatory bowel disease. Gut. 1992;33:933–937. doi: 10.1136/gut.33.7.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinecker HC, Steffen M, Witthoeft T, Pflueger I, Schreiber S, MacDermott RP, Raedler A. Enhanced secretion of tumour necrosis factor-alpha. IL-6, and IL-1 beta by isolated lamina propria mononuclear cells from patients with ulcerative colitis and Crohn’s disease. Clin Exp Immunol. 1993;94:174–181. doi: 10.1111/j.1365-2249.1993.tb05997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carty E, De Brabander M, Feakins RM, Rampton DS. Measurement of in vivo rectal mucosal cytokine and eicosanoid production in ulcerative colitis using filter paper. Gut. 2000;46:487–492. doi: 10.1136/gut.46.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cominelli F, Pizarro TT. Interleukin-1 and interleukin-1 receptor antagonist in inflammatory bowel disease, Aliment Pharmacol Ther. 1996;10 (Suppl 2):49–53. doi: 10.1046/j.1365-2036.1996.22164020.x. discussion 54. [DOI] [PubMed] [Google Scholar]
- Mittal RD, Bid HK, Ghoshal UC. IL-1 receptor antagonist (IL-1Ra) gene polymorphism in patients with inflammatory bowel disease in India. Scand J Gastroenterol. 2005;40:827–831. doi: 10.1080/00365520510015629. [DOI] [PubMed] [Google Scholar]
- Heresbach D, Alizadeh M, Dabadie A, Le Berre N, Colombel JF, Yaouanq J, Bretagne JF, Semana G. Significance of interleukin-1beta and interleukin-1 receptor antagonist genetic polymorphism in inflammatory bowel diseases. Am J Gastroenterol. 1997;92:1164–1169. [PubMed] [Google Scholar]
- Nemetz A, Nosti-Escanilla MP, Molnar T, Kope A, Kovacs A, Feher J, Tulassay Z, Nagy F, Garcia-Gonzalez MA, Pena AS. IL1B gene polymorphisms influence the course and severity of inflammatory bowel disease. Immunogenetics. 1999;49:527–531. doi: 10.1007/s002510050530. [DOI] [PubMed] [Google Scholar]
- Cominelli F, Nast CC, Clark BD, Schindler R, Lierena R, Eysselein VE, Thompson RC, Dinarello CA. Interleukin 1 (IL-1) gene expression, synthesis, and effect of specific IL-1 receptor blockade in rabbit immune complex colitis. J Clin Invest. 1990;86:972–980. doi: 10.1172/JCI114799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cominelli F, Nast CC, Duchini A, Lee M. Recombinant interleukin-1 receptor antagonist blocks the proinflammatory activity of endogenous interleukin-1 in rabbit immune colitis. Gastroenterology. 1992;103:65–71. doi: 10.1016/0016-5085(92)91096-m. [DOI] [PubMed] [Google Scholar]
- Al-Sadi R, Ye D, Dokladny K, Ma TY. Mechanism of IL-1beta-induced increase in intestinal epithelial tight junction permeability. J Immunol. 2008;180:5653–5661. doi: 10.4049/jimmunol.180.8.5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Sadi RM, Ma TY. IL-1beta causes an increase in intestinal epithelial tight junction permeability. J Immunol. 2007;178:4641–4649. doi: 10.4049/jimmunol.178.7.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma TY, Iwamoto GK, Hoa NT, Akotia V, Pedram A, Boivin MA, Said HM. TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am J Physiol Gastrointest Liver Physiol. 2004;286:G367–G376. doi: 10.1152/ajpgi.00173.2003. [DOI] [PubMed] [Google Scholar]
- Madara JL, Stafford J. Interferon-gamma directly affects barrier function of cultured intestinal epithelial monolayers. J Clin Invest. 1989;83:724–727. doi: 10.1172/JCI113938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruewer M, Utech M, Ivanov AI, Hopkins AM, Parkos CA, Nusrat A. Interferon-gamma induces internalization of epithelial tight junction proteins via a macropinocytosis-like process. FASEB J. 2005;19:923–933. doi: 10.1096/fj.04-3260com. [DOI] [PubMed] [Google Scholar]
- Marano CW, Lewis SA, Garulacan LA, Soler AP, Mullin JM. Tumor necrosis factor-alpha increases sodium and chloride conductance across the tight junction of CACO-2 BBE, a human intestinal epithelial cell line. J Membr Biol. 1998;161:263–274. doi: 10.1007/s002329900333. [DOI] [PubMed] [Google Scholar]
