Abstract
Randomized controlled trials have shown improved short-term bleeding outcomes for bivalirudin relative to unfractionated heparin (UFH) in patients undergoing percutaneous coronary intervention (PCI) for stable angina and acute coronary syndrome. This study analyzed the impact of bivalirudin based anticoagulation strategy versus UFH based anticoagulation strategy on long-term bleeding complications and major adverse cardiac events in patients undergoing PCI in routine clinical practice. Between September 2005 and April 2009, 3367 consecutive patients who underwent PCI for stable angina or Non ST-segment elevation acute coronary syndrome at Brigham and Women’s Hospital were studied. Of these patients, 2228 (66%) patients received UFH and 1139 (34%) received bivalirudin. We compared the bleeding complication and major adverse cardiac event rates at discharge, 30-days and 1-year. In a propensity-score matched analysis, bivalirudin based anticoagulation strategy was associated with lower bleeding complications at 30 days (7.0% vs. 13.7%, p=0.001) and 1-year (12.7% vs.18.9%, p=0.013). The major adverse cardiac event rates was not significantly different between the groups at discharge, 30-days and 1-year (6.4% vs. 8.3%, p=0.103; 9.4% vs.10.9%, p=0.449; 12.1% vs.14.8%, p=0.235 respectively). There was no difference in all-cause mortality rates between the two groups (0.9% vs. 0.8%, p=0.808 at discharge; 1.9% vs. 3.6%, p=0.112 at 30-days; 3.6% vs. 5.5%, p=0.195 at 1 year). In conclusion, in a real-world cohort of patients undergoing PCI, Bivalirudin based anticoagulation strategy is associated with significant reduction in the risk of bleeding complications after 30-days and 1-year compared to UFH based anticoagulation strategy with no increase in the risk for major adverse cardiac events.
Introduction
Optimal antithrombotic treatment in patients undergoing percutaneous coronary intervention (PCI) is crucial to balance the risk of post-PCI bleeding versus ischemic complications. Bivalirudin, a direct thrombin inhibitor has been extensively investigated as an intra-procedural antithrombotic therapy in patients with stable angina, Non ST-segment elevation acute coronary syndrome (NSTE ACS), and ST-segment elevation myocardial infarction (STEMI). [1–4] Bivalirudin, when used with or without glycoprotein IIb/IIIa inhibitors (GPI) during PCI has been found to be superior to Unfractionated heparin (UFH) with or without GPI in reducing 30-day bleeding complications without significant increase in the rate of ischemic events. [1–4] Moreover, studies have shown that Bivalirudin is non-inferior to UFH for the rates of 1-year mortality.[5–7] Other studies have shown the 1-year bleeding and mortality rates to be superior in bivalirudin treated patients undergoing PCI for acute ST-segment elevation myocardial infarction (STEMI) compared with UFH treated patients. [8] Whether this bleeding safety profile with bivalirudin over UFH in patients with stable angina and NSTE ACS is preserved at 1 year in routine clinical practice is not consistently reported. We sought to compare the risk of bleeding and major adverse cardiac events (MACE) at discharge, 30-days and 1-year, in a prospective cohort of patients who received UFH based or bivalirudin based anticoagulation strategy during PCI for stable angina or NSTE ACS in routine clinical practice.
Methods
We prospectively evaluated consecutive patients undergoing PCI between September 2005 and April 2009 at Brigham and Women’s Hospital. All patients who received either UFH-based or bivalirudin-based anticoagulation at the time of PCI for stable angina, or NSTE ACS were included for analysis. Exclusion criteria included STEMI, chronic total occlusions, and patients who received both heparin and bivalirudin. If a patient had multiple PCI procedures during their admission, only the index procedure was included in the analysis. Patients were divided into 3 cohorts based on the length of follow-up: inpatients (in-patient follow-up group), patients who had 30-day follow-up (30-day group), and patients who had 1-year follow-up (1-year group). Informed written consent was obtained from all of the patients, and the study was approved by the local institutional review board.
A prospective catheterization laboratory database, based on the American College of Cardiology-National Cardiovascular Data Registry data collection tool, was used to record clinical and procedural data elements for each patient. [9,10] Thirty-day and 1-year outcomes were collected for PCI cases performed from September 2006 to March 2009 and September 2005 and April 2008, respectively. The 30-day and 1-year outcomes were obtained through telephone interviews where patients were surveyed regarding post procedural complications including bleeding, blood transfusions, repeat hospitalizations, access site complications, repeat cardiac catheterization or PCI, and myocardial infarction.
