Abstract
Pituitary adenylate cyclase-activating peptide (PACAP) is an important neuropeptide and immunomodulator in various tissues. Although this peptide and its receptors (ie, VPAC1R, VPAC2R, and PAC1R) are expressed in human skin, their biological roles are unknown. Therefore, we tested whether PACAP regulates vascular responses in human skin in vivo. When injected intravenously, PACAP induced a significant, concentration-dependent vascular response (ie, flush, erythema, edema) and mediated a significant and concentration-dependent increase in intrarectal body temperature that peaked at 2.7°C. Topical application of PACAP induced marked concentration-dependent edema. Immunohistochemistry revealed a close association of PACAP-immunoreactive nerve fibers with mast cells and dermal blood vessels. VPAC1R was expressed by dermal endothelial cells, CD4+ and CD8+ T cells, mast cells, and keratinocytes, whereas VPAC2R was expressed only in keratinocytes. VPAC1R protein and mRNA were also detected in human dermal microvascular endothelial cells. The PACAP-induced change in cAMP production in these cells demonstrated VPAC1R to be functional. PACAP treatment of organ-cultured human skin strongly increased the number of CD31+ vessel cross-sections. Taken together, these results suggest that PACAP directly induces vascular responses that may be associated with neurogenic inflammation, indicating for the first time that PACAP may be a crucial vascular regulator in human skin in vivo. Antagonists to PACAP function may be beneficial for the treatment of inflammatory skin diseases with a neurogenic component.
Evidence is accumulating that neuropeptides play an essential role in skin–nervous system interactions.1,2 Sensory fibers have been shown to be involved in inflammatory skin diseases such as urticaria,3,4,5,6 psoriasis,7 rosacea, and atopic dermatitis.8,9,10 Pituitary adenylate cyclase activating polypeptide (PACAP) is a regulatory peptide that belongs to the vasoactive intestinal peptide (VIP)/secretin family and exists in two forms, PACAP1-27 and PACAP1-38, which have similar biological activity.11,12,13 In peripheral tissues of mammals, PACAP1-38 is by far the predominant form, but the proportions of PACAP1-27 and PACAP1-38 vary among different organs.13,14 Thus far, PACAP has been localized in nerve fibers of various peripheral tissues as well as in lymphoid tissues and immunocompetent cells,15,16,17 colocalizing with important neuropeptides such as substance P or calcitonin-gene related peptide.
Its distribution suggests PACAP may play an important role in both the sensory and autonomic nervous system18,19 and is involved in pain, immunomodulation, and inflammatory processes.20 For example, PACAP induced the release of histamine21 and serotonin22 from human mast cells (MC) and mediated vasodilatation23 and plasma extravasation23 in rodents. In these species, PACAP administration led to vasodilatation and plasma extravasation23,24 and induced a significant relaxation in precontracted vessels as well as cAMP accumulation. Interestingly, the PACAP-induced effects were five times higher than the cAMP concentration induced by VIP, peptide histidine methionine (PHM), and peptide histidine valine (PHV).25 However, the role of PACAP in human neurogenic inflammation and vasoregulation in human skin is still unknown.
The biological effect of PACAP is mediated by PACAP/VIP G protein–coupled receptors. Three distinct receptor subtypes with different affinities for VIP or PACAP have been observed: vasoactive intestinal peptide/pituitary adenylate cyclase activating polypeptide-receptor-1 (VPAC1R) and VPAC2 receptors show equal affinity for PACAP and VIP, whereas the PAC1 receptor shows affinity only for PACAP.26 In humans, VPAC1R and VPAC2R are expressed in various tissues such as lung, brain, heart, and liver.27,28 Both receptors are also found in immune or skin cells, including keratinocytes, endothelial cells, and monocytes.20,29,30,31,32,33,34
Both PACAP and VIP appear to contribute to experimentally-induced arthritis,35 suggesting PACAP has an anti-inflammatory role during chronic inflammation. However, animal in vivo studies indicate that PACAP also has a proinflammatory role in acute inflammation by modulating vasodilatation and plasma extravasation,22,23,24 two phenomena that are observed clinically as edema and erythema in many inflammatory skin diseases. Both symptoms in particular characterize the pathology of urticaria, a disease in which angioedema and spontaneous wheals rapidly emerge.36 Our hypothesis is that PACAP may be involved in the regulation of vascular and immune responses in human skin and may be important in the pathology of urticaria and atopic dermatitis, lesional human skin diseases characterized by vasoregulative dysfunction, and neurogenic inflammation.
To test this hypothesis, the aims of this study were to i) examine the effects of PACAP infusion on the modulation of skin vasculature in humans in vivo, ii) determine the role of PACAP on blood circulation in human skin in vivo, iii) define whether PACAP induces vasodilatation in human skin in vivo, iv) determine and localize the expression of PACAP and functional VPAC receptors in human skin, lesional skin, and cultured dermal microvascular endothelial cells, v) verify whether PACAP is functional in human dermal microvascular endothelial cells, and vi) verify whether PACAP modulates the function of blood vessels in three-dimensional skin organ cultures.
Materials and Methods
Materials
PACAP1-27, PACAP1-38, PACAP6-38, or VIP6-28 were generated with an automatic peptide synthesizer (Novasym Crystal, Novabiochem, Cambridge, UK). VIP1-28-NH2 was ordered from Bissendorf Biochem, Hannover, Germany. Human serum albumin (20%) was purchased from Behring Comp., Marburg, Germany. Instruments for the detection of intrarectal body temperature (RT) and blood flow were from Braun Comp., Melsungen, Germany. Other reagents were from Sigma, Deisendorf, Germany.
Healthy Volunteers
Thirty-three healthy men (average age, 26.7 ± 5.2 years) participated in this study after informed consent was obtained. Permission for human studies was given by the Ethical Committees of the Universities of Goettingen, Bochum and Muenster, Germany, in accordance with the ethical standards of the 1964 Declaration of Helsinki. All volunteers completed the study without complications. For immunohistochemical studies, at least six patients and healthy skin tissues were used (three men and three women; average age was 24 ± 6 for atopic dermatitis and 43 ± 9 for urticaria). For real-time PCR, healthy skin from at least four patients was used for each experiment.
Infusion of PACAP and Effects on Vascular Function in Vivo
After a basal period of 15 minutes, subjects received a continuous infusion of 7.5, 15, 30, 100 pmol/kg b.w./h sterile PACAP1-27, diluted in 2% human serum albumin. Alternatively, VIP1-28 (20 or 100 pmol/kg b.w./h) was infused in the same manner as PCAP. Body temperature (intrarectal body temperature, RT) was measured at defined time points. After each experiment, venous blood was obtained, centrifuged, and stored at −20°C until use.
Full-Field Laser Perfusion Imaging
Laser perfusion imaging was conducted in an air-conditioned (temperature 22 ± 0.5°C, humidity 49 ± 5%) and undisturbed environment. Participants were seated comfortably in a reclining chair, with their lower forearms placed in a vacuum cushion (HEK Medical, Lübeck, Germany) for immobilization. Before recording began, all participants were instructed to breathe evenly, to remain silent, and to avoid any body movement throughout the experiment. Two spots 5 cm apart on each forearm were chosen for stimulation and 50 μl of Ringer’s solution containing PACAP (1 × 10−9 to 1 × 10−6 mol/L; Bachem, Weil am Rhein, Germany) was injected in random order into the volar forearm of subjects. The full-field laser perfusion imager (FLPI; Moor Instruments Ltd, Axminster, UK) (free from vibrations), was mounted perpendicular to, and at a distance of 25 cm from, the skin surface. The image focus of the FLPI was adjusted to cover a rectangular area of 8 × 8 cm2 (152 × 113 pixels) around the stimulation site. For FLPI image capture, a rectangular cardboard (4.0 cm2) was placed on the skin during the first three images for standardized area calibration. After the cardboard was removed, a further series of five images was recorded as baseline.
RNA Extraction from Tissue and Quantitative Real-Time PCR
Total RNA was extracted from 4-mm punch biopsies taken from healthy adult volunteers using RNeasy mini and micro extraction kits from Qiagen (Courtaboeuf Cedex, France) according to the manufacturer’s instructions. RNA quantity was measured using the Quant-it RNA assay kit (Molecular Probes, Inc., Eugene, OR), and 200 ng of extracted RNA was then used for synthesizing cDNA using high capacity cDNA archive kits (Applied Biosystems, Courtaboeuf, France). cDNA was subjected to the ready-to-use TaqMan Gene Expression Assay (Applied Biosystems). Assay identification numbers of interest were Hs00270351 for VPAC1R and Hs00173643. qRT-PCR was performed using Master Mix (Universal PCR Master Mix from Applied Biosystems) according to manufacturer’s instructions. qRT-PCR was performed in a 7900 HT Cycler (Applied Biosystems) for 40 cycles of amplification. Data are expressed in terms of cycle threshold (Ct), and mRNA expression was determined using the delta Ct method, where normalization was performed using the mean of four housekeeping genes, RNA 18S, GAPDH, ACTB, and HPRT1. The normalized Ct was calculated according to following formula:
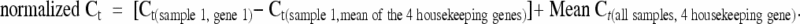 |
RNA Isolation and Quantitative RT PCR for VPACRs in HDMEC
Human dermal microvascular endothelial cells (HDMEC) were cultured with endothelial growth medium (Promocell, Heidelberg, Germany), grown to 80% confluence, and harvested. RNA was prepared with the Trizol method.9 For quantitative RT-PCR, HDMEC were pelleted and total RNA was extracted using the RNA solubility buffer TRIzol (Life Technologies, Karlsruhe, Germany), according to the manufacturer’s instructions. To avoid DNA contamination, total RNA was treated with 10 U RQ1RNase-free DNase I (Promega, Mannheim, Germany) for 30 minutes at 37°C. The amount of total RNA was measured spectrophotometrically.
