Abstract
The present analyses were done to define the role of fetuin-A (Fet) in mammary tumorigenesis using the polyoma middle T antigen (PyMT) transgenic mouse model. We crossed Fet-null mice in the C57BL/6 background with PyMT mice in the same background and after a controlled breeding protocol obtained PyMT/Fet+/+, PyMT/Fet+/−, and PyMT/Fet−/− mice that were placed in control and experimental groups. Whereas the control group (PyMT/Fet+/+) formed mammary tumors 90 days after birth, tumor latency was prolonged in the PyMT/Fet−/− and PyMT/Fet+/− mice. The majority of the PyMT/Fet−/− mice were tumor-free at the end of the study, at approximately 40 weeks. The pathology of the mammary tumors in the Fet-null mice showed extensive fibrosis, necrosis, and squamous metaplasia. The preneoplastic mammary tissues of the PyMT/Fet−/− mice showed intense phopho-Smad2/3 staining relative to control tissues, indicating that transforming growth factor-β signaling is enhanced in these tissues in the absence of Fet. Likewise, p19ARF and p53 were highly expressed in tumor tissues of PyMT/Fet−/− mice relative to the controls in the absence of Fet. The phosphatidylinositol 3-kinase/Akt signaling pathway that we previously showed to be activated by Fet, on the other hand, was unaffected by the absence of Fet. The data indicate that Fet is a powerful modulator of breast tumorigenesis in this model system and has the potential to modulate breast cancer progression in humans.
Mounting evidence suggests that fetuin-A is a multifunctional regulator of normal physiology as well as pathophysiology.1,2,3,4 We previously demonstrated that it is required for optimal in vitro and in vivo growth of Lewis lung carcinoma cells, and our studies suggested that it influences tumor growth via phosphatidylinositol 3 (PI3)-kinase/Akt signaling.3 It is necessary to elucidate whether or not it plays a crucial role in the growth of both normal and tumor cells not only in culture (where it is a major growth supplement in most growth medium) but also in vivo and especially during the transformation and progression of tumors.
Fetuin-A is a major 63-kDa plasma protein that makes up approximately 45% of noncollagenous glycoproteins synthesized by the liver and secreted into the serum.5 The concentration of fetuin-A in adult humans is approximately 0.5 mg/ml, whereas the concentration in fetal blood in both mouse and human is much higher (∼1.5–2 mg/ml).6 The concentration of fetuin-A in 4-week-old Fet+/+ and Fet+/− mice was estimated by enzyme-linked immunosorbent assay to be 0.84 and 0.28 mg/ml, respectively.7 It is a conserved member of the cysteine protease inhibitors, also known as cystatins, and contains both N-linked and O-linked carbohydrate moieties8 and can be phosphorylated on at least four different sites.9 A number of physiological functions have been assigned to fetuin-A including brain development,6 inhibition of ectopic calcification,10,11 and bone remodeling.2 Mice lacking fetuin-A are born phenotypically normal and fertile; however, they exhibit calcification of the kidney, testis, skin, heart, and vasculature.2 Fetuin-A has been shown to mimic transforming growth factor (TGF-β) receptor because of the presence of the TGF-β receptor II homology 1 domain (TRH1)12 and therefore has the potential to compete with epithelial cells for TGF-β. The potential sequestration of TGF-β by fetuin-A would modulate TGF-β signaling in breast epithelial cells as previously reported for intestinal epithelial cells.4 In addition, fetuin-A binds to and interacts with other cell surface receptors such as the insulin receptor.13 It also interacts with lipids such as phospholipids14 and free fatty acids.15 These interacting protein and nonprotein factors make it very difficult to obtain pure fetuin-A for in vitro mechanistic studies and thus indicate the need for in vivo studies.16 The ability of fetuin-A to interact with a number of key cellular receptors and growth factors suggests that it can promote or attenuate growth signaling pathways.
To understand the potential role of fetuin-A in the transformation, promotion, and progression of breast cancer, we crossed C57BL/6 mice whose fetuin-A gene had been knocked out with C57BL/6 transgenic mice for polyoma virus middle T oncogene (PyMT). The PyMT model has been shown to recapitulate many processes found in human breast cancer progression, especially in the pattern of expression of biomarkers associated with poor prognosis.17 For the PyMT oncogene to transform breast epithelial cells, one of the key requirements is the inactivation of the ARF-p53 tumor suppressor pathway.18 We hereby report that the absence of fetuin-A in the PyMT-C57BL/6 mice significantly reduces mammary tumor incidence and prolongs tumor latency. In addition, we show increased TGF-β signaling in the preneoplastic mammary acini of PyMT/Fet−/− mice relative to those of PyMT/Fet+/+ mice of the same age that are already showing signs of transformation, implying that TGF-β signaling suppresses mammary tumorigenesis in the fetuin-A-null PyMT mice. Interestingly, our data also show low expression of p19 and p53 in mammary tissues of PyMT/Fet+/+ mice in comparison with those of PyMT/Fet−/− mice, in which these proteins are expressed at high levels.
Materials and Methods
All of the reagents were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise stated.
Cells
The breast cancer cell line BT-549 was a kind gift from Dr. Avraham Raz (Karmonos Cancer Research Institute, Detroit, MI). It was routinely maintained in Dulbecco’s modified Eagle’s medium/F12 medium containing 10% fetal bovine serum.
Breeding
We purchased female C57BL/6 Fet+/+ mice from Harlan Teklad (Madison, WI). Male transgenic PyMT C57BL/6 Fet+/+ mice, purchased from Mayo Clinic (Rochester, MN), were crossed with female Fet−/− C57BL/6 mice, a gift from Dr. Willi Jahnen-Dechent (University Clinic, Aachen, Germany). The PyMT/Fet+/− mice were back-crossed to obtain the three experimental and control genotypes. The female mice were genotyped by tail clipping3 and transferred to the experimental (PyMT/Fet−/− and PyMT/Fet+/−) and control PyMT/Fet+/+ groups. They were housed in the animal care facility at Meharry Medical College, a fully Association for Assessment and Accreditation of Laboratory Animal Care-accredited facility. All animal experiments were performed according to the protocols approved by the Institutional Animal Care and Usage Committee and following the Guide for the Care and Use of Laboratory Animals. Mice rooms were maintained at 22°C with 12 hours of light and 12 hours of dark cycled daily. Animals had 24 hours access to nonsoy standard rodent feed and water.
The experimental (PyMT/Fet−/− and PyMT/Fet+/−, 10 animals/group) and control (PyMT/Fet+/+, 10 animals/group) groups were allowed to grow and mature for designated time points. They were palpated weekly, and tumor volumes were estimated by calipers. The animals were euthanized by CO2 asphyxiation at designated time points or when the tumor endpoint of 15 mm (tumor length) was reached or when the animals were moribund. Normal and tumorigenic mammary tumor tissues were excised, fixed, and processed for histopathology or frozen at −80°C until needed. Palpable tumors were usually discovered at approximately 90 days after birth in PyMT/Fet+/+ mice and 120 days for PyMT/Fet+/− and PyMT/Fet−/− mice. Perpendicular measurements (length and width) were taken for each palpable tumor. Tumor volumes were estimated according to the formula V = (L/2) × (W)2, where V represents volume, L represents length, and W represents width.
Genotyping
Genotyping of fetuin-A wild-type and null animals was done as described previously.3 To genotype for PyMT, DNA was isolated from mouse tail clippings (0.5 cm) at the time of pup weaning (21 days) using the DNeasy blood and tissue kit (Qiagen, Valencia, CA) following the manufacturer’s recommendation. DNA was prepared by proteinase K digestion and extraction.19 Primers used were PyMT sense (5′-GGAAAGTCACTAGGAGCAGGG-3′) and PyMT antisense (5′-GGAAGCAAGTACTTCACAAGGG-3′).