Percutaneous coronary interventions were performed according to standard protocol. [11–13] Unless contraindicated, all patients who underwent PCI received aspirin and clopidogrel according to the standard American College of Cardiology/American Heart Association recommendations. [11,12] Bivalirudin was recommended as the anticoagulant of choice for patients treated for stable coronary artery disease and low risk unstable angina. Patients presenting with high-risk unstable angina and non-ST elevation myocardial infarction were treated with bivalirudin at the discretion of the interventional cardiologist. Patients undergoing thrombectomy or rotational atherectomy, and those patients already on heparin with an activated clotting time greater than 180 seconds were recommended to be treated with UFH. Final anticoagulation treatment decisions were made at the discretion of the treating interventional cardiologist. Patients in the bivalirudin group received a bolus of 0.75 mg/kg of bivalirudin before the guide wire crossed the lesion followed by an infusion of 1.75 mg/kg/hour for the duration of the procedure. Patients in the UFH group received a bolus of 60–80 units/kg plus an infusion of 12 units/kg/hour to achieve an activated clotting time of 250 to 300 seconds during PCI. When a GPI was considered, either eptifibatide or abciximab was used at standard recommended doses. Patients who received GPI along with UFH or Bivalirudin were also included in our study as such anticoagulation strategy was compared in the Acute Catheterization and Urgent Intervention Triage Strategy (ACUITY) and Randomized Evaluation in PCI Linking Angiomax to Reduced Clinical Events (REPLACE-2) trials. [1,2] When stenting occurred, it was at the discretion of the treating physician as to whether the patient received a bare-metal or drug-eluting stent. Standard post procedural therapy included aspirin (81mg to 325mg indefinitely), and clopidogrel (75mg per day for at least 1 month, in patients with bare-metal stents, and for at least 12 months in patients with drug-eluting stents, unless contraindicated).
Primary outcomes of the study included a composite bleeding endpoint at discharge, 30-days and 1-year. Secondary outcome was defined as a composite outcome of death, recurrent myocardial infarction and repeat revascularization (MACE). Composite bleeding endpoint for the inpatient cohort was defined as any access site bleeding (defined as external bleeding from the access site requiring a blood transfusion and/or prolongation of the hospital stay, and/or cause a drop in hemoglobin > 3.0 gm/dl; or a hematoma >10 cm for femoral access or >2 cm for radial access; or >5 cm for brachial access) or any bleeding from a gastrointestinal, genitourinary, retroperitoneal or unknown source that resulted in >3gms/dl drop in hemoglobin and/or requiring blood transfusion and/or prolongation of hospital stay. Composite bleeding endpoint for the 30-day and 1-year follow-up cohort was defined as any bleeding requiring hospitalization or blood transfusion or physician visit/intervention. Peri-procedural myocardial infarction was defined by the presence of at least 1 of the following criteria: 1. Evolutionary ST-segment elevation, development of new Q-waves in 2 or more contiguous ECG leads, or new left bundle branch. 2. Biochemical evidence of myocardial necrosis (Creatine Kinase-MB fraction > 3x the upper limit of normal).