One microgram RNA was subjected to reverse transcription using a reverse transcription system and random primer (Promega) at a final reaction volume of 20 μl containing 5 mmol/L MgCl2, 1× reverse transcriptase buffer, 1 mmol/L each dNTP, 1 U/μl RNAsin, 20 U AMV reverse transcriptase, and 0.5 μg random primers. Tubes with reaction mixtures were incubated for 10 minutes at room temperature, then at 42°C for 30 minutes, followed by 5 minutes of enzyme inactivation at 95°C, and chilled on ice for 5 minutes. cDNA was stored at −20°C until experiments were performed.
For quantitative RT-PCR amplifications the following primer pairs were used:
VPAC1R forward primer: 5′-GTACACTACATCATGTTCGCCTTC-3′, reverse primer: 5′-AGTAGAGGATAGCCACCACAAAAC-3′; VPAC2R forward primer: 5′-CATAAGCAAAAACTGTACGAGTGAC-3′, reverse primer: 5′-GACACTGTAGCCCAGGGTATAAAT-3′; PAC1R forward primer: 5′-AATCCACTACACAGTATTTGCCTT-3′, reverse primer: 5′-CTCACCATTCAGAAAACAGTAGAGA-3′.
The PCR products for VPAC1R, VPAC2R and PAC1R were amplified from the same undiluted complementary DNA using the following program: 1 cycle 50°C, 2 minutes; followed by 1 cycle of 95°C, 15 minutes and 40 cycles of 95°C, 15 seconds and 60°C, 1 minute.
Tissue Preparation, Immunohistochemistry, and Double-Immunofluorescence
Punch biopsies (4 mm) from healthy adult volunteers as well as from patients with chronic urticaria (n = 12) or atopic dermatitis (n = 8) were done under local anesthesia, fixed by immersion in Bouin’s solution or Stefanini’s solution for 4–6 hours, washed, and stored in 30% sucrose/PBS for 24 hours at 4°C.9 Additional punch biopsies were done on postoperative tissue (n = 15) and prepared the same way. Frozen sections (10–14 μm) were preincubated for 30 minutes with normal serum and incubated overnight at 4°C with mouse monoclonal antibodies against PACAP1-38 (1:10; kindly provided by Dr. J. Fahrenkrug, Department of Clinical Biochemistry, Copenhagen, Denmark); mouse monoclonal antibodies against PACAP1-27 or PACAP1-38 (1:200;37), or rabbit polyclonal antibodies against PACAP1-38 and PACAP1-27 (1:1000, Euro Diagnostica, Malmo, Sweden). A monoclonal antibody against human tryptase was purchased from Chemicon (Ochsenhausen, Germany). The sites of localization of antibodies to PACAP were revealed by the indirect or double-immunofluorescence technique as previously described.9,33 The following controls were included in each study: i) preabsorption of antibodies at working dilutions with an excess of the respective antigen (10−5 mol/L, 48 hours before reaction); ii) application of isotype-specific Ig antibody. The antisera used showed no cross-reactivity with other antibodies tested such as VIP (1:2000), calcitonin-gene related peptide (1:3000), or substance P (1:2000) (all from Euro Diagnostica, Malmö, Sweden). After being washed in a dark chamber, slides were mounted in Vectashield (Vector Laboratories, Burlingame, CA) and examined using a Leica microscope (Leica DMR, Heidelberg, Germany). For immunohistochemistry against receptors, specimens were obtained as described38,39 and incubated with primary antibodies against VPAC receptors: VPAC1R, 1:500; VPAC2R, 1:500, kindly provided by Dr. E. J. Goetzl, UCSF, San Francisco, USA) or PAC1R (1:100, Chemicon, Germany). The next day, slides were washed 3× in PBS buffer for 30 minutes and incubated with a secondary antibody (goat-anti-mouse IgG, 1:200) for 60 minutes, then washed, and incubated with AEC for 15 minutes, as described.38 In controls, primary polyclonal antibodies were preincubated for 24–48 hours with corresponding peptides (10–100 μmol/L) used for immunization or matched monoclonal Ig control antibodies were used to elucidate background staining.
For double-immunofluorescence analysis, VPAC1R antibody (rabbit anti-VPAC1, 375 μg/ml, Dr. E. J. Goetzl, UCSF, San Francisco, USA) or VPAC1 (2VIPR-2H8, Santa Cruz Biotechnology, Inc., Santa Cruz, CA) was incubated at 4°C overnight. The next day, slides were washed in PBS buffer and incubated with the secondary antibody Alexa Fluor 488 chicken anti-rabbit IgG (1:200) (Molecular Probes, Inc.) for 60 minutes at room temperature. For costaining, VPAC1R staining (same dilution) was performed together with antibodies against CD31 (blood endothelial cells, monoclonal mouse anti-human CD31 Clone JC70A, 1:50, DAKO, Glostrup, Denmark), mouse anti-human smooth muscle actin (Clone 1A4, 1:300, DAKO), CD45 (T-cells, monoclonal mouse anti-human CD45R, 1:50, DAKO), CD4 (T-helper cells, mouse anti-human CD4 IgG1, 1:20, Biozol, Germany), CD8 (suppressor/cytotoxic T cells, monoclonal mouse anti-human CD8 Clone: C8/144B, Isotype: IgG1, kappa, 1:50, DAKO), CD68 (macrophages, Human CD68, 1:20, DAKO), and mast cell tryptase (Monoclonal Mouse Anti-Human Mast Cell Tryptase, 1:100, DAKO). Slides were incubated overnight at 4°C, washed with PBS buffer, and again incubated with the secondary antibodies against Alexa Fluor 488 chicken anti-rabbit IgG (1:200 in PBS) and Alexa Fluor 555 donkey anti-mouse IgG (1:200 in PBS) (Molecular Probes, Inc.) for 60 minutes at room temperature. After being washed in a dark chamber, slides were mounted in Vectashield (Vector Laboratories).
Cell Culture and cAMP EIA-Assay
HDMEC were grown in endothelial cell basal medium according to manufacturer’s instructions (PromoCell, Heidelberg, Germany) supplemented with 5% fetal calf serum, 0.1 ng per ml epidermal growth factor, 1.0 μg per ml hydrocortisone, 0.02 μg per ml gentamicin-25, and endothelial cell growth supplement, 2 ml, amphotericin-B 2.5 ng per ml. Cells in passage four were used for experiments. To verify that cultured HDMEC were free of contaminating cells, HDMEC were characterized by their typical cobblestone morphology using light microscopy. Cells for passage four were grown in 100-mm Petri dishes. Trypsin (Life Technology, Karlsruhe, Germany) was used for detaching the cells during the splitting at passages one to three. Accutase (PAA Laboratories, Coelbe, Germany) was used for detaching the cells at passage four. Lyophilized PACAP1-38 (Sigma, Deisendorf, Germany) was diluted in the appropriate volume of HDMEC assay medium before use.
In selected studies, HDMEC were kept in FCS-deficient medium for 24 hours before stimulation.40 VPAC1R antagonists41,42 (VIP6-28; PACAP6-27 (Bachem) were applied to cells in a 100-fold concentration 3 hours before neuropeptide was added, following the procedure of Rácz et al.43 Experiments were performed four times (two double experiments). IBMX (3-isobutyl-1-methylxanthin; Sigma; 4 mmol/L) was used to measure total intracellular cAMP due to receptor stimulation. After 24 hours, cells were harvested and extracted with 1.7 ml 65% ethanol in PBS, and, after centrifugation, were split into aliquots (300 μl and 1400 μl). Afterward, aliquots were lyophilized. The extract of the smaller aliquot was used for protein concentration measurements (Lowry), and diluted at 1:15 in lysis buffer for cAMP EIA analysis (Amersham Pharmacia Biotech, Freiburg, Germany), according to the manufacturer’s protocol without acetylation.
Human Full-Thickness Skin Organ Culture and Subsequent Histology
Human full-thickness skin organ culture was performed as described previously.44 Skin biopsies (three per group) were treated for 72 hours with vehicle (Williams’s E medium) or 100 nmol/L PACAP1-38. Skin punches were embedded on day 4 after initial cultivation. All incubation steps in the subsequent staining procedure were interspersed by washing with Tris-buffered saline (TBS, 0.05 mol/L, pH 7.6; 3 × 5 minutes). Nonspecific binding was blocked by using an avidin-biotin blocking kit solution (Vector Laboratories) and by 5% bovine normal serum in TBS. Thereafter, sections were incubated with the primary antibody (mouse anti-CD31, 1:30, DAKO), diluted in TBS containing 1% bovine normal serum for 45 minutes, followed by biotinylated secondary antibodies (goat anti-mouse, Beckmann-Coulter, Krefeld, Germany; 1:200 in TBS containing 4% bovine normal serum; 30 minutes). Then, the ABC-AP complex was added to the slides (Vector Laboratories; 1:100; 30 minutes), followed by staining for alkaline phosphatase, and counterstaining in Mayer’s hemalaun.45 Giemsa staining was used on paraffin-embedded longitudinal skin sections to identify MC by their characteristic morphology and the presence of metachromatic granules.46 Data were expressed as number of Giemsa-positive cells per visual field, counting 15–18 visual fields. MCs were classified as “degranulated” when five or more extracellularly located metachromatic granules could be detected histochemically when magnified.