A TaqPCR Master Mix Kit was used for amplification according to the manufacturer’s procedure (Qiagen), and the PCR products were separated in 2% agarose gels).
Real-Time RT-PCR
To quantify the expression of mRNA for collagens in the tumors from PyMT/Fet+/+ and PyMT/Fet−/− animals, total RNA was extracted from the frozen tissues with an RNeasy Mini Kit (Qiagen). To synthesize the first strand of cDNA, an iScript cDNA kit was used according to the manufacturer’s instructions (Bio-Rad, Hercules, CA). The primers (purchased from Invitrogen, Carlsbad, CA) were collagen (Col) 1a1 sense (5′-CACCCTCAAGAGCCTGAGTC-3′), Col1a1 antisense (5′-CAGCGGGCTGAGTAGGGAAC-3′), Col1a2 sense oligonucleotide (5′-CCGTGCTTCTCAGAACATCA-3′), Col1a2 antisense oligonucleotide (5′-TCCAGAGGTGCAATGTCAAG-3′), Col4a1 sense oligonucleotide (5′-CCTTTGTACCGTTGCATCCT-3′), Col4a1 antisense oligonucleotide (5′-AAAGGGAGAAAGAGGCTTGC-3′), Col4a2 sense oligonucleotide (5′-GATGCATGCAGTGTGCTTCT-3′), Col4a2 antisense oligonucleotide (5′-ATGCTGGTGAGGGCTAGAGA-3′), glyceraldehyde-3-phosphate dehydrogenase (GAPDH) sense oligonucleotide (5′-GTGTTCCTACCCCCAATGTG-3′), and GAPDH antisense oligonucleotide (5′-CCCTGTTGCTGTAGCCGTAT-3′). PCR reactions were performed in duplicate. The PCR for cDNA from the mRNA protocol was 50°C for 50 minutes, 94°C for 2 minutes, 94°C for 30 seconds, 55°C for 30 seconds, 68°C for 1 minute, and 72°C for 7 minutes for 35 cycles. Real-time PCR was accomplished with an iQ SYBR Green Supermix kit following the manufacturer’s protocol (Bio-Rad). The copies of targeted cDNA were normalized to GAPDH.
Blood Collection
To obtain blood, the mice were anesthetized by isoflurane, followed by orbital bleeding. The blood was allowed to clot at room temperature and then centrifuged at maximum speed to obtain serum.
Histopathological and Immunohistochemical Analysis
H&E staining was performed on paraffin-embedded 3-μm tissue sections after processing as described below. The sections were analyzed in a blinded examination by two clinical and one veterinarian pathologists from Meharry Medical College Department of Pathology. Mouse mammary tumors and lung tissues were excised, fixed in 4% formalin or Bouin’s reagent (Ricca Chemical, Arlington, TX) overnight, and embedded in paraffin. Sections (3 μm) were cut from the paraffin blocks. The slides were deparaffinized in xylene (Labsco, Louisville, KY) and rehydrated with decreasing graded alcohol (Labvision Thermo Scientific, Fremont, CA). Antigen retrieval was performed by a PT Module Plus machine using 100× citrate buffer, pH 6, at 95 to 96°C in a water bath for 20 minutes and cooled to room temperature (Labvision Thermo Scientific). Endogenous peroxidase blocking was accomplished with 3% hydrogen peroxide for 30 minutes (Labvision Thermo Scientific). Nonspecific background was eliminated by incubating tissue slides in BEAT Blocking Solution A for 30 minutes and then BEAT Blocking solution B for 10 minutes (HistoMouse-MAX Kit, Invitrogen, Carlsbad, CA).
The following primary antibodies and stains were used after antigen retrieval: rabbit polyclonal CDNKN2A/P19ARF (Abcam, Cambridge, MA); rabbit polyclonal p53 (FL-393, Santa Cruz Biotechnology, Santa Cruz, CA); rabbit polyclonal phospho-Akt (Ser-473, Cell Signaling, Danvers, MA); rabbit polyclonal anti-fetuin-A (Santa Cruz Biotechnology); rabbit monoclonal anti-Smad2 (Abcam); rabbit anti-extracellular signal-regulated kinase (ERK) 2 and anti-phospho-ERK1/2 (Santa Cruz Biotechnology); anti-proliferating cell nuclear antigen (PCNA) (Invitrogen); anti-Cas-3 (Cell Signaling); von Kossa staining (Newcomer Supply, Middleton, WI) that included 1% silver nitrate, 5% sodium thiosulfate, and nuclear fast red; and a trichrome staining kit (Newcomer Supply).
Tissue sections were washed in Tris-buffered saline Tween 20 (LabvisionThermo Scientific) followed by incubation for 10 minutes in secondary antibody conjugated to peroxidase (HistoMouse-MAX Kit). Sections were treated with diaminobenzidine as the substrate (Dako, Glostrup, Denmark). Sections were counterstained with hematoxylin for 5 minutes (Dako). Slides were then dehydrated through a graded series of alcohol, cleared in xylene, and mounted with coverslips. Negative controls were performed using Tris-buffered saline instead of primary antibody.
To assay for cellular senescence, cryo-tissue sections of frozen mammary tumors from fetuin-A wild-type and null animals were washed with PBS twice and incubated in fixative solution (25% glutaraldehyde) for 10 minutes, washed with PBS, and incubated in staining solution provided in a Senescence β-Galactosidase Staining Kit (Calbiochem, Darmstadt, Germany) overnight at 37°C covered with Parafilm. The slides were counterstained with hematoxylin for 5 minutes and mounted with a coverslip. Cells undergoing senescence stained blue. All of the experiments were repeated three times, and one of the representative results is shown.
Processing Tumor Tissues for Protein Analysis
Total cell lysates from frozen tissues were prepared from breast tumors from PyMT/Fet+/+ and PyMT/Fet−/− mice by solubilization and sonication in ice-cold radioimmunoprecipitation assay buffer (50 mmol/L Tris-HCl, pH 8.0, 150 mmol/L NaCl, 1 mmol/L EDTA, 1% Nonidet NP-40, 0.25% sodium deoxycholate, protease inhibitor cocktail [Sigma P8340], and 1 mmol/L phenylmethylsulfonyl fluoride). The homogenate was incubated on ice for 30 minutes and centrifuged at 500 × g for 5 minutes at 4°C to remove tissue clumps. The supernatant was centrifuged at 10,000 × g for 10 minutes at 4°C to separate detergent-solubilized proteins from the insoluble chromatin pellet. The pellet was resuspended in the same buffer and sonicated on ice. The protein concentrations of the detergent-insoluble proteins and the sonicated pellet were determined using the Bradford assay (Bio-Rad) and analyzed by SDS-polyacrylamide gel electrophoresis and Western blotting described previously3 with the exception that the exposure of membranes probed with anti-p53 antibody was performed overnight at room temperature.
Statistical Analysis
Statistical analysis was performed by one-way analysis of variance using the software GraphPad Prism (version 4). Values of P < 0.05 were considered significant. In comparing the fetuin-A null versus wild-type or heterozygous animals, we calculated and arrived at the number of 10 null and 10 wild-type mice to achieve a power of 0.99 (two-sided type 1 error 0.05).