Statistical analysis was performed using the Statistical Analysis System version 9.2 (SAS Institute Inc., Cary, NC) and SPSS for Windows version 17.0 (SPSS Inc., Chicago, IL). Data are expressed as mean ± S.D. for continuous variables and as percentages for categorical variables. Student’s t test was used to compare continuous variables, and the Chi square test or Fisher’s Exact Test was used to compare categorical variables. A p value <0.05 was considered to indicate statistical significance. To prevent selection bias, a propensity-score matching analysis using greedy matching protocol was performed for the propensity to use a given antithrombotic agent. [14,15] To calculate the propensity score, the following 31 variables were entered into a non-parsimonious logistic regression model [16]: Age, body mass index, female, smoking, diabetes, hypertension, hyperlipidemia, prior myocardial infarction, prior PCI, prior coronary artery bypass graft, history of congestive heart failure, history of cerebro-vascular disease, history of peripheral vascular disease, presence of cardiogenic shock, renal failure, salvage PCI, non ST-segment elevation myocardial infarction, unstable angina, high risk lesion, left main coronary artery PCI, presence of coronary thrombus, International normalized ratio, calcified coronary disease, use of drug-eluting stent, number of diseased vessels, rotablator use, maximum diameter of the vascular access sheath, access via femoral artery, access via radial artery, use of femoral venous access, use of vascular closure device. Subjects were matched using caliper width equal to 0.6 of the standard deviation of the logit of the propensity score. [17–19]
Results
The study patient flow for inclusion and follow-up, and the results of the propensity-score matching are shown in Figure 1. Of the 4825 patients who underwent PCI between 09/01/2005 to 04/30/2009, 1458 patients were excluded from this analysis based on the exclusion criteria noted above. Of the 3367 patients included in the final analysis, 2228 (66%) patients had received UFH based anticoagulation strategy and 1139 patients (34%) had received bivalirudin based anticoagulation strategy during PCI. GPI was used in 877 patients (39%) in the UFH group and in 44 patients (4%) in the Bivalirudin group. Inhospital outcomes were available for the entire cohort. One year follow-up interviews were begun in September 2006 for patients who underwent PCI the year prior. Therefore, 30-day outcomes were not available for the first year. Similarly, 1-year outcomes were not available for the cases performed in the last twelve months of the study period. As a result, 2224 patients were eligible for 30-day follow up and 2590 patients were eligible for 1-year follow up. The response rate for the 30-day and 1-year follow up was 67% (1488/2224) and 58% (1491/2590) respectively. A propensity-score matching algorithm successfully matched a total of 59% (1986/3367) of eligible inpatients, 63% (932/1488) of eligible patients with 30-day follow up, and 59% (878/1491) of eligible patients with 1-year follow up.
Figure 1.
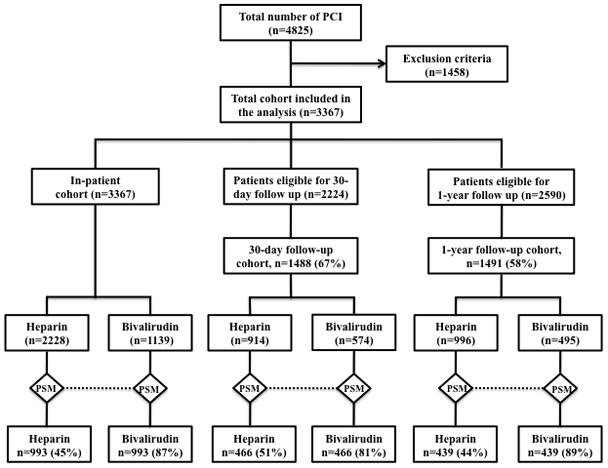
Flow-Chart Showing The Enrolment And Follow-Up Of Patients In The Study. PCI = Percutaneous Coronary Intervention, PSM = Propensity-Score Matching
Baseline characteristics of the study cohort and the propensity-score matched in-patient cohort are shown in Table 1. After propensity-score matching, baseline characteristics were more evenly distributed, though there were still a higher proportion of UFH treated patients with GPI (30.4% vs. 4.1%, p<0.001) or rotational atherectomy (5.0% vs. 1.8%, p<0.001). The difference in the baseline characteristics of patients who did and did not respond to follow-up telephone inquiries is shown in Table 2.
Table 1.