Statistical Analysis
Data were analyzed using Statistica 6.0 software package (Statsoft, Tulsa, OK). Values were calculated by multivariate analysis of variance and repeated measures or by Student-Newman-Keuls test (multiple groups). P values <0.05 were considered significant. Data are depicted as mean ± SEM (SEM) throughout the manuscript.
Results
Peripheral Effects of PACAP and VIP in Normal Human Skin in Vivo
Intrarectal Body Temperature and Vasodilatation
Within 5 minutes after PACAP1-27 administration RT rapidly increased by + 1.2°C ± 0.15 over baseline, reaching a plateau of +1.8°C ± 0.26 after 30 minutes (Figure 1A). This effect in human skin was concentration-dependent (Figure 1B). Accordingly, i.v. infusion of PACAP1-27 at a low 7.5 pmol/kg b.w./h concentration resulted in a significant increase in RT (+1.25°C ± 0.15) over baseline (32.8°C ± 0.25), a concentration of 15 pmol/kg b.w./h resulted in an increase of +2.1°C ± 0.2, and a concentration of 30 pmol/kg b.w./h resulted in an increase of +2.4°C ± 0.3 (Figure 1B). Immediately after the infusion of 30 pmol/kg b.w./h concentration stopped, RT slowly decreased (Figure 1B).
Figure 1.
Changes in intrarectal body temperature [ΔT (°C)] compared with basal value (0 = 33.51 ± 0.25°C). A: Continuous infusion of 100 pmol/kg b.w./h PACAP1-27. Mean ± SEM, *P < 0.05 (n = 10). B: Continuous infusion of 7.5, 15, or 30 pmol/kg b.w./h PACAP1-27. Mean ± SEM, *P < 0.05 (n = 9). Mean time until first flush symptoms occurred was 46.03 ± 5.30 minutes. C: Bolus injection of 315 pmol/kg b.w. PACAP1-27. Mean ± SEM, *P < 0.05 (n = 8). D: Different conditions and concentrations of continuous infusion. Mean ± SEM, *P < 0.05 (n = 10). E: Changes in intrarectal body temperature [ΔT (°C)] compared to basal (32.75 ± 0.25°C) after continuous infusion of 20 or 100 pmol/kg b.w./h VIP1-28. Mean ± SEM, *P < 0.05 (n = 7). F: Normal skin in a healthy volunteer before intravenous application of PACAP1-27. G: Flush phenomenon in a healthy volunteer after intraveneous application of PACAP1-27. Cutaneous erythema and facial as well as a marked periorbital edema was observed within minutes, with a peak at 30 minutes after application (representative from n = 33 volunteers).
When the effect of a single bolus exposure to PACAP1-27 was compared to that of continuous infusion by intravenous injection of 315 pmol/kg b.w. PACAP1-27, we found that the bolus resulted in a rapid and significant increase of RT that peaked after 30 minutes (+2.7°C ± 0.9), followed by a plateau phase (Figure 1C). After 60 minutes, RT decreased slightly but remained elevated until the final measurement after 120 minutes. Bolus exposure resulted in a significantly higher increase in RT than did an infusion of 100 pmol/kg b.w./h PACAP1-27 (Figure 1D).
Intravenous infusion of 20 pmol/kg b.w./h VIP1-28 did not result in a significant increase in RT over basal temperature (32.75°C ± 0.25), but infusion of 100 pmol/kg b.w./h VIP1-28 did result in a continuous increase of RT (+1.36°C ± 0.33) that peaked after 60 minutes (Figure 1E); longer time periods were not measured.
Participants in both experimental groups (bolus versus continuous infusion) developed erythema (“flushing”) and edema (Figure 1F), whereas control injections of 2% human serum albumin in 0.9% saline did not cause either phenomena. In nine of ten healthy volunteers, erythema was predominantly in the face and upper trunk (Figure 1G). The approximate duration before first signs of erythema occurred after administration of 100 pmol/kg b.w./h PACAP1-27 was 46.03 ± 5.3 minutes. This erythema persisted for approximately 6 hours. Therefore, doses higher than 100 pmol/kg b.w./h PACAP1-27 could not be used in our in vivo experiments.
To test whether the erythema and edema were due to a local vascular effect, rather than a secondary systemic effect, we measured systemic characteristics such as pulse (beats per min) and blood pressure RR syst./diast. (mm Hg). No measurable differences in either were detected (data not shown).
To further address the effects of PACAP on local vascular function, we administered it intradermally into the volar forearm of volunteers (n = 6 per group). In each participant, a significant flare reaction developed within minutes after intradermal application of PACAP1-38 (Figure 2, A and B). The flare intensity and area of flare development were dependent on the PACAP concentration in that the largest and most persistent flare was observed after the highest injected PACAP concentration of 10−6 mol/L, although significant differences in the NaCl controls were found at nanomolar concentrations (Figure 2, A and B). Thus, PACAP-induced changes are most likely attributable to a local effect in cutaneous vasculature.
Figure 2.
A: Sequence of laser Doppler images recorded in response to intradermal injections of saline (upper panel) or PACAP at various doses (10−9–10−6 mol/L; panels 2–5). Laser Doppler images were obtained at 30-second intervals for 5 minutes. Flare development is indicated by color code where dark blue represents baseline. PACAP concentration-dependently mediates very strong vasodilatation (panels 3–5, concentration as indicated). White line represents 1-cm scale (data not normalized). Displayed series of laser Doppler images represents results of six independent studies. B: Concentration-dependent flare response in human skin to intradermal injection of PACAP (n = 6).
Expression of VPAC1R, VPAC2R, and PAC1R in Lesional Human Skin
Because PACAP affects vasoregulation, edema, and erythema of normal human skin, we examined the expression of PACAP-activated receptors (VPAC1R, VPAC2R, and PAC1R) in tissue extracts of nonlesional human skin by using quantitative real-time PCR. In whole tissue extracts, no expression was detected for VPAC2R, moderate expression was shown for PAC1R, and strong expression was found for VPAC1R (see Supplemental Figure 1 at http://ajp.amjpathol.org). We furthermore analyzed and compared the receptor expression profiles in the skins’ functional entities (see Supplemental Figure 1 at http://ajp.amjpathol.org). The dermis, epidermis, and sebaceous glands were strongly positive for VPAC1R RNA expression, whereas hair follicles expressed VPAC1R RNA in strong but comparably lower amounts. For VPAC2R RNA, only moderate expression levels were detected in the dermis, hair follicles and sebaceous glands, whereas no expression was detectable for the epidermis of non-lesional human skin (see Supplemental Figure 2 at http://ajp.amjpathol.org). PAC1R RNA expression closely paralleled VPAC2R RNA expression levels, except for a stronger expression signal in the dermis.
Distribution of PACAP in Urticaria Tissues by Double-Immunofluorescence
We and others20,33 have previously described immunoreactivity for PACAP1-38 in normal human skin and its enhanced concentration in lesional human skin from patients with psoriasis.33 As demonstrated above, our results clearly show that PACAP is involved in vasoregulation, edema, and erythema of normal human skin. We next examined the distribution of PACAP in human skin of patients with urticaria who suffer from acute wheal (edema) and flare (erythema) reactions reflecting vascular reactions such as vasodilatation and plasma extravasation. Similar to what occurs in normal human skin,33 PACAP-positive fibers were found in the dermis of urticaria patients (Figure 3, A–C) by immunofluorescence. And similar to what occurs in patients with psoriasis,33 immunopositive staining for PACAP1-38 was also found in the epidermis (Langerhans cells) of urticaria patients (Figure 3A). We also observed a close anatomical association of PACAP-positive nerve fibers along with mast cells (Figure 3B), indicating that PACAP may modulate vascular responses by activating mast cells (Figure 3B). Moreover, PACAP1-38 immunoreactivity was observed around the dermal blood vessels of urticaria patients (Figure 3C). Preabsorption control studies prevented positive staining for PACAP, confirming specificity of the immune reaction (Figure 3D).
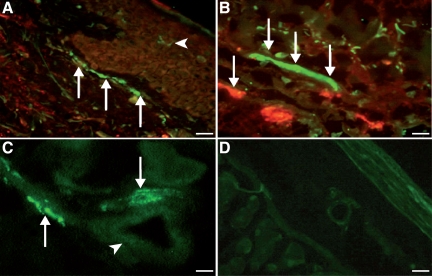
Figure 3.