Results
Fetuin-A Attenuates Tumor Latency in PyMT Transgenic Mice
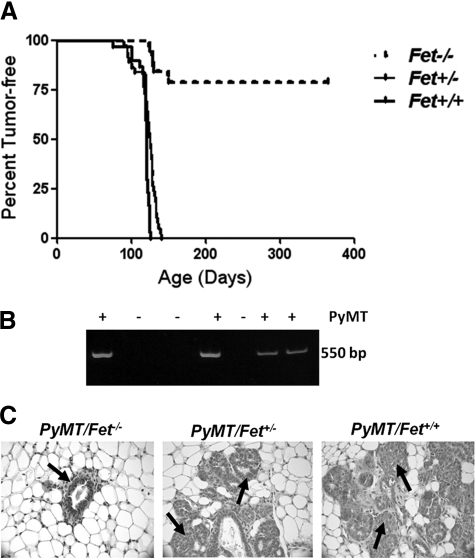
The onset of mammary tumors expressing the PyMT transgene (Figure 1B) has been observed as early as 30 days postpartum.20 Based on our earlier studies showing that fetuin-A promotes the growth of Lewis lung carcinoma cells in vivo,3 we questioned in the present studies whether fetuin-A modulates mammary transformation and/or promotion of mammary tumors. Our breeding protocol produced a total of 20 female PyMT/Fet−/−, 38 PyMT/Fet+/−, and 34 PyMT/Fet+/+ mice. Of the 20 PyMT/Fet−/− animals, 4 had tumor burden at 150 days of age and had to be euthanized. The remainder did not develop tumors for the duration of the study (Figure 1A). Palpable tumors began to appear by 90 days (12 weeks) in both PyMT/Fet+/− and PyMT/Fet+/+ mice (Figure 1A). However, tumor latency was significantly delayed in PyMT/Fet−/− mice such that the earliest palpable tumors in these animals appeared in 120-day (17-week)-old mice (Figure 1A). All of the PyMT/Fet+/+ and PyMT/Fet+/− animals reached their end points of tumor burden by 150 days and had to be euthanized.
Figure 1.
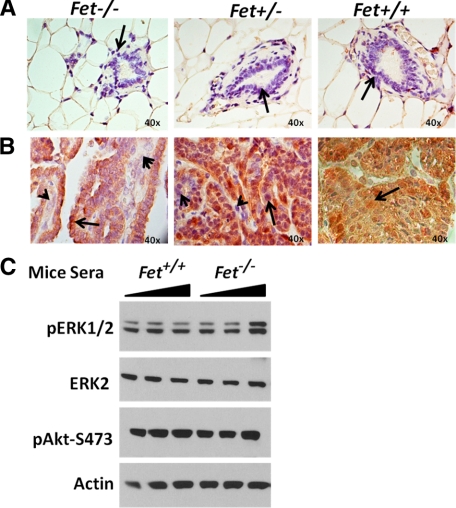
Lack of fetuin-A decreases mammary tumor incidence and increases tumor latency in PyMT/Fet−/− relative to PyMT/Fet+/+ and PyMT/Fet+/− mice. A: Kaplan-Meier survival curve showing that all of the PyMT/Fet+/+ and PyMT/Fet+/− mice in the study succumbed to mammary tumors by approximately 180 days of age, whereas more than 60% of the PyMT/Fet−/− mice were tumor-free at the end of the experiment at approximately 380 days of age. B: A typical genotyping assay revealed the 550-bp band of the PyMT oncogene. C: Mammary tissues from 60-day-old mice. Note that the acinus of PyMT/Fet−/− looks normal (arrow), whereas the acini of PyMT/Fet+/− and PyMT/Fet+/+ exhibit typical changes of multifocal intraepithelial neoplasia containing multifocal areas of glandular differentiation (arrows).
Histopathological evaluation of mammary glands of PyMT/Fet−/− mice at 60 days of age showed no significant morphological changes. The tissues of PyMT/Fet+/− and PyMT/Fet+/+ mice at 60 days of age on the other hand, showed hyperplastic acini and exhibited multiple areas of mammary intraepithelial neoplasia infiltrating the adjacent adipose tissue (Figure 1C).
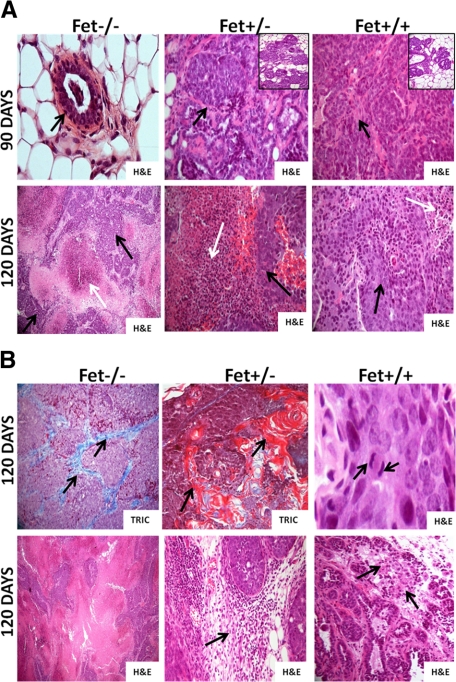
At 90 days of age, mammary tissues of PyMT/Fet−/− appeared normal (same as 60 days) (Figure 2A, top panel, H&E). In contrast, mammary tissues of PyMT/Fet+/− and PyMT/Fet+/+ mice at 90 days of age exhibited tumor masses characterized by solid sheets of neoplastic cells that are tightly packed with slit-like curvilinear spaces. Tumor sections also show multifocal areas of cystic spaces with luminal papillary projections lined by hyperplastic epithelium covering a fibrovascular stalk. In all tumor masses examined at the three time points, neoplastic cells were poorly differentiated, exhibited cellular atypia, and contained variable amounts of fibrovascular stroma with absence of polarization to basement membrane (Figure 2A, top panel, H&E). There was mild to moderate mononuclear cell infiltration along the tumor margins observed in PyMT/Fet+/− and PyMT/Fet+/+. The mammary tumor tissues of PymT/Fet+/− and PyMT/Fet+/+ also showed hyperplastic acini and exhibited multiple areas of mammary intraepithelial neoplasia (Figure 2A, top panel, insets). Additional changes at 90-day-old in PyMT/Fet+/− mice included multifocal regions of necrosis with plexiform sheets of neoplastic cells.
Figure 2.
Histopathology of mammary tissues of PyMT/Fet−/−, PyMT/Fet+/−, and PyMT/Fet+/+ at 90 and 120 days of age. A, top panel: Normal acinus (arrow) in a PyMT/Fet−/− mouse at 90 days of age (original magnification, ×40). The mammary tumor tissues of PyMT/Fet+/− and PyMT/Fet+/+ mice at 90 days of age clearly show tumor masses (arrows original magnification, ×20). There are also several hyperplastic acini and mammary intraepithelial neoplasm (insets). A, bottom panel: Tumors of PyMT/Fet−/− (original magnification, ×10), PyMT/Fet+/−, and PyMT/Fet+/+ (original magnification, ×20) mice show expansile tumor masses and extensive to moderate areas of necrosis (white arrows) interspersed with islands of neoplastic cells (black arrows). B, top panel: Mammary tumors show accumulation of collagen fibers particularly in tumors in PyMT/Fet−/− mice (stained blue with trichrome [TRIC], arrows). Tumor tissues of PyMT/Fet−/− and PyMT/Fet+/− also show squamous metaplasia and parakeratosis with concentric lamellar keratin consistent with keratin pearls (stained red with trichome; original magnification, ×20). Mitotic figures (arrows) for PyMT/Fet+/+ tumors of 3 to 5 per high-power field are shown (original magnification, ×40). B, bottom panel: tumor tissues in PyMT/Fet−/− mice show a medullary pattern (original magnification, ×10). This panel also shows mild to moderate mononuclear cell infiltration (arrows) in the tumor tissues of PyMT/Fet+/− and PyMT/Fet+/+ mice (original magnification, ×20).