Baseline Characteristics Of All In-Patients In The Unfractionated Heparin And Bivalirudin Group
| Variable | Before propensity-score matching | After propensity-score matching | ||||
|---|---|---|---|---|---|---|
| Heparin (N=2228) | Bivalirudin (N=1139) | P Value | Heparin (N=993) | Bivalirudin (N=993) | P Value | |
| Age (years), mean ± SD | 68.2 ± 12 | 68.6 ± 11.7 | 0.304 | 68.6 ± 11.3 | 68.9 ± 11.7 | 0.506 |
| Female | 678 (30.4%) | 339 (29.8%) | 0.690 | 294 (29.6%) | 303 (30.5%) | 0.660 |
| BMI (kg/m2), mean ± SD | 29.4 ± 6.2 | 29.2 ± 5.5 | 0.304 | 29.1 ± 5.35 | 29.2 ± 5.6 | 0.829 |
| Diabetes mellitus | 765 (34.3%) | 407 (35.7%) | 0.421 | 356 (35.9%) | 348 (35%) | 0.707 |
| Hx of Renal Insufficiency | 164 (7.4%) | 70 (6.1%) | 0.190 | 71 (7.2%) | 63 (6.3%) | 0.474 |
| Renal Dialysis | 68 (3.1%) | 16 (1.4%) | 0.004 | 26 (2.6%) | 15 (1.5%) | 0.083 |
| CVD History | 224 (10.1%) | 118 (10.3%) | 0.781 | 108 (10.9%) | 101 (10.2%) | 0.609 |
| Prior PCI | 694 (31.1%) | 391 (34.3%) | 0.062 | 339 (34.1%) | 340 (34.2%) | 0.962 |
| Prior CABG | 474 (21.3%) | 215 (18.9%) | 0.103 | 205 (20.6%) | 200 (20.1%) | 0.781 |
| Prior MI | 709 (31.8%) | 359 (31.5%) | 0.858 | 332 (33.4%) | 317 (31.9%) | 0.473 |
| CSA History | 601 (27%) | 514 (45.1%) | <0.001 | 413 (41.6%) | 420 (42.3%) | 0.750 |
| HF History | 358 (16.1%) | 155(13.6%) | 0.06 | 143 (14.4%) | 136 (13.7%) | 0.651 |
| Hypertension | 1909 (85.7%) | 1020(89.6%) | 0.002 | 891 (89.7%) | 882 (88.8%) | 0.514 |
| Hyperlipidemia | 1978 (88.8%) | 1060 (93%) | <0.001 | 917 (92.3%) | 919 (92.5%) | 0.865 |
| Smoker | 1505 (67.5%) | 744 (65.3%) | 0.194 | 663 (66.8%) | 655 (66%) | 0.704 |
| PVD History | 309 (13.9%) | 143 (12.6%) | 0.290 | 136 (13.7%) | 127 (12.8%) | 0.551 |
| NSTEMI | 736 (33%) | 128(11.2%) | <0.001 | 136 (13.7%) | 128 (12.9%) | 0.597 |
| Unstable angina | 506 (22.7%) | 201 (17.6%) | 0.001 | 199 (20%) | 198 (19.9%) | 0.955 |
| GPI Use | 877 (39.4%) | 44 (3.9%) | <0.001 | 302 (30.4%) | 41 (4.1%) | <0.001 |
| High Risk Lesion | 630 (28.2%) | 184 (16.2%) | <0.001 | 173 (17.4%) | 180 (18.1%) | 0.681 |
| Left Main PCI | 95 (4.3%) | 26 (2.3%) | 0.003 | 25 (2.5%) | 26 (2.6%) | 0.887 |
| PCI with no stent | 152 (6.8%) | 76 (6.7%) | 0.870 | 78 (7.9%) | 71 (7.2%) | 0.551 |
| Drug-eluting stent | 1729 (77.6%) | 868 (76.2%) | 0.361 | 752 (75.7%) | 756 (76.1%) | 0.834 |
| Thrombus | 184 (8.3%) | 38 (3.3%) | <0.001 | 41 (4.13%) | 35 (3.52%) | 0.452 |
| No. of narrowed coronary arteries | 1.78±1.11 | 1.78±1.04 | 1.00 | 1.78±1.1 | 1.8±1.04 | 0.754 |
| Calcified Lesion | 204 (9.2%) | 52 (4.6%) | <0.001 | 55 (5.5%) | 51 (5.1%) | 0.690 |
| Rotablator Use | 126 (5.7%) | 18 (1.6%) | <0.001 | 50 (5.0%) | 18 (1.8%) | <0.001 |
| Radial artery Access | 152(6.8%) | 19(1.7%) | <0.001 | 22 (2.22%) | 19 (2%) | 0.636 |
| Venous Access | 353 (15.8%) | 154 (13.5%) | 0.075 | 152 (15.3%) | 144 (14.5%) | 0.614 |
| Vascular Closure Device | 1752(78.7%) | 958(84.1%) | <0.001 | 826 (83.2%) | 838 (84.4%) | 0.465 |
All values are expressed in n (%) unless stated otherwise. BMI= Body Mass Index, CABG = Coronary Artery Bypass Grafting, CSA = Chronic Stable Angina, CVD = Cardiovascular Disease, GPI=Glycoprotein IIb/IIIa Inhibitor, HF = Heart Failure, MI= Myocardial Infarction, NSTEMI = Non ST-segment elevation myocardial infarction, PCI= Percutaneous Coronary Intervention, PVD = Peripheral Vascular Disease.