Double-immunofluorescence staining for PACAP (polyclonal, green) and mast cell tryptase (monoclonal, red) in tissues from patients with urticaria (n = 6). A: Marked immunostaining for PACAP (green) in nerve fibers close to the dermal-epidermal border of human skin tissue (arrows). In the environment of epidermal nerve fibers, single PACAP-positive dendritic-like cells (Langerhans cells) of urticaria patients were detected (arrowhead, red; ×20; Scale bar = 40 μm). B: In the upper dermis, marked immunofluorescence staining for PACAP in nerve fibers (arrows) closely accompanied by tryptase-positive mast cells (red; ×40; Scale bar = 31.5 μm). C: PACAP-positive nerve fibers (arrows) close to dermal blood vessels (arrowhead, ×40; Scale bar = 31.5 μm). D: Negative control (pre-immune absorption control) showing absence of PACAP in nerve fibers and endothelial cells demonstrating specificity of immunostaining (×10; Scale bar = 45 μm).
Immunohistochemical Detection of VPAC1R in Endothelial Cells and Cell Infiltrates of Lesional Human Skin Tissues, Urticaria, and Atopic Dermatitis
To determine the distribution of VPACRs in urticaria tissues, we analyzed skin biopsies by immunohistochemistry. Blood vessels showed the strongest staining (Figure 4, A and B). Higher magnification revealed intense staining of VPAC1R in dermal endothelial cells and certain leukocytes (Figure 4, C–E). The negative controls did not exhibit epidermal staining for VPAC1R (Figure 4F).
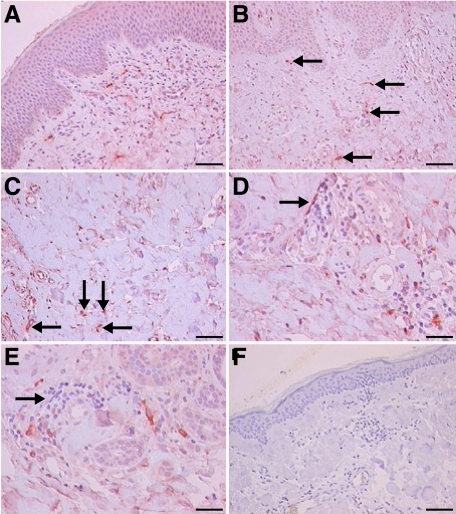
Figure 4.
Immunohistochemical detection of VPAC1R in urticaria human skin (n = 6). A: Overview shows intense dermal staining of VPAC1R in blood vessels and occasionally in leukocytes and weak staining for VPAC1R in keratinocytes and certain fibroblasts (×10; Scale bar = 45 μm). B: Higher magnification shows intense staining of endothelial cells, mast cells and lymphocytes for VPAC1R (arrows) (×20; Scale bar = 40 μm). C: Weaker staining for VPAC1R in the deeper dermis, small blood vessels, and leukocytes (arrows) as compared to superficial dermis (×40; Scale bar = 31.5 μm). D and E: Negative to weak staining for VPAC1R in dermal connective tissue, and occasionally staining of lymphocytes and mast cells (arrows, ×40; Scale bar = 31.5 μm). F: Control tissue (pre-immune absorption) demonstrates absence of VPAC1R in the skin, verifying specificity (×10; Scale bar = 45 μm). Experiments were performed as described in Materials and Methods.
In atopic dermatitis patients (Figure 5, A and B), significant immunohistochemical staining of endothelial cells, keratinocytes, and leukocytes was observed for VPAC1R. In contrast, the controls (preimmune absorption, Figure 5C) showed negative staining for VPAC1R in these tissues. Furthermore, no immunoreactivity as well as RNA message (mRNA) was observed in human skin endothelium when VPAC2R or PAC1R antibodies or primers were used, respectively (data not shown). Thus, VPAC1R is the main receptor for PACAP-induced signaling in human skin endothelial cells and is expressed by dermal microvascular endothelial cells during the disease state.
Figure 5.
Immunohistochemical distribution of VPAC1R in the tissue of patients with atopic dermatitis (n = 6). A: Intensive staining of endothelial cells, keratinocytes and leukocytes was observed (arrows, ×20; Scale bar = 40 μm). B: Higher magnification shows intensive staining for VPAC1R in dermal endothelial cells (arrows, ×40; Scale bar = 31.5 μm). C: Control tissue (pre-immune absorption) shows negative staining for VPAC1R (×10; Scale bar = 45 μm). In addition, no immunoreactivity was observed in human skin endothelium using VPAC2R or PAC1R antibodies (data not shown).
To further specify this staining pattern in the epidermis and dermis, coimmunostaining studies of VPAC1R were performed in lesional human skin of patients with atopic dermatitis together with antibodies against CD31 (blood endothelial cells, Figure 6, A and H) and α smooth muscle cells (α-SMA, data not shown), CD45 (T-cells, data not shown), CD4+ (T helper cells, Figure 6C), CD8+ (cytotoxic T cells, Figure 6D), CD68 (macrophages, Figure 6E), and mast cell tryptase (Figure 6F). Codistribution of VPAC1R was found with CD31+ endothelial cells (Figure 6, A and H), CD4+ and CD8+ T cells (Figure 6C, D, and H), and mast cells (Figure 6F) in inflamed lesions. Furthermore, smooth muscle cells were positive for VPAC1R expression but displayed a markedly lower signal when compared to endothelial cells (data not shown). Staining was pronounced in keratinocytes of atopic dermatitis skin tissues (Figure 6G) but weak in normal skin (data not shown). Control tissue was negative for VPAC1R (Figure 6I). Thus, an additional indirect effect by vasoregulatory mediators deriving from keratinocytes, CD4+ and CD8+ T cells, or mast cells cannot be excluded.
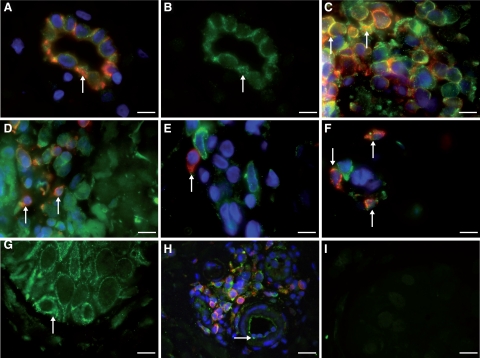
Figure 6.
Double immunofluorescence of VPAC1R (green) in human skin sections of patients with atopic dermatitis (n = 6). A: Colocalization of VPAC1R and CD31+ in dermal vascular endothelial cells of atopic dermatitis patients (arrow). No staining of smooth muscle cells against VPAC1R was seen. B: Membrane staining for VPAC1R (green) in endothelial cells of the same postcapillary venule as Figure 6A (arrow). C: Colocalization of VPAC1R (green) in many, but not all, CD4+ T-helper cells (yellow, arrow) of dermal T-cell infiltrate (stained against CD4+, red). D: Immunofluorescence of VPAC1R (green) in the same infiltrate as (A), but now against CD8+ T-cells. Rare colocalization (yellow, arrow) of VPAC1R (green) in CD8+ cytotoxic T cells (red) of the infiltrate E: No colocalization of VPAC1R (green) in tissue macrophages within dermal infiltrate stained against CD68 (red, arrow). F: Weak colocalization (yellow) in a few mast cells stained for tryptase (red, arrow) and VPAC1R (green). G: Localization of VPAC1R in basal and suprabasal keratinocytes (green, arrow). H: Staining of endothelial cells (green) for VPAC1R (arrow). Note surrounding CD4+ T cells (red), colocalization in yellow. I: Control tissue (preimmune absorption) demonstrates absence of VPAC1R in the skin, verifying specificity. Experiments were performed as described in Materials and Methods. Magnification, ×100; Scale bars = 12 μm.
HDMEC Express Functional VPAC1R
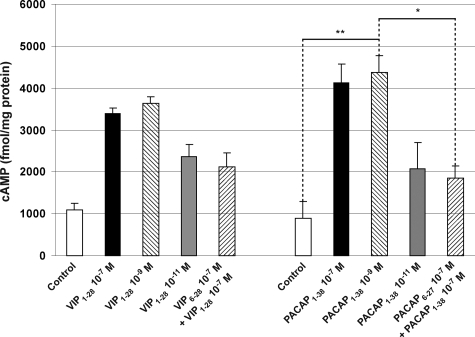
To confirm that HDMEC express functional VPAC1R, we kept HDMEC in FCS/nutrient-deficient medium for 24 hours before treating them with specific agonists and/or antagonists to PACAP receptors. PACAP1-38 at lower concentration than VIP stimulated a rapid increase in cAMP in HDMEC (Figure 7). PACAP1-38 (EC50 ∼10 nmol/L) or VIP (EC50 ∼5 × 10 nmol/L) were almost equipotent (data not shown). Dose-response experiments (Figure 7) showed that the concentration of VIP and PACAP peaked at 10−9 mol/L with respect to receptor activation in HDMEC. The corresponding neuropeptide inhibitors (truncated peptides) were administered in 100-fold concentrations (VIP6-28 10−7 mol/L and PACAP6-27), as described in other studies.21,47,48,49 There was a weak to no cAMP response at various concentrations of the VPAC1R antagonists VIP6-28 or PACAP6-27 (100× as concentrated as the corresponding agonist at the optimal concentration). Pretreatment with the antagonist occurred 3 minutes before agonist stimulation. Thus, PACAP specifically and effectively induced cAMP activation in HDMEC. Since HDMEC express VPAC1R, it is the crucial receptor mediating the PACAP-induced responses in human cutaneous endothelial cells.