The mammary tumor sections obtained from 120-day-old PyMT/Fet−/−, PyMT/Fet+/−, and PyMT/Fet+/+ mice were characterized by expansile masses with pushing margins and cystic acini as well as solid sheets of neoplastic cells and cellular atypia; mitotic figures for PyMT/Fet+/+ tumors were 3 to 5 per high-power field (Figure 2B, top panel, H&E). The mammary tumors of 120-day-old PyMT/Fet−/− mice and to a lesser extent of PyMT/Fet+/− and PyMT/Fet+/+ mice exhibited extensive to moderate areas of necrosis (Figure 2A, bottom panels, white arrows). At this age, PyMT/Fet−/− (Figure 2B, top panel, TRIC) and to a lesser extent PyMT/Fet+/− tumor tissues displayed robust accumulation of collagen fibers in the extracellular matrices compared with extracellular matrices in the mammary tumors of PyMT/Fet+/+ mice (trichrome staining). Additional characteristics of mammary tumors of PyMT/Fet−/− (∼40% of tumors) and PyMT/Fet+/− (∼30% of tumors) (Figure 2B, top panel, TRIC) mice were squamous metaplasia and keratin pearls, which were absent in tumors of 120-day-old PyMT/Fet+/+ mice. The data suggest that the reduced levels of fetuin-A give rise to a squamous metaplastic phenotype. This epithelial cell type was confirmed by cytokeratin-5 staining (data not shown). Studies have characterized this phenotype in mammary tissue as a more aggressive carcinoma with a worse prognosis.21 A medullary pattern variant of mammary tumors of 120-day-old PyMT/Fet−/− mice was observed in two of the four mice (Figure 2B, bottom panel, H&E). This variant is clinically characterized as less aggressive and carries a favorable prognosis as a more treatable carcinoma.22
Fetuin-A Moderately Promotes Mammary Tumor Growth in PyMT Mice
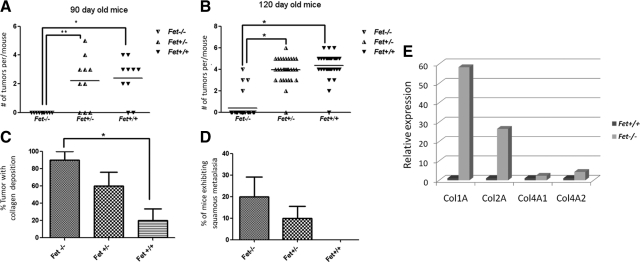
Having demonstrated that both PyMT/Fet+/+and PyMT/Fet+/− mice develop mammary tumors early (palpable tumors by 90 days of age) compared with the PyMT/Fet−/− mice, we next questioned the ability of fetuin-A to affect the growth rate of the tumors. Fetuin-A heterozygous animals should have at least half the serum concentration of fetuin-A (∼0.25 mg/ml) relative to that for the adult wild-type animals (0.5 mg/ml).2 At 90 days of age, the average number of tumors/mouse was basically the same in the two genotypes (Figure 3A). However, when one considers 120-day-old animals, the average number of tumors per mouse was higher in the PyMT/Fet+/+ mice compared with that in the fetuin-A heterozygous or null animals (Figure 3B). We also observed the tendency for tumors to fuse to form larger ones more frequently in the PyMT/Fet+/+ relative to the PyMT/Fet−/− and PyMT/Fet+/− mice. Although mammary tumors developed late in PyMT/Fet−/− mice (after 90 days), they grew rapidly once they reached a palpable size. Palpable tumors appeared in this genotype at ∼120 days (17 weeks). Within 20 days most of these reached a volume of ∼700 mm3 comparable to those harvested from PyMT/Fet+/− or PyM/Fet+/+ mice, which were first palpated at ∼90 days. As alluded to above, accompanying the growth of mammary tumors in PyMT/Fet−/− and to a lesser extent in PyMT/Fet+/− animals was the extensive fibrosis (Figure 3C). There was also evidence of squamous metaplasia in the mammary tumor tissues of PyMT/Fet−/− and PyMT/Fet+/− but not wild-type animals (Figure 3D). The fibrosis or collagen deposition implied a higher collagen synthesis in the mammary tissues of fetuin-A null and heterozygous relative fetuin-A wild-type animals. We therefore measured by real-time RT-PCR the collagen mRNA levels in the mammary tissues of 120-day-old PyMT/Fet−/− and PyMT/Fet+/+ animals that had been stored at −80°C. In three separate experiments, we determined that mRNA for collagen type 1 was almost 60-fold higher in PyMT/Fet−/− mammary tumor tissues compared with that in fetuin-A wild-type tissues. Collagen type 4 was also synthesized at a slightly higher rate in the null animals (Figure 3E). In one experiment, the difference in collagen type 1 mRNA expression was as high as 300-fold.
Figure 3.
Mammary tumor incidence at 90 and 120 days in the three PyMT genotypes. A: Number of palpable tumors/mouse in 90-day-old PyMT/Fet−/−, PyMT/Fet+/−, and PyMT/Fet+/+ mice (*P = 0.0002; **P = 0.0004). B: Number of palpable tumors/mouse in 120-day-old PyMT/Fet−/−, PyMT/Fet+/−, and PyMT/Fet+/+ mice. *P < 0.0001 C: Collagen deposition in the tumors of 120-day-old PyMT/Fet−/−, PyMT/Fet+/−, and PyMT/Fet+/+ mice as revealed by trichrome staining. *P < 0.05 D: Incidence of squamous metaplasia in the mammary tumors of 120-day-old PyMT/Fet−/− and PyMT/Fet+/− but not PyMT/Fet+/+ mice (mean ± SE, n = 10 mice per group; one-way analysis of variance). E: Real-time RT-PCR of collagen types 1 and 4 mRNA expression in the 120-day-old mammary tumors in PyMT/Fet−/− and PyMT/Fet+/+ animals.
Fetuin-A Attenuates TGF-β Signaling in Normal Mammary Epithelial Cells
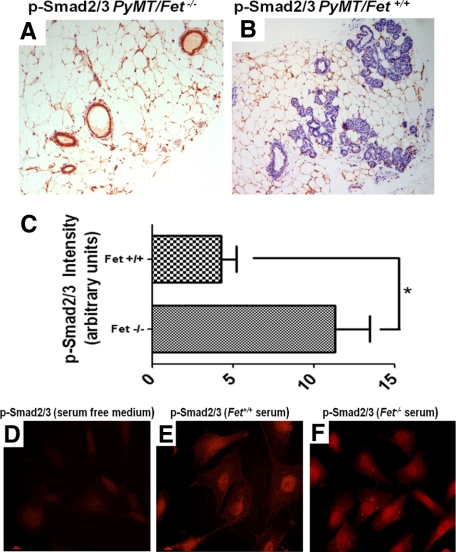
TGF-β, belongs to a family of proteins characterized as pleomorphic cytokines that regulate extracellular matrix production, wound healing, immune functions, cell proliferation, and differentiation.23,24 Fetuin-A has been shown to block TGF-β1 binding to the extracellular motifs of TGF-βII receptor, suppressing TGF-β signaling and inhibiting TGF-β induced epithelial-mesenchymal transition.4 TGF-β inhibits normal epithelial cell proliferation,25 but in many human cancers, TGF-β expression is elevated.26 To determine whether fetuin-A modulates TGF-β signaling during mammary carcinogenesis, we probed mammary tissue sections of PyMT/Fet−/− and PyMT/Fet+/+ mice with anti-phospho-Smad2/3 (P-Smad2/3), a downstream target of TGF-β as a readout. The preneoplastic mammary tissues of 60-day-old PyMT+/Fet−/− mice consistently stained positive for P-Smad2/3 in epithelial cells lining the acini (Figure 4A). In contrast, the tissues of 60-day-old PyMT/Fet+/+ mice were virtually devoid of P-Smad2/3, and the acini were partially or completely filled with neoplastic cells (Figure 4B). We quantified the P-Smad2/3 staining in Figure 4, A and B, comparing preneoplastic lesions of PyMT/Fet−/− and PyMT/Fet+/+ mice, respectively (Figure 4C). We also determined by indirect immunofluorescence, the propensity of TGF-β in the sera of PyMT/Fet+/+ and PyMT/Fet−/− animals to turn on the TGF-β signaling in human breast tumor cells. As expected, only background TGF-β signaling was observed in BT-549 cells incubated with serum-free medium (Figure 4D, control). There was accumulation of P-Smad2/3 in the nucleus but much less in the cytoplasm of BT-549 cells incubated with fetuin-A wild-type serum (Figure 4E, Fet+/+). There was more intense P-Smad2/3 labeling of the nucleus, which spilled over into the cytoplasm and basically filled BT-549 cells incubated with serum from fetuin-A null mice (Figure 4F, Fet−/−). This experiment clearly suggests that fetuin-A in serum has the capacity to sequester TGF-β away from the tumor cells. However, the staining was not as dramatic as in vivo (Figure 4, A and B).