Table 2.
Table Showing Difference In The Baseline Characteristics Between The Patients Who Had Follow Up And Patients Who Did Not Have Follow Up (30-Day Follow Up And 1-Year Follow Up)
| Variable | 30-day follow up | 1-year follow up | ||||
|---|---|---|---|---|---|---|
| 30-day follow-up (N=1488) | No 30-day follow-up (N=736) | P value | 1-year follow-up (N=1491) | No 1-year follow-up (N=1099) | P value | |
| Age (years), mean ± SD | 68.2±11.6 | 67.4±12.7 | 0.139 | 69.4±11.1 | 67.9±12.7 | 0.001 |
| Female | 451 (30.3%) | 230 (31.2%) | 0.651 | 428 (28.7%) | 349 (31.8%) | 0.094 |
| BMI (kg/m2), mean ± SD | 29.4±5.9 | 29.4±6.4 | 0.735 | 29.4±6 | 29.1±5.8 | 0.217 |
| Diabetes mellitus | 525 (35.3%) | 272 (36.9%) | 0.420 | 489 (32.8%) | 398 (36.2%) | 0.070 |
| Hx of Renal Insufficiency | 103 (6.9%) | 65 (8.8%) | 0.109 | 98 (6.6%) | 72 (6.5%) | 0.983 |
| Renal Dialysis | 33 (2.2%) | 25 (3.4%) | 0.101 | 30 (2%) | 25 (2.3%) | 0.647 |
| CVD History | 144 (9.7%) | 83 (11.3%) | 0.241 | 150 (10.1%) | 131 (11.9%) | 0.133 |
| Prior PCI | 498 (33.5%) | 237 (32.2%) | 0.550 | 476 (31.9%) | 352 (32%) | 0.955 |
| Prior CABG | 319 (21.4%) | 152 (20.7%) | 0.669 | 313 (21%) | 219 (19.9%) | 0.507 |
| Prior MI | 484 (32.5%) | 255 (34.6%) | 0.318 | 437 (29.3%) | 365 (33.2%) | 0.034 |
| CSA History | 523 (35.1%) | 226 (30.7%) | 0.037 | 525 (35.2%) | 333 (30.3%) | 0.009 |
| HF History | 222 (14.9%) | 136 (18.5%) | 0.032 | 206 (13.8%) | 163 (14.8%) | 0.465 |
| Hypertension | 1320 (88.7%) | 646 (87.8%) | 0.516 | 1290 (86.5%) | 940 (85.5%) | 0.473 |
| Hyperlipidemia | 1372 (92.2%) | 665 (90.4%) | 0.139 | 1337 (89.7%) | 980 (89.2%) | 0.683 |
| Smoker | 1010 (67.9%) | 484 (65.8%) | 0.317 | 1010 (67.7%) | 719 (65.4%) | 0.216 |
| PVD History | 212 (14.2%) | 113 (15.4%) | 0.487 | 182 (12.2%) | 159 (14.5%) | 0.093 |
| NSTEMI | 384 (25.8%) | 219 (29.8%) | 0.049 | 332 (22.3%) | 303 (27.6%) | 0.002 |
| Unstable angina | 278 (18.7%) | 164 (22.3%) | 0.045 | 302 (20.3%) | 264 (24%) | 0.022 |
| GPI Use | 319 (21.4%) | 181 (24.6%) | 0.094 | 424 (28.4%) | 359 (32.7%) | 0.021 |
| High Risk Lesion | 338 (22.7%) | 172 (23.4%) | 0.730 | 360 (24.1%) | 286 (26%) | 0.275 |
| Left Main PCI | 53 (3.6%) | 19 (2.6%) | 0.219 | 62 (4.2%) | 33 (3%) | 0.122 |
| PCI with no stent | 117 (7.9%) | 47 (6.4%) | 0.210 | 113 (7.6%) | 71 (6.5%) | 0.274 |
| Drug-eluting stent | 1051 (70.6%) | 525 (71.3%) | 0.733 | 1168 (78.3%) | 848 (77.2%) | 0.476 |
| Thrombus | 89 (6%) | 51 (6.9%) | 0.701 | 83 (5.6%) | 74 (6.7%) | 0.257 |
| No. of narrowed coronary arteries | 1.8±1.1 | 1.8±1.1 | 0.299 | 1.8±1.1 | 1.8±1.1 | 0.237 |
| Calcified disease | 104 (7%) | 65 (8.8%) | 0.123 | 125 (8.4%) | 73 (6.6%) | 0.099 |
| Rotablator use | 3 (0.2%) | 0 | 0.223 | 9 (0.6%) | 4 (0.4%) | 0.394 |