Figure 7.
In vitro measurement of changes in cAMP concentration in HDMEC after stimulation with the agonist VIP1-28 or PACAP1-38. Results show the concentration-dependent activation of VPAC1R in HDMEC by VIP1-28 and PACAP1-38, in contrast to the VPAC1R antagonists VIP6-28 and PACAP6-27, because PAC1R and VPAC2R are not expressed by HDMEC. *The change in cAMP concentration in HDMEC was reduced by approximately 50% by the inhibitor PACAP6-27 (Materials and Methods). This proves the functionality of VPAC1R. **After the application of PACAP 10−9 mol/L, the change in cAMP concentration in HDMEC was more than fourfold compared with the negative control. Experiments were performed at least three times.
PACAP Stimulates HDMEC Via VPAC1R But Not VPAC2R and PAC1R
To verify on the mRNA level that the PACAP-induced effects in human dermal endothelial cells are mediated via VPAC1R, we performed real-time PCR analysis for VPAC1R, VPAC2R, and PAC1R in HDMEC. In nonstimulated HDMEC, mRNA expression was strong for VPAC1R, not detected for VPAC2R, and mild for PAC1R (see Supplemental Figure 2 at http://ajp.amjpathol.org). To examine whether proinflammatory mediators could regulate the mRNA expression levels of VPAC1R and PAC1R, HDMEC were stimulated with TNFα or LPS (see Supplemental Figure 2 at http://ajp.amjpathol.org) for 6 hours and subjected to real-time PCR analysis. Both proinflammatory agents induced a marked down-regulation of VPAC1R and PAC1R mRNA when compared to the unstimulated controls, in which VPAC1R expression in HDMEC remained stronger than PAC1R expression. Taken together, these findings indicate a role of PACAP in vascular regulation under inflammatory conditions via VPAC1R and probably PAC1R.
PACAP Significantly Alters Cutaneous Vascular Arrangements
To further explore the functional consequences of PACAP treatment on vascular response, we used a skin organ culture assay and measured changes in the number of CD31+ (PECAM-1) vessel cross sections in the presence or absence of the neuropeptide.
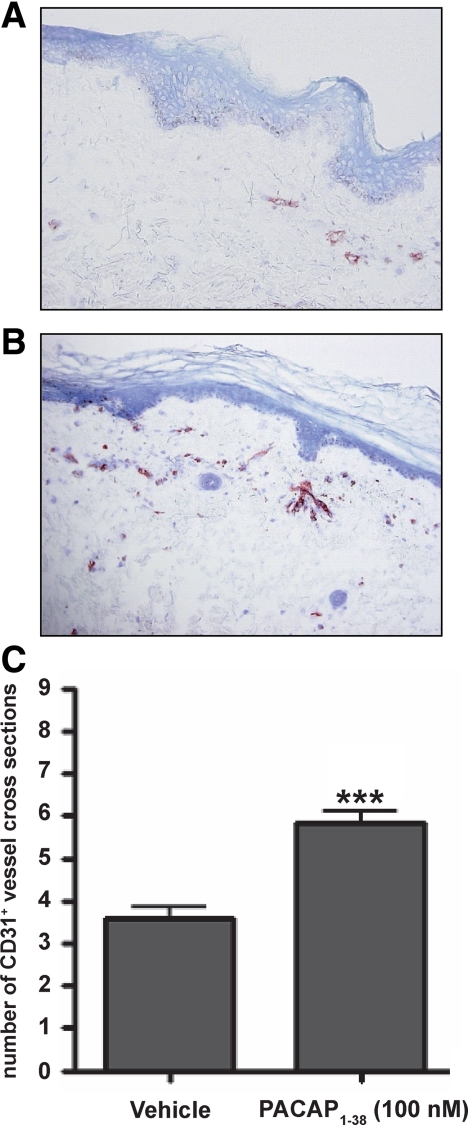
Multiple transversally cut vessels with a distinct central lumen were detectable in vehicle-treated samples 4 days after initiation of the explant cultures (Figure 8A). PACAP treatment led to a pronounced increase in the number of CD31+ vessel cross sections, indicating a proangiogenic effect of PACAP (Figure 8, B and C). The total number of histochemically detectable, Giemsa-positive mast cells in the connective sheet and the fraction of degranulated mast cells were not affected by PACAP treatment (see Supplemental Figure 3 at http://ajp.amjpathol.org). Therefore, mast cells appear not to be involved in the PACAP-induced angiogenesis (eg, by secretion of angiogenic cytokines and growth factors such as VEGF, bFGF, MMP9, CXCL8, heparin, and histamine). Taken together, these results suggest that PACAP directly induces vascular responses, thereby indicating for the first time that PACAP is a crucial vascular regulator in human skin in vivo.
Figure 8.
PACAP-stimulated organ-cultured skin show increased number of CD31+ vessel cross-sections. Three millimeter punch biopsies from the upper arm of healthy volunteer subjects (n = 3) were placed in organ culture for 24 hours and treated with either (A and C) vehicle or (B and C) 100 nmol/L PACAP. Thereafter, paraffin sections were subjected to immunohistochemical staining for CD31. (Student’s t-test, significant differences compared with vehicle control at ***P < 0.001).
Discussion
The results of our study support a role for PACAP in human skin function and disease by modulating significant vascular responses in vivo. First, we found that PACAP concentration-dependently induced significant vasodilatation, flushing, and edema in healthy humans in vivo, which occurred after 15 minutes and peaked after 30 minutes. Second, PACAP induced hyperthermia in human skin in vivo. Third, these effects were significantly stronger than those of VIP. Fourth, intradermal injection of PACAP at nanomolar concentrations led to a profound wheal and flare reaction resembling neurogenic inflammation. Fifth, application of PACAP to three-dimensional cultured skin samples increased the number of CD31+ vessel cross sections. Sixth, the immunoreactivity for VPAC1R was enhanced in blood vessels, keratinocytes, CD4+ T cells, or mast cells in atopic dermatitis patients. Finally, VPAC1R mRNA was the predominant receptor in human skin and was functional on cultured human dermal endothelial cells. Thus, systemic, local, and in vitro application of PACAP induces profound vascular responses in human skin.
In our investigation of the effects of systemic PACAP on body temperature, all healthy volunteers who received different concentrations via intravenous or bolus injection developed a concentration-dependent profound erythema, edema, and hyperemia, predominantly in the face, neck, and upper trunk. The restriction of these symptoms to these locations was clinically comparable to the so called “flush phenomenon” that can be observed in patients with rosacea, but also carcinoids, thus diseases which have a clear neurovascular component based on neuropeptide release (reviewed in 17). In patients with atopic dermatitis and urticaria (angioedema), vasodilatation, local hyperemia, and edema can be observed as part of the inflammatory response. However, in our study, patients and healthy volunteers reported no diarrhea, bowel sounds, hypotension, or constant skin reactions at the concentrations or time points investigated. Simultaneous measurements of blood pressure and pulse after PACAP infusion in our study did not result in hypotension or tachycardia, though these were observed in anesthetized rats11 or dogs,50 likely because of higher systemic concentrations.
In our study, a bolus injection of PACAP induced a more pronounced and more rapid intravasal effect than continuous i.v. infusion, which explains the steeper intrarectal body temperature curve observed after bolus injection. This effect was also concentration-dependent: the maximal increase of body temperature was obtained with 30 pmol/kg b.w./h after 30 minutes and continuously decreased after PACAP was withdrawn. This finding suggests that the vascular system in human skin is sensitive to PACAP stimulation even at low nanomolar (physiological) concentrations. Moreover, PACAP induced pronounced local changes in cutaneous vessel function, such as “flushing,” hyperemia, and edema. In contrast, no systemic vascular effects were observed even at high concentrations (bolus injection of 315 pmol/kg b.w). Thus, PACAP may play an important role by regulating dermal vascular functions in humans in vivo, especially in peripheral cutaneous microvascular vessels. This role is supported by studies in dogs, in which high doses of PACAP1-27 and PACAP1-38 induced hypertension after a transient hypotension, indicating a concentration-dependent effect of PACAP in all body vessels.50 Others,49 however, did not observe hypertension at concentrations of 10−12 mol/L, but showed that both PACAP1-38 and PACAP1-27 stimulated a marked vasodilatation in humans, which was 100× higher than that for VIP. In accordance with our study, a study investigating the systemic vasodepressive effect of PACAP in rodents demonstrated that the effects of PACAP were significantly weaker than those of VIP, indicating a limited systemic vasodepressive effect of PACAP both in rodents and humans at moderate physiological concentrations.51,52 Recently, we showed that PACAP-immunoreactive nerve fibers can be found in close proximity to dermal blood vessels,33 suggesting that PACAP can also induce direct local effects in human skin.
Beside flushing and edema, systemic PACAP also induces hyperthermia. Studies in rats suggest that it does so via a cyclooxygenase-dependent centrally-regulated pathway.53 In those studies, intracerebroventricular administration of PACAP1-38 induced a dose-related increase in colon temperature. This effect was abolished by pretreatment with PACAP1-38 antiserum and the cyclooxygenase inhibitor noraminophenazone. The impact of additional effects mediated by CNS-derived PACAP on the vascular function in human skin in vivo remains unknown.
