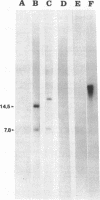
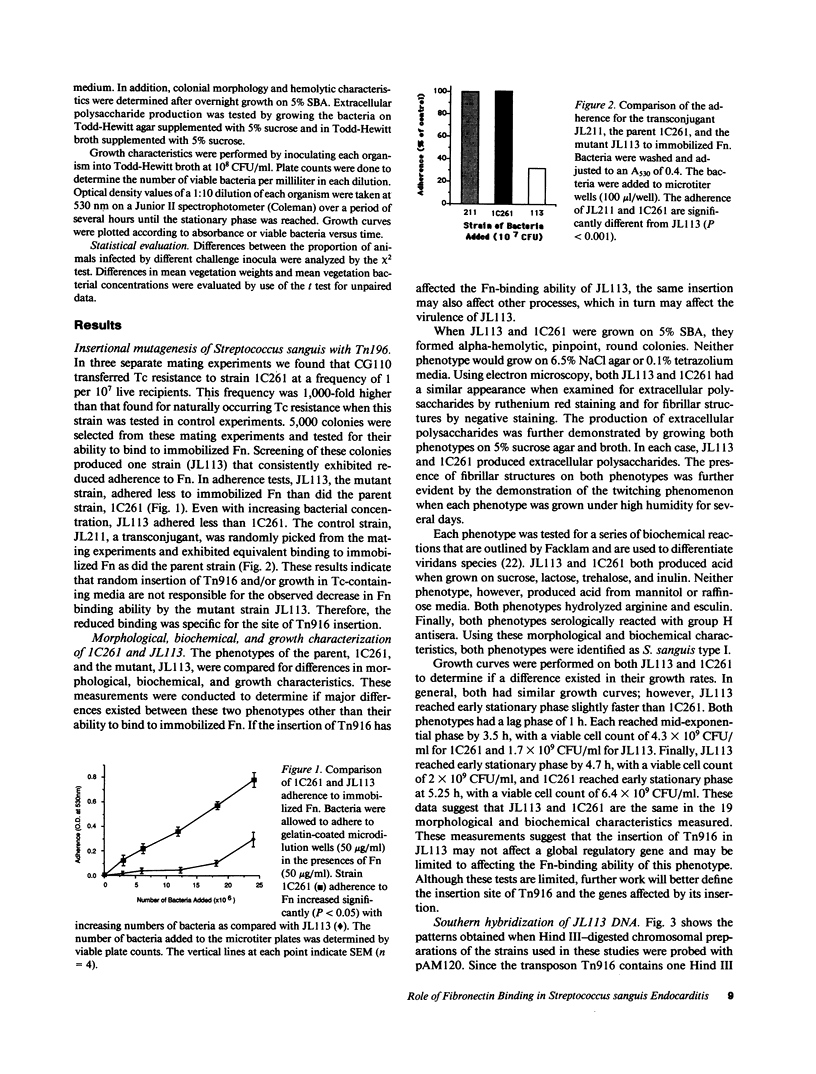
Abstract
Inactivation of fibronectin (Fn) binding by insertional mutagenesis of Streptococcus sanguis with Tn916 reduces virulence of this bacterium in the rat model of infective endocarditis (IE). Transconjugants were screened for Fn adherence using an ELISA adherence test. One transconjugant had a decreased adherence to immobilized Fn. Southern hybridization demonstrated that the insertion occurred only once in this mutant. The parent strain and mutant strain JL113 were used as challenge strains in a rat endocarditis model. These experiments demonstrated that the mutant had a reduced ability (P less than 0.05) to produce IE. Spontaneous excision of Tn916 from JL113 produced strains identical to both the parental and mutant phenotypes. One strain (JLR-19) that retained the mutant phenotype and one (JLR-15) that regained the parental phenotype for Fn binding were tested for their ability to produce IE. These strains demonstrated that the ability to bind Fn and to produce IE were correlated after Tn916 excision. The reduced virulence of the mutant suggested that adherence of S. sanguis to immobilized Fn plays an important role in the production of IE.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baddour L. M., Christensen G. D., Hester M. G., Bisno A. L. Production of experimental endocarditis by coagulase-negative staphylococci: variability in species virulence. J Infect Dis. 1984 Nov;150(5):721–727. doi: 10.1093/infdis/150.5.721. [DOI] [PubMed] [Google Scholar]
- Baddour L. M., Christensen G. D., Lowrance J. H., Simpson W. A. Pathogenesis of experimental endocarditis. Rev Infect Dis. 1989 May-Jun;11(3):452–463. doi: 10.1093/clinids/11.3.452. [DOI] [PubMed] [Google Scholar]
- Baddour L. M. Twelve-year review of recurrent native-valve infective endocarditis: a disease of the modern antibiotic era. Rev Infect Dis. 1988 Nov-Dec;10(6):1163–1170. doi: 10.1093/clinids/10.6.1163. [DOI] [PubMed] [Google Scholar]
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Gawron-Burke C. Conjugative transposons and the dissemination of antibiotic resistance in streptococci. Annu Rev Microbiol. 1986;40:635–659. doi: 10.1146/annurev.mi.40.100186.003223. [DOI] [PubMed] [Google Scholar]
- Courtney H. S., Ofek I., Simpson W. A., Hasty D. L., Beachey E. H. Binding of Streptococcus pyogenes to soluble and insoluble fibronectin. Infect Immun. 1986 Sep;53(3):454–459. doi: 10.1128/iai.53.3.454-459.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durack D. T., Beeson P. B. Experimental bacterial endocarditis. I. Colonization of a sterile vegetation. Br J Exp Pathol. 1972 Feb;53(1):44–49. [PMC free article] [PubMed] [Google Scholar]