Figure 4.
TGF-β signaling determined by staining for P-Smad2/3 in mammary tissues of mice and human cells. A and B: Intensity of P-Smad2/3 staining in the mammary tissues of 60-day-old PyMT/Fet−/− (A) and PyMT/Fet+/+(B) mice (original magnification, ×10). C: Intensity of P-Smad2/3 staining represented as arbitrary units. *P = 0.008 D–F: TGF-β signaling in BT-549 breast tumor cells incubated in the absence of serum (D) and in the presence of serum from PyMT/Fet+/+ (E) and PyMT/Fet−/− (F) animals.
Fetuin-A Modulates the ARF-p53 Tumor Suppressor Pathway in the Mammary Tumors
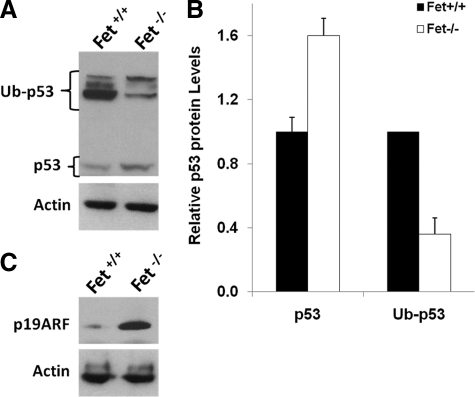
It has been reported that for polyoma middle T antigen to transform mammary epithelial cells, the ARF-p53 tumor suppressor pathway needs to be inactivated.18 We therefore examined the expression by Western blotting of ARF (commonly known as p19 in rodents and p14 in humans) and p53 proteins in the lysates of PyMT/Fet−/− and PyMT/Fet+/+ mammary tumors (Figure 5, A and C). Our data showed that mammary tumors from PyMT/Fet+/+ mice had scant p19 and p53 expression, whereas the tumors from PyMT/Fet−/− mice had heavy expression of both p19 and p53 (Figure 5, A and C). Polyubiquitinated forms of p53 were more abundant in the tissues of PyMT/Fet+/+ mice with the implication that the protein was less stable here, but stabilized in the mammary tissues of PyMT/Fet−/− mice (Figure 5, A and B). The clear difference in the expression of these tumor suppressor proteins in the fetuin-A wild-type and null tumors suggests that fetuin-A is directly or indirectly responsible for the down-regulation of p19 and p53 in PyMT/Fet+/+ mammary gland and consequently the attenuation of tumor latency in these animals. Because there is strong cross-talk between TGF-β and p53,27 it is likely that TGF-β signaling in PyMT/Fet−/− tumors was responsible for the increased expression of p53 and p19 proteins. Strong expression of p19 and p53 in PyMT/Fet−/− mammary tissues probably contributed to the suppression of tumorigenicity in this environment and prolonged the latency in animals in which tumors did develop. It is also conceivable that the process of PyMT tumorigenesis may begin early when the serum concentration of fetuin-A is as high as 2 mg/ml, suggesting that the p53 in the tumor tissues of the PyMT/Fet+/+ is destined for degradation and therefore is unstable.28
Figure 5.
Fetuin-A-mediated inactivation of ARF-P19 and degradation of P53. Equal amounts of detergent-solubilized proteins of mammary tumors from the fetuin-A wild-type and null mice prepared as described in Materials and Methods were separated on a 4 to 12% SDS gel and transferred to nitrocellulose membranes, and the membranes were probed with antibodies to p53 (A) or p19ARF (C). Actin was used as a loading control. Note that the exposure of membranes probed with anti-p53 antibody was performed overnight at room temperature. Ub-p53, ubiquitinylated p53. B: Densitometric analysis of the p53 and Ub-p53 bands of whole tissue lysates. Bars represent means and SD of densitometric ratios relative to Fet+/+ from blots of two representative experiments.
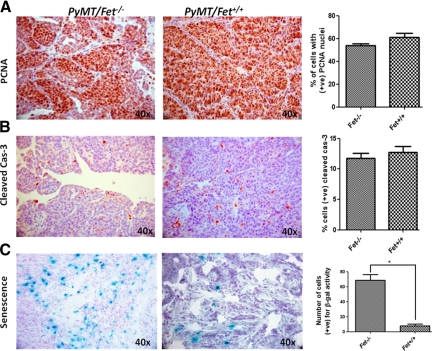
Of note is the observation that mammary tumors of PyMT/Fet−/− mice had necrotic areas and displayed unique phenotypes such as squamous metaplasia, implying that they were more differentiated than the fetuin-A wild-type tumors. The data suggest that necrosis and differentiation are due to the heavy expression of p19 and more stable p53 that may lead to cell cycle arrest and apoptosis.29 We therefore examined the expression of markers of cellular proliferation (PCNA), apoptosis (cleaved caspase-3), and senescence (β-galactosidase). Interestingly, cellular proliferation was not significantly affected by the absence of fetuin-A as was expected (Figure 6A). Likewise, apoptosis occurred at the same frequency in PyMT/Fet−/− and PyMT/Fet+/+ tumors (Figure 6B). However, there was extensive senescence in the tumor tissues of PyMT/Fet−/− mice but only marginal senescence in the fetuin-A wild-type mammary tumors (Figure 6C). This observation was quite striking, and similar data were obtained in two other sets of mammary tumors.
Figure 6.
Proliferation, apoptotic, and senescence markers in mammary tumors from PyMT/Fet−/− and PyMT/Fet+/+ animals. Mammary tumors (120 days old) from fetuin-A null and qjwild-type PyMT animals were sectioned and processed for immunohistochemical analysis as described in Materials and Methods. The slides were stained for PCNA (proliferation) (A) and cleaved caspase-3 (cas-3, apoptosis) (B). C: The slides were also stained for β-galactosidase activity that was quantified as number of cells positive for β-galactosidase (β-gal) activity per field (*P < 0.004). Original magnification, ×40. +ve, positive.