| Radial artery Access | 74 (4.9%) | 38 (5.2%) | 0.847 | 64 (4.3%) | 38 (3.4%) | 0.280 |
| Venous Access | 226 (15.2%) | 103 (14%) | 0.456 | 233 (15.6%) | 169 (15.4%) | 0.862 |
| Vascular Closure Device | 1210 (81.3%) | 608 (82.6%) | 0.458 | 1210 (81.2%) | 913 (83%) | 0.209 |
All values are expressed in n (%) unless stated otherwise. BMI= Body Mass Index, CABG = Coronary Artery Bypass Grafting, CSA = Chronic Stable Angina, CVD = Cardiovascular Disease, GPI=Glycoprotein IIb/IIIa Inhibitor, HF = Heart Failure, MI= Myocardial Infarction, NSTEMI = Non ST-segment elevation myocardial infarction, PCI= Percutaneous Coronary Intervention, PVD = Peripheral Vascular Disease.
The primary and secondary outcomes of this analysis for both the entire patient cohorts as well as the propensity-score matched groups are summarized in Table 3, and Figures 2 and 3. The composite bleeding end point during the in-hospital stay was found to be higher in the UFH group than in the bivalirudin group (4.8% vs. 1.8%, p<0.001 in the unadjusted patient cohort and 3.5% vs. 2.1%, p=0.058 in the propensity-score matched cohort). This difference in the composite bleeding end point was largely due to higher incidence of non-access site bleeding (bleeding from unknown location requiring a transfusion and/or prolong the hospital stay, and/or cause a drop in hemoglobin >3.0 gm/dl) noted in the UFH group than in the bivalirudin group. All other bleeding outcomes were not significantly different between the groups. At 30-days and 1-year, bleeding complications were significantly higher in the UFH group than in the bivalirudin group after propensity-score matching (13.7% vs. 7.1%, p=0.001 at 30-days and 18.9% vs. 12.8%, p=0.013 at 1-year). This difference in the composite bleeding end point at 30-days and 1-year was largely due to the bleeding that required physician visit or intervention.
Table 3.
Primary End-Points In Unfractionated Heparin And Bivalirudin Group (Before And After Propensity-Score Matching)
| Before propensity-score matching | After propensity score matching | |||||
|---|---|---|---|---|---|---|
| In-patient outcomes | N=2228 | N=1139 | N=3367 | N= 993 | N= 993 | N=1986 |
| Heparin | Bivalirudin | P value | Heparin | Bivalirudin | P-value | |
| Composite Bleeding End-point | 106 (4.76%) | 21 (1.84%) | <0.001 | 35 (3.52%) | 21 (2.11%) | 0.058 |
| Gastrointestinal Bleeding | 11 (0.49%) | 2 (0.17%) | 0.159 | 2 (0.2%) | 2 (0.2%) | 1.00 |
| Retroperitoneal Bleeding | 5 (0.22%) | 1 (0.08%) | 0.374 | 1(0.1%) | 1 (0.1%) | 1.00 |
| Access site hematoma | 9 (0.4%) | 2 (0.17%) | 0.272 | 2(0.2%) | 2 (0.2%) | 1.00 |
| Genitourinary Bleeding | 1 (0.04%) | 0 | 0.475 | 0 | 0 | - |