- Durack D. T., Beeson P. B., Petersdorf R. G. Experimental bacterial endocarditis. 3. Production and progress of the disease in rabbits. Br J Exp Pathol. 1973 Apr;54(2):142–151. [PMC free article] [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977 Jul 15;20(1):1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- Facklam R. R. Physiological differentiation of viridans streptococci. J Clin Microbiol. 1977 Feb;5(2):184–201. doi: 10.1128/jcm.5.2.184-201.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawron-Burke C., Clewell D. B. Regeneration of insertionally inactivated streptococcal DNA fragments after excision of transposon Tn916 in Escherichia coli: strategy for targeting and cloning of genes from gram-positive bacteria. J Bacteriol. 1984 Jul;159(1):214–221. doi: 10.1128/jb.159.1.214-221.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasty D. L., Courtney H. S., Simpson W. A., McDonald J. A., Beachey E. H. Immunochemical and ultrastructural mapping of the gelatin-binding and cell-attachment regions of human plasma fibronectin with monoclonal antibodies. J Cell Sci. 1986 Mar;81:125–141. doi: 10.1242/jcs.81.1.125. [DOI] [PubMed] [Google Scholar]
- Henriksen S. D., Henrichsen J. Twitching motility and possession of polar fimbriae in spreading Streptococcus sanguis isolates from the human throat. Acta Pathol Microbiol Scand B. 1975 Apr;83(2):133–140. doi: 10.1111/j.1699-0463.1975.tb00083.x. [DOI] [PubMed] [Google Scholar]
- Herzberg M. C., Brintzenhofe K. L., Clawson C. C. Aggregation of human platelets and adhesion of Streptococcus sanguis. Infect Immun. 1983 Mar;39(3):1457–1469. doi: 10.1128/iai.39.3.1457-1469.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerényi T., Voss B., Rauterberg J., Fromme H. G., Jellinek H., Hauss W. H. Connective tissue proteins on the injured endothelium of the rat aorta. Exp Mol Pathol. 1985 Oct;43(2):151–161. doi: 10.1016/0014-4800(85)90036-x. [DOI] [PubMed] [Google Scholar]
- Kuypers J. M., Proctor R. A. Reduced adherence to traumatized rat heart valves by a low-fibronectin-binding mutant of Staphylococcus aureus. Infect Immun. 1989 Aug;57(8):2306–2312. doi: 10.1128/iai.57.8.2306-2312.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowrance J. H., Hasty D. L., Simpson W. A. Adherence of Streptococcus sanguis to conformationally specific determinants in fibronectin. Infect Immun. 1988 Sep;56(9):2279–2285. doi: 10.1128/iai.56.9.2279-2285.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher D. F., Proctor R. A. Binding and factor XIIIa-mediated cross-linking of a 27-kilodalton fragment of fibronectin to Staphylococcus aureus. Science. 1980 Aug 22;209(4459):927–929. doi: 10.1126/science.7403857. [DOI] [PubMed] [Google Scholar]
- Ouaissi M. A., Cornette J., Capron A. Trypanosoma cruzi: modulation of parasite-cell interaction by plasma fibronectin. Eur J Immunol. 1985 Nov;15(11):1096–1101. doi: 10.1002/eji.1830151106. [DOI] [PubMed] [Google Scholar]