- Wang F, Graham WV, Wang Y, Witkowski ED, Schwarz BT, Turner JR. Interferon-gamma and tumor necrosis factor-alpha synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am J Pathol. 2005;166:409–419. doi: 10.1016/s0002-9440(10)62264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Sadi R, Boivin M, Ma T. Mechanism of cytokine modulation of epithelial tight junction barrier. Front Biosci. 2009;14:2765–2778. doi: 10.2741/3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayburgh DR, Barrett TA, Tang Y, Meddings JB, Van Eldik LJ, Watterson DM, Clarke LL, Mrsny RJ, Turner JR. Epithelial myosin light chain kinase-dependent barrier dysfunction mediates T cell activation-induced diarrhea in vivo. J Clin Invest. 2005;115:2702–2715. doi: 10.1172/JCI24970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayburgh DR, Musch MW, Leitges M, Fu YX, Turner JR. Coordinated epithelial NHE3 inhibition and barrier dysfunction are required for TNF-mediated diarrhea in vivo. J Clin Invest. 2006;116:2682–2694. doi: 10.1172/JCI29218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrieta MC, Madsen K, Doyle J, Meddings J. Reducing small intestinal permeability attenuates colitis in the IL10 gene-deficient mouse. Gut. 2009;58:41–48. doi: 10.1136/gut.2008.150888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen KL, Malfair D, Gray D, Doyle JS, Jewell LD, Fedorak RN. Interleukin-10 gene-deficient mice develop a primary intestinal permeability defect in response to enteric microflora. Inflamm Bowel Dis. 1999;5:262–270. doi: 10.1097/00054725-199911000-00004. [DOI] [PubMed] [Google Scholar]
- Ferrier L, Mazelin L, Cenac N, Desreumaux P, Janin A, Emilie D, Colombel JF, Garcia-Villar R, Fioramonti J, Bueno L. Stress-induced disruption of colonic epithelial barrier: role of interferon-gamma and myosin light chain kinase in mice. Gastroenterology. 2003;125:795–804. doi: 10.1016/s0016-5085(03)01057-6. [DOI] [PubMed] [Google Scholar]
- Miehsler W, Puspok A, Oberhuber T, Vogelsang H. Impact of different therapeutic regimens on the outcome of patients with Crohn’s disease of the upper gastrointestinal tract. Inflamm Bowel Dis. 2001;7:99–105. doi: 10.1097/00054725-200105000-00004. [DOI] [PubMed] [Google Scholar]
- Wild GE, Waschke KA, Bitton A, Thomson AB. The mechanisms of prednisone inhibition of inflammation in Crohn’s disease involve changes in intestinal permeability, mucosal TNFalpha production and nuclear factor kappa B expression. Aliment Pharmacol Ther. 2003;18:309–317. doi: 10.1046/j.1365-2036.2003.01611.x. [DOI] [PubMed] [Google Scholar]
- Wyatt J, Vogelsang H, Hubl W, Waldhoer T, Lochs H. Intestinal permeability and the prediction of relapse in Crohn’s disease. Lancet. 1993;341:1437–1439. doi: 10.1016/0140-6736(93)90882-h. [DOI] [PubMed] [Google Scholar]
- Thalhamer T, McGrath MA, Harnett MM. MAPKs and their relevance to arthritis and inflammation. Rheumatology (Oxford) 2008;47:409–414. doi: 10.1093/rheumatology/kem297. [DOI] [PubMed] [Google Scholar]
- Symons A, Beinke S, Ley SC. MAP kinase kinase kinases and innate immunity. Trends Immunol. 2006;27:40–48. doi: 10.1016/j.it.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Dunne A, O'Neill LA. The interleukin-1 receptor/Toll-like receptor superfamily: signal transduction during inflammation and host defense. Sci STKE. 2003;2003:re3. doi: 10.1126/stke.2003.171.re3. [DOI] [PubMed] [Google Scholar]
- Li Q, Engelhardt JF. Interleukin-1beta induction of NFkappaB is partially regulated by H2O2-mediated activation of NFkappaB-inducing kinase. J Biol Chem. 2006;281:1495–1505. doi: 10.1074/jbc.M511153200. [DOI] [PubMed] [Google Scholar]
- Takeuchi J, Hirota K, Itoh T, Shinkura R, Kitada K, Yodoi J, Namba T, Fukuda K. Thioredoxin inhibits tumor necrosis factor- or interleukin-1-induced NF-kappaB activation at a level upstream of NF-kappaB-inducing kinase. Antioxid Redox Signal. 2000;2:83–92. doi: 10.1089/ars.2000.2.1-83. [DOI] [PubMed] [Google Scholar]