| Other Bleeding* | 79 (3.54%) | 16 (1.4%) | <0.001 | 30 (3.02%) | 16 (1.61%) | 0.037 |
| Bleeding requiring Blood Tx. | 35 (1.58%) | 6 (0.53%) | 0.009 | 6 (0.6%) | 6 (0.6%) | 1.00 |
| Death/MI/Revascularization | 157 (7%) | 97 (8.5%) | 0.127 | 64 (6.4%) | 83 (8.3%) | 0.103 |
| Peri-procedural MI | 121 (5.4%) | 89 (7.8%) | 0.007 | 51 (5.1%) | 75 (7.5%) | 0.027 |
| Repeat PCI | 22(0.9%) | 1 (0.08%) | 0.003 | 10 (1%) | 0 | 0.002 |
| Coronary artery bypass graft | 2 (0.09%) | 0 | 0.312 | 0 | 0 | - |
| Death | 25 (1.12%) | 9 (0.8%) | 0.362 | 8 (0.81%) | 9 (0.91%) | 0.808 |
| 30-Day outcomes | N=914 | N=574 | N= 1488 | N= 466 | N= 466 | N=932 |
| Heparin | Bivalirudin | P value | Heparin | Bivalirudin | P value | |
| Composite Bleeding End-point | 121 (13.2%) | 41 (7.14%) | <0.001 | 64 (13.73%) | 33(7.08%) | 0.001 |
| Hospitalization due to bleeding | 14 (1.53%) | 7 (1.22%) | 0.619 | 10 (2.14%) | 4 (0.85%) | 0.106 |
| Bleeding requiring blood Tx. | 9 (0.98%) | 7 (1.22%) | 0.669 | 5 (1.07%) | 4 (0.85%) | 0.738 |
| Death/MI/Revascularization | 98 (10.7%) | 59 (10.3%) | 0.786 | 44 (9.4%) | 51 (10.9%) | 0.449 |
| Myocardial Infarction | 41 (4.5%) | 42 (7.3%) | 0.021 | 22 (4.7%) | 36 (7.7%) | 0.058 |
| Repeat PCI | 21 (2.3%) | 8 (1.4%) | 0.220 | 7 (1.5%) | 7 (1.5%) | 1.000 |
| Coronary artery bypass graft | 2 (0.22%) | 2 (0.35%) | 0.638 | 2 (0.42%) | 1 (0.21%) | 0.563 |
| Death | 42 (4.6%) | 9 (1.57%) | 0.002 | 17 (3.64%) | 9 (1.93%) | 0.112 |
| 1-Year Outcomes | N=996 | N=495 | N=1491 | N= 439 | N= 439 | N=878 |
| Heparin | Bivalirudin | P value | Heparin | Bivalirudin | P value | |
| Composite Bleeding End-point | 186 (18.7%) | 61 (12.32%) | 0.002 | 83 (18.9%) | 56 (12.75%) | 0.013 |
| Hospitalization due to bleeding | 35(3.51%) | 19(3.83%) | 0.752 | 16 (3.64%) | 17 (3.87%) | 0.859 |
| Bleeding requiring blood Tx. | 19 (1.9%) | 9 (1.81%) | 0.905 | 6 (1.36%) | 7 (1.59%) | 0.780 |
| Death/MI/Revascularization | 137 (13.7%) | 68 (13.7%) | 0.993 | 53 (12.1%) | 65 (14.8%) | 0.235 |
| Myocardial Infarction | 51 (5.1%) | 37 (7.5%) | 0.069 | 21 (4.8%) | 35 (8%) | 0.053 |
| Repeat PCI | 27 (2.7%) | 10 (2%) | 0.419 | 8 (1.8%) | 9 (2%) | 0.807 |
| Coronary artery bypass graft | 7(0.7%) | 5 (1.01%) | 0.532 | 2 (0.45%) | 5 (1.13%) | 0.255 |
| Death | 59 (5.92%) | 16 (3.23%) | 0.025 | 24 (5.46%) | 16 (3.64%) | 0.195 |
All values are expressed in n (%). Blood Tx = Blood transfusion, MI = Myocardial Infarction, PCI = Percutaneous Coronary Intervention.
Other Bleeding = Bleeding occurred at other or unknown locations (than mentioned above) during or after the cath lab visit until hospital discharge that required a transfusion and/or prolong the hospital stay, and/or cause a drop in hemoglobin >3.0 gm/dl.
Figure 2.