Fetuin-A Does Not Affect the PI3 Kinase/Akt Pathway in Mammary Tissues
We previously demonstrated that one of the cellular activities of fetuin-A is the mediation of PI3 kinase/Akt activation in vitro, as determined by the phosphorylation of Akt on Ser-473.3 We therefore questioned whether the absence of fetuin-A in the mammary tumors of fetuin-A-null mice results in the down-regulation of the PI3 kinase/Akt signaling. Because PI3 kinase/Akt is an important housekeeping pathway in tumors, there are several pathways through which it can be activated and so, at best, we only expected a moderate down-regulation in the absence of fetuin-A or a reduced concentration. We compared the activity of the PI3 kinase/Akt pathway in the mammary tissues of normal non-PyMT Fet−/−, Fet+/−, and Fet+/+ relative to tumors from PyMT/Fet−/−, PyMT/Fet+/−, and PyMT/Fet+/+ C57BL/6 mice. As expected, normal mammary tissues (60-day-old mice, non-PyMT controls) did not show activation of the pathway (Figure 7A). On the contrary, PI3 kinase/Akt was active in the mammary tumor tissues of 120-day-old PyMT/Fet−/−, PyMT/Fet+/−, and PyMT/Fet+/+ animals (Figure 7B). Serum fetuin-A is normally associated with a number of proteins such as α-2-macroglobulin, which are also capable of activating the PI3 kinase/Akt signaling pathway.30 We questioned whether serum proteins from PyMT/Fet+/+ activated the PI3 kinase/Akt and mitogen-activated protein kinase pathways in the human breast carcinoma cell line (BT-549) more robustly compared with similar concentrations of serum proteins from PyMT/Fet−/− mice. The data indicated that the presence of fetuin-A in the PyMT/Fet+/+ serum did not make much of a difference in the activation/phosphorylation of either Akt. However, pERK1/2 was up-regulated at higher concentrations of serum proteins from Fet−/− animals (Figure 7C).
Figure 7.
Expression of phospho-Akt-Ser-473 in the PyMT mammary tissues. A and B: Negligible expression of phospho-Akt-Ser-473 in normal mammary acini (A, arrows) (original magnification, ×40) and strong expression in mammary tumor sections (B, arrows) (original magnification, ×40). There were, however, some cells in the tumor sections of Fet−/− and Fet+/− animals in which phospho-Akt expression was weak (arrowheads). C: Breast carcinoma cells (BT-549) were incubated with graded doses (9.3–930 μg/ml) of serum proteins from PyMT/Fet+/+ and PyMT/Fet−/− mice for 30 minutes, followed by lysis of cells and resolution in SDS-polyacrylamide gel electrophoresis and transferred to immobile membrane. The membranes were probed with anti-phospho-Erk1/2 (pERK1/2), Erk2, phospho-Akt-473 (pAkt-S473), and actin. There was no marked difference in the level of activation of either Erk or Akt mediated by serum proteins from fetuin-A wild-type and null (minus fetuin-A) mice.
Discussion
The present studies demonstrate for the first time that lack of fetuin-A reduces the incidence of mammary tumors in the PyMT transgenic mouse model for breast cancer by more than 60% and increases tumor latency in this model. Taken together, our data demonstrate that fetuin-A plays a key role in the polyoma middle T oncogenesis. Because fetuin-A is an abundant serum protein, these studies bring into focus its potential role as a significant modulator of breast cancer in humans. The fact that mammary tumors in PyMT/Fet+/− mice, in which the serum concentration of fetuin-A is approximately 0.25 mg/ml, grew at a slightly slower rate than in the fetuin-A wild-type animals further underscored its importance in breast cancer development.
The interaction of fetuin-A with TGF-β is well documented as is the involvement of TGF-β in the transformation of mammary epithelial cells. Fetuin-A is known to have a TGF-β binding motif12 and has the potential to radically alter TGF-β signaling not only in breast alveolar cells but also in the stromal compartment. Numerous studies support the notion that TGF-β has a powerful tumor-suppressive role in most epithelial derived tumors such as breast cancer.31,32,33 The promiscuous binding of TGF-β to fetuin-A in the extracellular compartment of mammary tumors would divert it away from the epithelial cells, which would then be transformed more easily. Our data support this notion. In most of the precancerous tissues we examined, TGF-β signaling (using P-Smad2/3 staining as a readout) was restricted to the tissues of PyMT/Fet−/− mice, with negligible TGF-β signaling in similar mammary tissues of PyMT/Fet+/+ or PyMT/Fet+/− mice. The distinct difference in P-Smad2/3 expression in the epithelial tissues of fetuin-A wild-type versus null mice has been demonstrated by others.4 Interestingly, the present studies also support the notion that after undergoing transformation, mammary tumor tissues became refractory to the suppressive signals of TGF-β, which can quickly change roles and become a tumor promoter instead.33
Other studies in which the TGF-β receptor II was ablated in the alveolar progenitor cells of the mammary gland have also demonstrated that lack of TGF-β signaling attenuates tumor latency in the PyMT model.34 The readily available TGF-β in PyMT/Fet−/− mammary stroma due to lack of fetuin-A could also be responsible for the fibrosis seen in these tumors. Indeed this observation was confirmed by almost 60-fold higher expression of collagen type I mRNA in the tumor tissues of PyMT/Fet−/− mice relative to that in the mRNA in the mammary tumors of fetuin-A wild-type animals. TGF-β signaling, particularly in stromal fibroblasts, is known to enhance collagen synthesis.35 Whereas TGF-β via its receptor suppresses breast cancer transformation and can prolong tumor latency in the PyMT models, the mice in these studies eventually succumb to the disease.34 In our study, on the other hand, the lack of fetuin-A not only prolonged tumor latency, it significantly reduced the incidence of mammary tumors in PyMT/Fet−/− mice relative to that in the controls.
A number of studies have clearly demonstrated that there is considerable cross-talk between TFG-β and ARF-p53.27,36,37 For example, Smad2/3 and p53 have been shown to physically interact and jointly regulate the transcription of several TGF-β target genes.38 Experiments also suggest that p53 activation conveys cues from extracellular signals within the TGF-β gene expression program and support the idea that p53 acts as an integration node between Ras/mitogen-activated protein kinase and TGF-β pathways.27 The strong in vivo TGF-β signaling in the absence of fetuin-A most likely induces the p19(Arf) transcription in the mammary tissues,39 resulting in the up-regulation of p53. The up-regulated p53 in the absence of fetuin-A, in turn, would amplify TGF-β signaling.37 The polyoma virus (Py) genome encodes three proteins known as T antigens (large T, middle T, and small T). The middle T antigen (PyMT) considered to be the major transforming oncogene, is a membrane-associated protein that can bind and activate a number of key signaling molecules including members of the Src, Ras, PI3 kinase/Akt, and mitogen-activated protein kinase pathways.40 These pathways are also altered in human breast cancers.41 It has been reported that whereas the Py early region that encodes all of the three Py T antigens will transform primary rodent cells, transformation by PyMT is possible only if p53 is inactivated.18 By extending these original observations to the in vivo environment, it becomes clear that for PyMT to transform the mammary epithelial cells in PyMT transgenic mice, inactivation of the ARF-p53 cascade is necessary. The dogmatic explanation for this is that p19ARF binds to the ubiquitin ligase MDM2 and this prevents the ubiquitination of p53 and hence its degradation. The dynamics of the in vivo inactivation of this cascade is yet to be defined.