- Kim YJ, Hwang SY, Oh ES, Oh S, Han IO. IL-1beta, an immediate early protein secreted by activated microglia, induces iNOS/NO in C6 astrocytoma cells through p38 MAPK and NF-kappaB pathways. J Neurosci Res. 2006;84:1037–1046. doi: 10.1002/jnr.21011. [DOI] [PubMed] [Google Scholar]
- Oetjen E, Blume R, Cierny I, Schlag C, Kutschenko A, Kratzner R, Stein R, Knepel W. Inhibition of MafA transcriptional activity and human insulin gene transcription by interleukin-1beta and mitogen-activated protein kinase kinase kinase in pancreatic islet beta cells. Diabetologia. 2007;50:1678–1687. doi: 10.1007/s00125-007-0712-2. [DOI] [PubMed] [Google Scholar]
- Ye D, Ma I, Ma TY. Molecular mechanism of tumor necrosis factor-alpha modulation of intestinal epithelial tight junction barrier. Am J Physiol Gastrointest Liver Physiol. 2006;290:G496–G504. doi: 10.1152/ajpgi.00318.2005. [DOI] [PubMed] [Google Scholar]
- Pomerantz JL, Baltimore D. Two pathways to NF-kappaB. Mol Cell. 2002;10:693–695. doi: 10.1016/s1097-2765(02)00697-4. [DOI] [PubMed] [Google Scholar]
- Baldwin AS., Jr The NF-kappa B and I kappa B proteins: new discoveries and insights, Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- Aliaga JC, Deschenes C, Beaulieu JF, Calvo EL, Rivard N. Requirement of the MAP kinase cascade for cell cycle progression and differentiation of human intestinal cells. Am J Physiol. 1999;277:G631–G641. doi: 10.1152/ajpgi.1999.277.3.G631. [DOI] [PubMed] [Google Scholar]
- Magro F, Fraga S, Ribeiro T, Soares-da-Silva P. Intestinal Na+-K+-ATPase activity and molecular events downstream of interferon-gamma receptor stimulation. Br J Pharmacol. 2004;142:1281–1292. doi: 10.1038/sj.bjp.0705895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Wang X, Lasson A, Borjesson A, Leveau P, Haraldsen P, Andersson R. Roles of platelet-activating factor, interleukin-1beta and interleukin-6 in intestinal barrier dysfunction induced by mesenteric arterial ischemia and reperfusion. J Surg Res. 1999;87:90–100. doi: 10.1006/jsre.1999.5746. [DOI] [PubMed] [Google Scholar]
- Barreau F, Ferrier L, Fioramonti J, Bueno L. Neonatal maternal deprivation triggers long term alterations in colonic epithelial barrier and mucosal immunity in rats. Gut. 2004;53:501–506. doi: 10.1136/gut.2003.024174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarnegar B, Yamazaki S, He JQ, Cheng G. Control of canonical NF-kappaB activation through the NIK-IKK complex pathway. Proc Natl Acad Sci USA. 2008;105:3503–3508. doi: 10.1073/pnas.0707959105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M, Delhase M. The I kappa B kinase (IKK) and NF-kappa B: key elements of proinflammatory signalling. Semin Immunol. 2000;12:85–98. doi: 10.1006/smim.2000.0210. [DOI] [PubMed] [Google Scholar]
- Ma TY, Boivin MA, Ye D, Pedram A, Said HM. Mechanism of TNF-{alpha} modulation of Caco-2 intestinal epithelial tight junction barrier: role of myosin light-chain kinase protein expression. Am J Physiol Gastrointest Liver Physiol. 2005;288:G422–G430. doi: 10.1152/ajpgi.00412.2004. [DOI] [PubMed] [Google Scholar]
- Ma TY, Hoa NT, Tran DD, Bui V, Pedram A, Mills S, Merryfield M. Cytochalasin B modulation of Caco-2 tight junction barrier: role of myosin light chain kinase. Am J Physiol Gastrointest Liver Physiol. 2000;279:G875–G885. doi: 10.1152/ajpgi.2000.279.5.G875. [DOI] [PubMed] [Google Scholar]
- Madara JL, Barenberg D, Carlson S. Effects of cytochalasin D on occluding junctions of intestinal absorptive cells: further evidence that the cytoskeleton may influence paracellular permeability and junctional charge selectivity. J Cell Biol. 1986;102:2125–2136. doi: 10.1083/jcb.102.6.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]