In our in vivo study, using moderate physiological concentrations, we observed a marked edematous skin reaction, a characteristic of neurogenic inflammation (Figure 2). This correlates well with the observation in rats that PACAP induces plasma extravasation at certain concentrations in vivo.23 In humans, however, plasma extravasation is obviously difficult to quantify. This effect may be direct, indirect, or a combination of both, because PACAP-positive nerve fibers can activate both blood vessels and mast cells. PACAP may contribute to vasoregulation as well as neurogenic inflammation in human skin in vivo.
We found that PACAP modulates vascular responses in human skin that were pronounced in the face and upper trunk, but the flushing and edema and the marked increase in body temperature noted after PACAP were not observed after VIP. Thus, at comparable concentrations, VIP may influence systemic blood flow (stronger than PACAP), body temperature (weaker than PACAP), but does not produce a skin flush phenomenon with erythema and edema that was characteristic after PACAP.
To further elucidate the local cutaneous effects of PACAP in human skin, we studied the ability of PACAP1-38 to induce a flare reaction in human skin in vivo by Laser-Doppler-Video-Imaging. Our results clearly show that PACAP1-38 at nanomolar concentrations induces a profound flare reaction in human skin in vivo. Thus, local release of PACAP by sensory nerve endings in the skin may contribute to flushing and neurogenic inflammation. A further detailed analysis in humans is hampered by the lack of a potent, specific antagonist that could be used. PACAP6-27 or PACAP6-38, unfortunately, also exert agonistic effects on other G protein–coupled and neuropeptide receptors, which limits the interpretation of such potential experiments.
To characterize the impact of PACAP on blood vessel anatomy and function, mast cell degranulation, and epidermal changes, we stimulated cultured three-dimensional human skin biopsies with PACAP1-38. Our data show that PACAP treatment leads to a de novo formation of new blood vessels (Figure 8). Again, the lack of an appropriate antagonist limits our ability to interpret whether this effect can be specifically inhibited by blocking the VPAC1R pathway.
To analyze the crucial receptor through which PACAP exerts its effects in human skin, we next explored the distribution of PACAP and PACAP receptors in urticaria and atopic dermatitis, two human skin diseases that show a neuronal component and characteristics of neurogenic inflammation. PACAP-immunoreactive nerve fibers were found in close proximity to dermal blood vessels and mast cells in patients with urticaria (Figure 3B), a disease characterized by transient but intensive vasodilatation and plasma extravasation (neurogenic inflammation). Thus, PACAP may directly regulate endothelial cell function in dermal blood vessels, although additional effects of PACAP via activation of mast cells, T cells, or keratinocytes cannot be excluded in human skin. PACAP1-27 was already localized in dermal and epidermal sensory nerve fibers of rat skin.18 We and others20,33 detected immunoreactivity for PACAP1-38 in the dermis of normal human skin. In psoriasis patients, we found an increased immunoreactivity for PACAP1-38 in dermal nerve fibers at the dermal–epidermal junction, around blood vessels, and in the epidermis,33 suggesting a role for PACAP in cutaneous inflammation. We further confirmed these data by in situ hybridization,52 showing that the VPAC1 receptor is highly expressed by human dermal microvascular endothelial cells and is the predominant receptor in human skin tissue and HDMEC cells. The fact that VPAC1R may be the predominant receptor for PACAP activation in human skin is also supported by our real-time PCR data. Comparison of skin tissues and different compartments in human skin show that VPAC1R mRNA is the predominantly found PACAP receptor mRNA, whereas VPAC2R mRNA is only moderately expressed and PAC1R expression appears to be absent in most cutaneous structures and is only detected in the dermis. Thus, VPAC1R is also the predominant receptor for PACAP on HDMEC. PACAP may directly and efficiently modulate the function of human dermal microvascular endothelial cells by activating VPAC1R. Moreover, preincubation of HDMEC with proinflammatory mediators like TNFα and LPS markedly down-regulated the VPAC1R in these cells. This finding is in agreement with the finding that PACAP, similarly to somatostatin, may be an anti-inflammatory mediator under chronic inflammatory conditions.54,55,56,57 Thus, PACAP may exert proinflammatory effects during skin homeostasis (ie, temperature regulation, flushing) and the early phase of inflammation (ie, vasodilatation and edema to recruit leukocytes to the site of inflammation), but may exert anti-inflammatory effects during chronic inflammation. This is currently under investigation.
We cannot exclude the possibility that PACAP may also exert indirect effects on blood vessels by activating mast cells, for example. Our results show that in human skin, PACAP-positive nerve fibers are closely associated with mast cells, T cells, and keratinocytes, which express VPAC1R and generate several mediators involved in vasoregulation and neurogenic inflammation.1 Animal studies also indicate that PACAP can mediate vasoregulatory effects by inducing the release of vasodilatators such as histamine23 or serotonin21,47 from mast cells. However, data from our three-dimensional skin culture system indicate that PACAP does not induce mast cell degranulation in that setting (see Supplemental Figure 3 at http://ajp.amjpathol.org). Another good candidate is nitric oxide, one of the most potent vasodilatators in humans.58,59 The precise analysis of the PACAP-mediated effects in human skin, however, requires further clarification.
In summary, the neuropeptide PACAP undoubtedly affects the human cutaneous vascular system and causes a marked vasodilatation, edema, and flush phenomenon as well as hyperthermia in vivo. Therefore, skin sensory nerves contribute to vascular regulation in humans in vivo, and PACAP may be an essential neuromediator of neurovascular and neuroimmune interactions in humans during health and disease. Thus, inhibition of this potent neuropeptide and its receptors may be a novel strategy for the treatment of inflammatory skin diseases that have a neurogenic component. Therefore, future quantitative studies of PACAP using microdialysis10 from patients with urticaria, rosacea, atopic dermatitis, erythroderma, or carcinoid syndrome, for example, may be helpful to define the specific role of PACAP as a vasoactive agent in human skin in vivo under physiological or pathophysiological circumstances. Moreover, animal studies using specific receptor antagonists and PACAP and/or VPAC1R gene-deficient mice are currently under investigation to further clarify the role of PACAP and VPAC receptors in skin function. Together, PACAP and PACAP receptors may be important mediators of cutaneous vasoregulation and neurogenic inflammation under physiological and pathophysiological conditions, and inhibition of PACAP-induced effects on human dermal microvascular endothelial cells may be beneficial for the treatment of the cutaneous neurogenic inflammation found in diseases such as atopic dermatitis, urticaria, or rosacea.
Acknowledgments
The technical help of Astrid Becker, Heike Hinte, Andrea Poppe, Karin Baer, and Christian Meß is gratefully acknowledged.
Footnotes
Address reprint requests to Martin Steinhoff, M.D., Ph.D., Departments of Dermatology and Surgery, University of California San Francisco, 513 Parnassus Ave, Room S-1268, San Francisco, CA 94143. E-mail: martin_steinhoff@web.de.
Supported by grants from the Interdisciplinary Center for Clinical Research (Stei3/034/09); DFG (STE 1014/2-1); SFB 293 (A14); CE.R.I.E.S, Paris; Rosacea foundation, USA (M.S.).