There are other pathways that can be altered by fetuin-A to radically change tumor growth and metastasis. One property of fetuin-A in particular that has been overlooked is its ability to stabilize matrix metalloproteinases in the extracellular matrix. These enzymes play critical, albeit complex, roles in tumor invasion and metastasis.42 The interaction of fetuin-A with these enzymes allows them to remain active for long periods in vivo and prevents their inactivation by autolysis.43 In this way they can propel the “tumor islands” to invade the stroma and link up or fuse as they grow bigger and bigger and eventually metastasize to distant organs. We demonstrated previously that the serum of fetuin-A wild-type C57BL/6 mice has more active matrix metalloproteinase 2 and 9 than the serum from fetuin-A null mice.43 Thus, the extensive fibrosis seen in the PyMT/Fet−/− and PyMT/Fet+/− mammary tumors could be due not only to higher levels of collagen mRNA expression in the absence of fetuin-A but also to low levels of active matrix metalloproteinases in the stromal microenvironment necessary to remodel the collagens.
Last, we expected, based on our earlier data showing that fetuin-A is a significant mediator of PI3 kinase/Akt activation, that this pathway would be up-regulated in the mammary tissues of the PyMT/Fet+/+ mice but not the fetuin null mice. However, this does not seem to be the case. From the data we can conclude that the constitutive activation of PI3/Akt pathway is driven by PyMT oncogenesis and does not seem to be influenced by the presence of fetuin-A.
In summary, our data implicate fetuin-A in the modulation of PyMT oncogenesis in the mammary tissues. The data suggest that stronger TGF-β signaling in the absence of fetuin-A is translated via cross-talk into elevated expression of ARF-p53, whereas the sequestration of TGF-β by fetuin-A, leading to attenuation of its signaling in the epithelial cells, inactivates the ARF-p53. This attenuation of TGF-β signaling shortens the latency of mammary tumorigenesis with implications for the development of breast cancer in humans. Thus, fetuin-A is emerging as a key player in the oncogenic transformation of not only breast cancer but also of other tumors whose initiation and promotion are under the tight control of TGF-β. Furthermore, fetuin-A by virtue of its association with other key signaling pathways is likely to play other roles in tumor progression.
Acknowledgments
We thank Ms. Kiesha Williams for help with histopathology, immunohistochemistry and with graphics.
Footnotes
Address reprint requests to Josiah Ochieng, Ph.D., Department of Biochemistry and Cancer Biology, Meharry Medical College, 1005 D.B. Todd Blvd., Nashville, TN 37208. E-mail: jochieng@mmc.edu.
Supported by the Department of Defense (grants W81XWH-07-1-0254 and 1SC1CA134018-03 to J.O.).
None of the authors disclosed any relevant financial relationships.
References
- Nie Z. Fetuin: its enigmatic property of growth promotion. Am J Physiol. 1992;263:C551–C562. doi: 10.1152/ajpcell.1992.263.3.C551. [DOI] [PubMed] [Google Scholar]
- Jahnen-Dechent W, Schinke T, Trindl A, Muller-Esterl W, Sablitzky F, Kaiser S, Blessing M. Cloning and targeted deletion of the mouse fetuin gene. J Biol Chem. 1997;272:31496–31503. doi: 10.1074/jbc.272.50.31496. [DOI] [PubMed] [Google Scholar]
- Kundranda MN, Henderson M, Carter KJ, Gorden L, Binhazim A, Ray S, Baptiste T, Shokrani M, Leite-Browning ML, Jahnen-Dechent W, Matrisian LM, Ochieng J. The serum glycoprotein fetuin-A promotes Lewis lung carcinoma tumorigenesis via adhesive-dependent and adhesive-independent mechanisms. Cancer Res. 2005;65:499–506. [PubMed] [Google Scholar]
- Swallow CJ, Partridge EA, Macmillan JC, Tajirian T, DiGuglielmo GM, Hay K, Szweras M, Jahnen-Dechent W, Wrana JL, Redston M, Gallinger S, Dennis JW. α2HS-glycoprotein, an antagonist of transforming growth factor β in vivo, inhibits intestinal tumor progression. Cancer Res. 2004;64:6402–6409. doi: 10.1158/0008-5472.CAN-04-1117. [DOI] [PubMed] [Google Scholar]
- Triffitt JT, Gebauer U, Ashton BA, Owen ME, Reynolds JJ. Origin of plasma α2-HS-glycoprotein and its accumulation in bone. Nature. 1976;262:226–227. doi: 10.1038/262226a0. [DOI] [PubMed] [Google Scholar]
- Brown WM, Saunders NR, Mollgard K, Dziegielewska KM. Fetuin—an old friend revisited. Bioessays. 1992;14:749–755. doi: 10.1002/bies.950141105. [DOI] [PubMed] [Google Scholar]
- Schafer C, Heiss A, Schwarz A, Westenfeld R, Ketteler M, Floege J, Muller-Esterl W, Schinke T, Jahnen-Dechent W. The serum protein α2-Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J Clin Invest. 2003;112:357–366. doi: 10.1172/JCI17202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiro RG, Bhoyroo VD. Structure of the O-glycosidically linked carbohydrate units of fetuin. J Biol Chem. 1974;249:5704–5717. [PubMed] [Google Scholar]
- Jahnen-Dechent W, Trindl A, Godovac-Zimmermann J, Muller-Esterl W. Posttranslational processing of human α2-HS glycoprotein (human fetuin). Evidence for the production of a phosphorylated single-chain form by hepatoma cells. Eur J Biochem. 1994;226:59–69. doi: 10.1111/j.1432-1033.1994.tb20026.x. [DOI] [PubMed] [Google Scholar]
- Hendig D, Schulz V, Arndt M, Szliska C, Kleesiek K, Gotting C. Role of serum fetuin-A, a major inhibitor of systemic calcification, in pseudoxanthoma elasticum. Clin Chem. 2006;52:227–234. doi: 10.1373/clinchem.2005.059253. [DOI] [PubMed] [Google Scholar]
- Schinke T, Amendt C, Trindl A, Poschke O, Muller-Esterl W, Jahnen-Dechent W. The serum protein α2-HS glycoprotein/fetuin inhibits apatite formation in vitro and in mineralizing calvaria cells. A possible role in mineralization and calcium homeostasis. J Biol Chem. 1996;271:20789–20796. doi: 10.1074/jbc.271.34.20789. [DOI] [PubMed] [Google Scholar]
- Demetriou M, Binkert C, Sukhu B, Tenenbaum HC, Dennis JW. Fetuin/α2-HS glycoprotein is a transforming growth factor-β type II receptor mimic and cytokine antagonist. J Biol Chem. 1996;271:12755–12761. doi: 10.1074/jbc.271.22.12755. [DOI] [PubMed] [Google Scholar]
- Mathews ST, Chellam N, Srinivas PR, Cintron VJ, Leon MA, Goustin AS, Grunberger G. α2-HSG, a specific inhibitor of insulin receptor autophosphorylation, interacts with the insulin receptor. Mol Cell Endocrinol. 2000;164:87–98. doi: 10.1016/s0303-7207(00)00237-9. [DOI] [PubMed] [Google Scholar]
- Tiffany JM, Blough HA. The interaction of fetuin with phosphatidylcholine monolayers. Characterization of a lipoprotein membrane system suitable for the attachment of myxoviruses. Biochem J. 1970;117:377–384. doi: 10.1042/bj1170377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayatte AJ, Kumbla L, Subbiah MT. Marked acceleration of exogenous fatty acid incorporation into cellular triglycerides by fetuin. J Biol Chem. 1990;265:5883–5888. [PubMed] [Google Scholar]