- Roberts R. B., Krieger A. G., Schiller N. L., Gross K. C. Viridans streptococcal endocarditis: the role of various species, including pyridoxal-dependent streptococci. Rev Infect Dis. 1979 Nov-Dec;1(6):955–966. doi: 10.1093/clinids/1.6.955. [DOI] [PubMed] [Google Scholar]
- Santoro J., Levison M. E. Rat model of experimental endocarditis. Infect Immun. 1978 Mar;19(3):915–918. doi: 10.1128/iai.19.3.915-918.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheld W. M., Strunk R. W., Balian G., Calderone R. A. Microbial adhesion to fibronectin in vitro correlates with production of endocarditis in rabbits. Proc Soc Exp Biol Med. 1985 Dec;180(3):474–482. doi: 10.3181/00379727-180-42205. [DOI] [PubMed] [Google Scholar]
- Scheld W. M., Valone J. A., Sande M. A. Bacterial adherence in the pathogenesis of endocarditis. Interaction of bacterial dextran, platelets, and fibrin. J Clin Invest. 1978 May;61(5):1394–1404. doi: 10.1172/JCI109057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schifferli D. M., Abraham S. N., Beachey E. H. Use of monoclonal antibodies to probe subunit- and polymer-specific epitopes of 987P fimbriae of Escherichia coli. Infect Immun. 1987 Apr;55(4):923–930. doi: 10.1128/iai.55.4.923-930.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sullam P. M., Drake T. A., Sande M. A. Pathogenesis of endocarditis. Am J Med. 1985 Jun 28;78(6B):110–115. doi: 10.1016/0002-9343(85)90373-0. [DOI] [PubMed] [Google Scholar]
- Switalski L. M., Ljungh A., Rydén C., Rubin K., Hök M., Wadström T. Binding of fibronectin to the surface of group A, C, and G streptococci isolated from human infections. Eur J Clin Microbiol. 1982 Dec;1(6):381–387. doi: 10.1007/BF02019939. [DOI] [PubMed] [Google Scholar]
- Switalski L. M., Murchison H., Timpl R., Curtiss R., 3rd, Hök M. Binding of laminin to oral and endocarditis strains of viridans streptococci. J Bacteriol. 1987 Mar;169(3):1095–1101. doi: 10.1128/jb.169.3.1095-1101.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. D., Baseman J. B., Alderete J. F. Fibronectin mediates Treponema pallidum cytadherence through recognition of fibronectin cell-binding domain. J Exp Med. 1985 Mar 1;161(3):514–525. doi: 10.1084/jem.161.3.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercellotti G. M., Lussenhop D., Peterson P. K., Furcht L. T., McCarthy J. B., Jacob H. S., Moldow C. F. Bacterial adherence to fibronectin and endothelial cells: a possible mechanism for bacterial tissue tropism. J Lab Clin Med. 1984 Jan;103(1):34–43. [PubMed] [Google Scholar]
- Vercellotti G. M., McCarthy J. B., Lindholm P., Peterson P. K., Jacob H. S., Furcht L. T. Extracellular matrix proteins (fibronectin, laminin, and type IV collagen) bind and aggregate bacteria. Am J Pathol. 1985 Jul;120(1):13–21. [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]