S.S. and J.B. contributed equally to this study.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Steinhoff M, Stander S, Seeliger S, Ansel JC, Schmelz M, Luger T. Modern aspects of cutaneous neurogenic inflammation. Arch Dermatol. 2003;139:1479–1488. doi: 10.1001/archderm.139.11.1479. [DOI] [PubMed] [Google Scholar]
- Cevikbas F, Steinhoff A, Homey B, Steinhoff M. Neuroimmune interactions in allergic skin diseases. Curr Opin Allergy Clin Immunol. 2007;7:365–373. doi: 10.1097/ACI.0b013e3282a644d2. [DOI] [PubMed] [Google Scholar]
- Blais C, Jr, Rouleau JL, Brown NJ, Lepage Y, Spence D, Munoz C, Friborg J, Geadah D, Gervais N, Adam A. Serum metabolism of bradykinin and des-Arg9-bradykinin in patients with angiotensin-converting enzyme inhibitor-associated angioedema. Immunopharmacology. 1999;43:293–302. doi: 10.1016/s0162-3109(99)00133-2. [DOI] [PubMed] [Google Scholar]
- Nussberger J, Cugno M, Cicardi M, Agostoni A. Local bradykinin generation in hereditary angioedema. J Allergy Clin Immunol. 1999;104:1321–1322. doi: 10.1016/s0091-6749(99)70030-8. [DOI] [PubMed] [Google Scholar]
- Doutre M. Physiopathology of urticaria. Eur J Dermatol. 1999;9:601–605. [PubMed] [Google Scholar]
- Singh LK, Pang X, Alexacos N, Letourneau R, Theoharides TC. Acute immobilization stress triggers skin mast cell degranulation via corticotropin releasing hormone, neurotensin, and substance P: a link to neurogenic skin disorders. Brain Behav Immun. 1999;13:225–239. doi: 10.1006/brbi.1998.0541. [DOI] [PubMed] [Google Scholar]
- Steinhoff M, Meinhardt A, Steinhoff A, Gemsa D, Bucala R, Bacher M. Evidence for a role of macrophage migration inhibitory factor in psoriatic skin disease. Br J Dermatol. 1999;141:1061–1066. doi: 10.1046/j.1365-2133.1999.03206.x. [DOI] [PubMed] [Google Scholar]
- Pincelli C, Fantini F, Romualdi P, Sevignani C, Lesa G, Benassi L, Giannetti A. Substance P is diminished and vasoactive intestinal peptide is augmented in psoriatic lesions and these peptides exert disparate effects on the proliferation of cultured human keratinocytes. J Invest Dermatol. 1992;98:421–427. doi: 10.1111/1523-1747.ep12499846. [DOI] [PubMed] [Google Scholar]
- Steinhoff M, Vergnolle N, Young SH, Tognetto M, Amadesi S, Ennes HS, Trevisani M, Hollenberg MD, Wallace JL, Caughey GH, Mitchell SE, Williams LM, Geppetti P, Mayer EA, Bunnett NW. Agonists of proteinase-activated receptor 2 induce inflammation by a neurogenic mechanism. Nat Med. 2000;6:151–158. doi: 10.1038/72247. [DOI] [PubMed] [Google Scholar]
- Steinhoff M, Neisius U, Ikoma A, Fartasch M, Heyer G, Skov PS, Luger TA, Schmelz M. Proteinase-activated receptor-2 mediates itch: a novel pathway for pruritus in human skin. J Neurosci. 2003;23:6176–6180. doi: 10.1523/JNEUROSCI.23-15-06176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata A, Arimura A, Dahl RR, Minamino N, Uehara A, Jiang L, Culler MD, Coy DH. Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem Biophys Res Commun. 1989;164:567–574. doi: 10.1016/0006-291x(89)91757-9. [DOI] [PubMed] [Google Scholar]
- Miyata A, Jiang L, Dahl RD, Kitada C, Kubo K, Fujino M, Minamino N, Arimura A. Isolation of a neuropeptide corresponding to the N-terminal 27 residues of the pituitary adenylate cyclase activating polypeptide with 38 residues (PACAP38). Biochem Biophys Res Commun. 1990;170:643–648. doi: 10.1016/0006-291x(90)92140-u. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Falluel-Morel A, Bourgault S, Basille M, Burel D, Wurtz O, Fournier A, Chow BK, Hashimoto H, Galas L, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev. 2009;61:283–357. doi: 10.1124/pr.109.001370. [DOI] [PubMed] [Google Scholar]
- Arimura A, Somogyvari-Vigh A, Miyata A, Mizuno K, Coy DH, Kitada C. Tissue distribution of PACAP as determined by RIA: highly abundant in the rat brain and testes. Endocrinology. 1991;129:2787–2789. doi: 10.1210/endo-129-5-2787. [DOI] [PubMed] [Google Scholar]
- Arimura A, Shioda S. Pituitary adenylate cyclase activating polypeptide (PACAP) and its receptors: neuroendocrine and endocrine interaction. Front Neuroendocrinol. 1995;16:53–88. doi: 10.1006/frne.1995.1003. [DOI] [PubMed] [Google Scholar]
- Delgado M, Ganea D. VIP and PACAP inhibit activation induced apoptosis in T lymphocytes. Ann NY Acad Sci. 2000;921:55–67. doi: 10.1111/j.1749-6632.2000.tb06951.x. [DOI] [PubMed] [Google Scholar]
- Roosterman D, Goerge T, Schneider SW, Bunnett NW, Steinhoff M. Neuronal control of skin function: the skin as a neuroimmunoendocrine organ. Physiol Rev. 2006;86:1309–1379. doi: 10.1152/physrev.00026.2005. [DOI] [PubMed] [Google Scholar]
- Moller K, Zhang YZ, Hakanson R, Luts A, Sjolund B, Uddman R, Sundler F. Pituitary adenylate cyclase activating peptide is a sensory neuropeptide: immunocytochemical and immunochemical evidence. Neuroscience. 1993;57:725–732. doi: 10.1016/0306-4522(93)90018-b. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Gonzalez BJ, Basille M, Yon L, Fournier A, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharmacol Rev. 2000;52:269–324. [PubMed] [Google Scholar]
- Odum L, Petersen LJ, Skov PS, Ebskov LB. Pituitary adenylate cyclase activating polypeptide (PACAP) is localized in human dermal neurons and causes histamine release from skin mast cells. Inflamm Res. 1998;47:488–492. doi: 10.1007/s000110050363. [DOI] [PubMed] [Google Scholar]
- Schmidt-Choudhury A, Furuta GT, Galli SJ, Schmidt WE, Wershil BK. Mast cells contribute to PACAP-induced dermal oedema in mice. Regul Pept. 1999;82:65–69. doi: 10.1016/s0167-0115(99)00041-5. [DOI] [PubMed] [Google Scholar]
- Warren JB, Larkin SW, Coughlan M, Kajekar R, Williams TJ. Pituitary adenylate cyclase activating polypeptide is a potent vasodilator and oedema potentiator in rabbit skin in vivo. Br J Pharmacol. 1992;106:331–334. doi: 10.1111/j.1476-5381.1992.tb14336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardell LO, Stjarne P, Wagstaff SJ, Agusti C, Nadel JA. PACAP-induced plasma extravasation in rat skin. Regul Pept. 1997;71:67–71. doi: 10.1016/s0167-0115(97)00027-x. [DOI] [PubMed] [Google Scholar]
- Cardell LO, Uddman R, Luts A, Sundler F. Pituitary adenylate cyclase activating peptide (PACAP) in guinea-pig lung: distribution and dilatory effects. Regul Pept. 1991;36:379–390. doi: 10.1016/0167-0115(91)90071-n. [DOI] [PubMed] [Google Scholar]
- Yao W, Sheikh SP, Ottesen B, Jorgensen JC. Vascular effects and cyclic AMP production produced by VIP. PHM, PHV, PACAP-27, PACAP-38, and NPY on rabbit ovarian artery, Peptides. 1996;17:809–815. doi: 10.1016/0196-9781(96)00080-0. [DOI] [PubMed] [Google Scholar]
- Zhou CJ, Shioda S, Yada T, Inagaki N, Pleasure SJ, Kikuyama S. PACAP and its receptors exert pleiotropic effects in the nervous system by activating multiple signaling pathways. Curr Protein Pept Sci. 2002;3:423–439. doi: 10.2174/1389203023380576. [DOI] [PubMed] [Google Scholar]
- Wei Y, Mojsov S. Tissue specific expression of different human receptor types for pituitary adenylate cyclase activating polypeptide and vasoactive intestinal polypeptide: implications for their role in human physiology. J Neuroendocrinol. 1996;8:811–817. doi: 10.1046/j.1365-2826.1996.05191.x. [DOI] [PubMed] [Google Scholar]
- Sreedharan SP, Huang JX, Cheung MC, Goetzl EJ. Structure, expression, and chromosomal localization of the type I human vasoactive intestinal peptide receptor gene. Proc Natl Acad Sci USA. 1995;92:2939–2943. doi: 10.1073/pnas.92.7.2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado M, Leceta J, Abad C, Martinez C, Ganea D, Gomariz RP. Shedding of membrane-bound CD14 from lipopolysaccharide-stimulated macrophages by vasoactive intestinal peptide and pituitary adenylate cyclase activating polypeptide. J Neuroimmunol. 1999;99:61–71. doi: 10.1016/s0165-5728(99)00105-8. [DOI] [PubMed] [Google Scholar]
- Delgado M, Munoz-Elias EJ, Gomariz RP, Ganea D. VIP and PACAP inhibit IL-12 production in LPS-stimulated macrophages: subsequent effect on IFNgamma synthesis by T cells. J Neuroimmunol. 1999;96:167–181. doi: 10.1016/s0165-5728(99)00023-5. [DOI] [PubMed] [Google Scholar]
- Delgado M, Munoz-Elias EJ, Gomariz RP, Ganea D. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide prevent inducible nitric oxide synthase transcription in macrophages by inhibiting NF-kappa B and IFN regulatory factor 1 activation. J Immunol. 1999;162:4685–4696. [PubMed] [Google Scholar]