- Westenfeld R, Schafer C, Smeets R, Brandenburg VM, Floege J, Ketteler M, Jahnen-Dechent W. Fetuin-A (AHSG) prevents extraosseous calcification induced by uraemia and phosphate challenge in mice. Nephrol Dial Transplant. 2007;22:1537–1546. doi: 10.1093/ndt/gfm094. [DOI] [PubMed] [Google Scholar]
- Lin EY, Jones JG, Li P, Zhu L, Whitney KD, Muller WJ, Pollard JW. Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model provides a reliable model for human diseases. Am J Pathol. 2003;163:2113–2126. doi: 10.1016/S0002-9440(10)63568-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomax M, Fried M. Polyoma virus disrupts ARF signaling to p53. Oncogene. 2001;20:4951–4960. doi: 10.1038/sj.onc.1204717. [DOI] [PubMed] [Google Scholar]
- Laird PW, Zijderveld A, Linders K, Rudnicki MA, Jaenisch R, Berns A. Simplified mammalian DNA isolation procedure. Nucleic Acids Res. 1991;19:4293. doi: 10.1093/nar/19.15.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy CT, Cardiff RD, Muller WJ. Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol Cell Biol. 1992;12:954–961. doi: 10.1128/mcb.12.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menes T, Schachter J, Morgenstern S, Fenig E, Lurie H, Gutman H. Primary squamous cell carcinoma (SqCC) of the breast. Am J Clin Oncol. 2003;26:571–573. doi: 10.1097/01.coc.0000045809.85995.3B. [DOI] [PubMed] [Google Scholar]
- Malyuchik SS, Kiyamova RG. Medullary breast carcinoma. Exp Oncol. 2008;30:96–101. [PubMed] [Google Scholar]
- Gold LI. The role for transforming growth factor-β (TGF-β) in human cancer. Crit Rev Oncog. 1999;10:303–360. [PubMed] [Google Scholar]
- Wakefield LM, Letterio JJ, Geiser AG, Flanders KC, O'Shaughnessy J, Roberts AB, Sporn MB. Transforming growth factor-βs in mammary tumorigenesis: promoters or antipromoters?. Prog Clin Biol Res. 1995;391:133–148. [PubMed] [Google Scholar]
- Moustakas A, Pardali K, Gaal A, Heldin CH. Mechanisms of TGF-β signaling in regulation of cell growth and differentiation. Immunol Lett. 2002;82:85–91. doi: 10.1016/s0165-2478(02)00023-8. [DOI] [PubMed] [Google Scholar]
- Shim KS, Kim KH, Han WS, Park EB. Elevated serum levels of transforming growth factor-β1 in patients with colorectal carcinoma: its association with tumor progression and its significant decrease after curative surgical resection. Cancer. 1999;85:554–561. doi: 10.1002/(sici)1097-0142(19990201)85:3<554::aid-cncr6>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Cordenonsi M, Montagner M, Adorno M, Zacchigna L, Martello G, Mamidi A, Soligo S, Dupont S, Piccolo S. Integration of TGF-β and Ras/MAPK signaling through p53 phosphorylation. Science. 2007;315:840–843. doi: 10.1126/science.1135961. [DOI] [PubMed] [Google Scholar]
- Xirodimas D, Saville MK, Edling C, Lane DP, Lain S. Different effects of p14ARF on the levels of ubiquitinated p53 and Mdm2 in vivo. Oncogene. 2001;20:4972–4983. doi: 10.1038/sj.onc.1204656. [DOI] [PubMed] [Google Scholar]
- Qian Y, Chen X. Tumor suppression by p53: making cells senescent. Histol Histopathol. 2010;25:515–526. doi: 10.14670/hh-25.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra UK, Pizzo SV. Activation of Akt/PDK signaling in macrophages upon binding of receptor-recognized forms of α2-macroglobulin to its cellular receptor: effect of silencing the CREB gene. J Cell Biochem. 2004;93:1020–1032. doi: 10.1002/jcb.20233. [DOI] [PubMed] [Google Scholar]
- Derynck R, Akhurst RJ, Balmain A. TGF-β signaling in tumor suppression and cancer progression. Nat Genet. 2001;29:117–129. doi: 10.1038/ng1001-117. [DOI] [PubMed] [Google Scholar]
- Siegel PM, Shu W, Cardiff RD, Muller WJ, Massague J. Transforming growth factor β signaling impairs Neu-induced mammary tumorigenesis while promoting pulmonary metastasis. Proc Natl Acad Sci USA. 2003;100:8430–8435. doi: 10.1073/pnas.0932636100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcellos-Hoff MH, Akhurst RJ. Transforming growth factor-β in breast cancer: too much, too late. Breast Cancer Res. 2009;11:202. doi: 10.1186/bcr2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierie B, Stover DG, Abel TW, Chytil A, Gorska AE, Aakre M, Forrester E, Yang L, Wagner KU, Moses HL. Transforming growth factor-β regulates mammary carcinoma cell survival and interaction with the adjacent microenvironment. Cancer Res. 2008;68:1809–1819. doi: 10.1158/0008-5472.CAN-07-5597. [DOI] [PubMed] [Google Scholar]
- Yee KO, Connolly CM, Pines M, Lawler J. Halofuginone inhibits tumor growth in the polyoma middle T antigen mouse via a thrombospondin-1 independent mechanism. Cancer Biol Ther. 2006;5:218–224. doi: 10.4161/cbt.5.2.2419. [DOI] [PubMed] [Google Scholar]
- Zhang S, Ekman M, Thakur N, Bu S, Davoodpour P, Grimsby S, Tagami S, Heldin CH, Landstrom M. TGFβ1-induced activation of ATM and p53 mediates apoptosis in a Smad7-dependent manner. Cell Cycle. 2006;5:2787–2795. doi: 10.4161/cc.5.23.3523. [DOI] [PubMed] [Google Scholar]
- Dupont S, Zacchigna L, Adorno M, Soligo S, Volpin D, Piccolo S, Cordenonsi M. Convergence of p53 and TGF-β signaling networks. Cancer Lett. 2004;213:129–138. doi: 10.1016/j.canlet.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Cordenonsi M, Dupont S, Maretto S, Insinga A, Imbriano C, Piccolo S. Links between tumor suppressors: p53 is required for TGF-β gene responses by cooperating with Smads. Cell. 2003;113:301–314. doi: 10.1016/s0092-8674(03)00308-8. [DOI] [PubMed] [Google Scholar]
- Freeman-Anderson NE, Zheng Y, McCalla-Martin AC, Treanor LM, Zhao YD, Garfin PM, He TC, Mary MN, Thornton JD, Anderson C, Gibbons M, Saab R, Baumer SH, Cunningham JM, Skapek SX. Expression of the Arf tumor suppressor gene is controlled by Tgfβ2 during development. Development. 2009;136:2081–2089. doi: 10.1242/dev.033548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluck MM, Schaffhausen BS. Lessons in signaling and tumorigenesis from polyomavirus middle T antigen. Microbiol Mol Biol Rev. 2009;73:542–563. doi: 10.1128/MMBR.00009-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pylayeva Y, Gillen KM, Gerald W, Beggs HE, Reichardt LF, Giancotti FG. Ras- and PI3K-dependent breast tumorigenesis in mice and humans requires focal adhesion kinase signaling. J Clin Invest. 2009;119:252–266. doi: 10.1172/JCI37160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhrmann A, Kammerer U, Kapp M, Dietl J, Anacker J. Expression of matrix metalloproteinases (MMPs) in primary human breast cancer and breast cancer cell lines: new findings and review of the literature. BMC Cancer. 2009;9:188. doi: 10.1186/1471-2407-9-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S, Lukyanov P, Ochieng J. Members of the cystatin superfamily interact with MMP-9 and protect it from autolytic degradation without affecting its gelatinolytic activities. Biochim Biophys Acta. 2003;1652:91–102. doi: 10.1016/j.bbapap.2003.08.004. [DOI] [PubMed] [Google Scholar]