- Delgado M, Sun W, Leceta J, Ganea D. VIP and PACAP differentially regulate the costimulatory activity of resting and activated macrophages through the modulation of B7.1 and B7.2 expression. J Immunol. 1999;163:4213–4223. [PubMed] [Google Scholar]
- Steinhoff M, McGregor GP, Radleff-Schlimme A, Steinhoff A, Jarry H, Schmidt WE. Identification of pituitary adenylate cyclase activating polypeptide (PACAP) and PACAP type 1 receptor in human skin: expression of PACAP-38 is increased in patients with psoriasis. Regul Pept. 1999;80:49–55. doi: 10.1016/s0167-0115(99)00010-5. [DOI] [PubMed] [Google Scholar]
- Groneberg DA, Rabe KF, Fischer A. Novel concepts of neuropeptide-based drug therapy: vasoactive intestinal polypeptide and its receptors. Eur J Pharmacol. 2006;533:182–194. doi: 10.1016/j.ejphar.2005.12.055. [DOI] [PubMed] [Google Scholar]
- Abad C, Martinez C, Leceta J, Gomariz RP, Delgado M. Pituitary adenylate cyclase-activating polypeptide inhibits collagen-induced arthritis: an experimental immunomodulatory therapy. J Immunol. 2001;167:3182–3189. doi: 10.4049/jimmunol.167.6.3182. [DOI] [PubMed] [Google Scholar]
- Schwartz LB. Mast cells and their role in urticaria. J Am Acad Dermatol. 1991;25:190–203. doi: 10.1016/s0190-9622(08)80468-9. discussion 203–194. [DOI] [PubMed] [Google Scholar]
- Schwarzhoff R, Schworer H, Fornefeld H, Morys-Wortmann C, Katsoulis S, Creutzfeldt W, Folsch UR, Schmidt WE. Specific monoclonal antibodies neutralize the action of PACAP 1-27 or PACAP 1-38 on intestinal muscle strips in vitro. Regul Pept. 1995;55:57–66. doi: 10.1016/0167-0115(94)00092-c. [DOI] [PubMed] [Google Scholar]
- Buddenkotte J, Stroh C, Engels IH, Moormann C, Shpacovitch VM, Seeliger S, Vergnolle N, Vestweber D, Luger TA, Schulze-Osthoff K, Steinhoff M. Agonists of proteinase-activated receptor-2 stimulate upregulation of intercellular cell adhesion molecule-1 in primary human keratinocytes via activation of NF-kappa B. J Invest Dermatol. 2005;124:38–45. doi: 10.1111/j.0022-202X.2004.23539.x. [DOI] [PubMed] [Google Scholar]
- Steinhoff M, Corvera CU, Thoma MS, Kong W, McAlpine BE, Caughey GH, Ansel JC, Bunnett NW. Proteinase-activated receptor-2 in human skin: tissue distribution and activation of keratinocytes by mast cell tryptase. Exp Dermatol. 1999;8:282–294. doi: 10.1111/j.1600-0625.1999.tb00383.x. [DOI] [PubMed] [Google Scholar]
- Shpacovitch VM, Brzoska T, Buddenkotte J, Stroh C, Sommerhoff CP, Ansel JC, Schulze-Osthoff K, Bunnett NW, Luger TA, Steinhoff M. Agonists of proteinase-activated receptor 2 induce cytokine release and activation of nuclear transcription factor kappaB in human dermal microvascular endothelial cells. J Invest Dermatol. 2002;118:380–385. doi: 10.1046/j.0022-202x.2001.01658.x. [DOI] [PubMed] [Google Scholar]
- Robberecht P, Gourlet P, De Neef P, Woussen-Colle MC, Vandermeers-Piret MC, Vandermeers A, Christophe J. Structural requirements for the occupancy of pituitary adenylate-cyclase-activating-peptide (PACAP) receptors and adenylate cyclase activation in human neuroblastoma NB-OK-1 cell membranes. Discovery of PACAP(6-38) as a potent antagonist. Eur J Biochem. 1992;207:239–246. doi: 10.1111/j.1432-1033.1992.tb17043.x. [DOI] [PubMed] [Google Scholar]
- Tams JW, Jorgensen RM, Holm A, Fahrenkrug J. Creation of a selective antagonist and agonist of the rat VPAC(1) receptor using a combinatorial approach with vasoactive intestinal peptide 6–23 as template. Mol Pharmacol. 2000;58:1035–1041. doi: 10.1124/mol.58.5.1035. [DOI] [PubMed] [Google Scholar]
- Racz B, Gasz B, Gallyas F, Jr, Kiss P, Tamas A, Szanto Z, Lubics A, Lengvari I, Toth G, Hegyi O, Roth E, Reglodi D. PKA-Bad-14-3-3 and Akt-Bad-14-3-3 signaling pathways are involved in the protective effects of PACAP against ischemia/reperfusion-induced cardiomyocyte apoptosis. Regul Pept. 2008;145:105–115. doi: 10.1016/j.regpep.2007.09.015. [DOI] [PubMed] [Google Scholar]
- Lu Z, Hasse S, Bodo E, Rose C, Funk W, Paus R. Towards the development of a simplified long-term organ culture method for human scalp skin and its appendages under serum-free conditions. Exp Dermatol. 2007;16:37–44. doi: 10.1111/j.1600-0625.2006.00510.x. [DOI] [PubMed] [Google Scholar]
- Handjiski BK, Eichmuller S, Hofmann U, Czarnetzki BM, Paus R. Alkaline phosphatase activity and localization during the murine hair cycle. Br J Dermatol. 1994;131:303–310. doi: 10.1111/j.1365-2133.1994.tb08515.x. [DOI] [PubMed] [Google Scholar]
- Paus R, Maurer M, Slominski A, Czarnetzki BM. Mast cell involvement in murine hair growth. Dev Biol. 1994;163:230–240. doi: 10.1006/dbio.1994.1139. [DOI] [PubMed] [Google Scholar]
- Schmidt-Choudhury A, Meissner J, Seebeck J, Goetzl EJ, Xia M, Galli SJ, Schmidt WE, Schaub J, Wershil BK. Stem cell factor influences neuro-immune interactions: the response of mast cells to pituitary adenylate cyclase activating polypeptide is altered by stem cell factor. Regul Pept. 1999;83:73–80. doi: 10.1016/s0167-0115(99)00054-3. [DOI] [PubMed] [Google Scholar]
- Seebeck J, Lowe M, Kruse ML, Schmidt WE, Mehdorn HM, Ziegler A, Hempelmann RG. The vasorelaxant effect of pituitary adenylate cyclase activating polypeptide and vasoactive intestinal polypeptide in isolated rat basilar arteries is partially mediated by activation of nitrergic neurons. Regul Pept. 2002;107:115–123. doi: 10.1016/s0167-0115(02)00072-1. [DOI] [PubMed] [Google Scholar]
- Warren JB, Donnelly LE, Cullen S, Robertson BE, Ghatei MA, Bloom SR, MacDermot J. Pituitary adenylate cyclase-activating polypeptide: a novel, long-lasting, endothelium-independent vasorelaxant. Eur J Pharmacol. 1991;197:131–134. doi: 10.1016/0014-2999(91)90511-n. [DOI] [PubMed] [Google Scholar]
- Ishizuka Y, Kashimoto K, Mochizuki T, Sato K, Ohshima K, Yanaihara N. Cardiovascular and respiratory actions of pituitary adenylate cyclase-activating polypeptides. Regul Pept. 1992;40:29–39. doi: 10.1016/0167-0115(92)90081-5. [DOI] [PubMed] [Google Scholar]
- Lam KS, Srivastava G. Sex-related differences and thyroid hormone regulation of vasoactive intestinal peptide gene expression in the rat brain and pituitary. Brain Res. 1990;526:135–137. doi: 10.1016/0006-8993(90)90259-e. [DOI] [PubMed] [Google Scholar]
- Usdin TB, Bonner TI, Mezey E. Two receptors for vasoactive intestinal polypeptide with similar specificity and complementary distributions. Endocrinology. 1994;135:2662–2680. doi: 10.1210/endo.135.6.7988457. [DOI] [PubMed] [Google Scholar]
- Pataki I, Adamik A, Jaszberenyi M, Macsai M, Telegdy G. Pituitary adenylate cyclase-activating polypeptide induces hyperthermia in the rat. Neuropharmacology. 2000;39:1303–1308. doi: 10.1016/s0028-3908(99)00209-9. [DOI] [PubMed] [Google Scholar]
- Delgado M, Abad C, Martinez C, Juarranz MG, Leceta J, Ganea D, Gomariz RP. PACAP in immunity and inflammation. Ann NY Acad Sci. 2003;992:141–157. doi: 10.1111/j.1749-6632.2003.tb03145.x. [DOI] [PubMed] [Google Scholar]
- Delgado M, Gonzalez-Rey E, Ganea D. VIP/PACAP preferentially attract Th2 effectors through differential regulation of chemokine production by dendritic cells. FASEB J. 2004;18:1453–1455. doi: 10.1096/fj.04-1548fje. [DOI] [PubMed] [Google Scholar]
- Helyes Z, Pozsgai G, Borzsei R, Nemeth J, Bagoly T, Mark L, Pinter E, Toth G, Elekes K, Szolcsanyi J, Reglodi D. Inhibitory effect of PACAP-38 on acute neurogenic and non-neurogenic inflammatory processes in the rat. Peptides. 2007;28:1847–1855. doi: 10.1016/j.peptides.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Martinez C, Abad C, Delgado M, Arranz A, Juarranz MG, Rodriguez-Henche N, Brabet P, Leceta J, Gomariz RP. Anti-inflammatory role in septic shock of pituitary adenylate cyclase-activating polypeptide receptor. Proc Natl Acad Sci USA. 2002;99:1053–1058. doi: 10.1073/pnas.012367999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Vessel tone and remodeling. Nat Med. 2006;12:16–17. doi: 10.1038/nm0106-16. [DOI] [PubMed] [Google Scholar]
- Lundberg JO, Weitzberg E. NO generation from nitrite and its role in vascular control. Arterioscler Thromb Vasc Biol. 2005;25:915–922. doi: 10.1161/01.ATV.0000161048.72004.c2. [DOI] [PubMed] [Google Scholar]