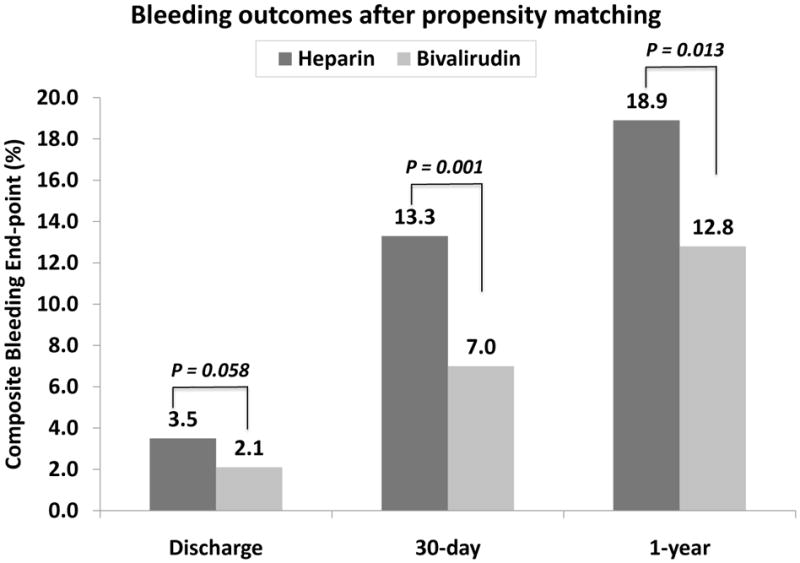
Bleeding outcomes after propensity-score matching
Figure 3.
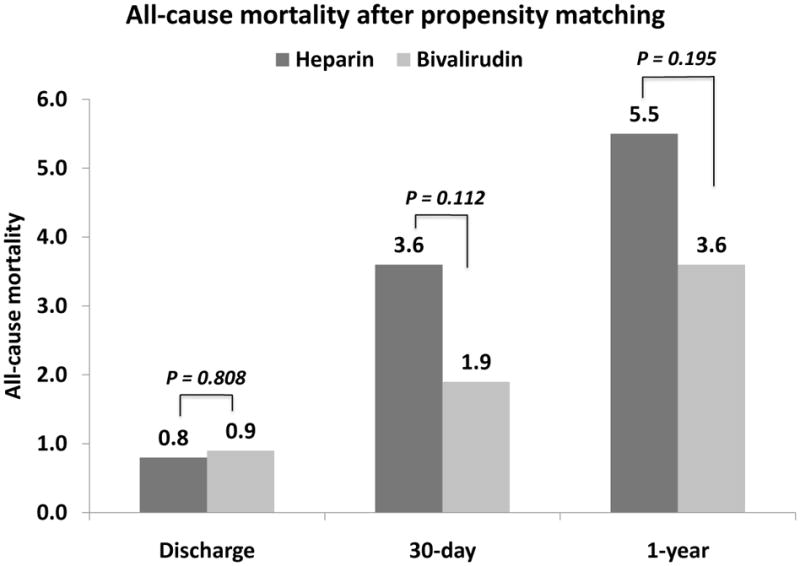
All-cause mortality after propensity-score matching.
The MACE rate was not significantly different between the groups at discharge, 30-days and 1-year (Table 3). All-cause mortality was not significantly different after propensity-score matching (3.6% vs. 1.9%, p=0.112 at 30-days and 5.5% vs. 3.6%, p=0.195 at 1-year). At discharge, peri-procedural myocardial infarction was significantly higher in the bivalirudin group (7.5% vs. 5.1%, p=0.027) but significantly more repeat PCI procedures were performed in the UFH group (1% vs. 0%, p=0.002).
Discussion
This study assessed the frequency of bleeding complications and MACE rates at discharge, 30-days and at 1year in patients without STEMI who received either an UFH-based or bivalirudin-based anticoagulation strategy during PCI in routine clinical practice. The results revealed a significantly lower incidence of bleeding complications at 30-days and 1-year in the bivalirudin group with similar MACE outcomes when compared with UFH based anticoagulation strategy.
Our data is consistent with data from randomized clinical trials including the Intracoronary Stenting and Antithrombotic Regimen: Rapid Early Action for Coronary Treatment (ISAR-REACT 3) trial, [3,7,20] that showed a significantly lower bleeding with bivalirudin when compared with UFH in patients with stable and unstable angina. The similar mortality outcomes in the two groups observed in this study are consistent with findings from the ACUITY [1,5] and the REPLACE-2 trials, [2,6] which evaluated patients who received UFH or bivalirudin in conjunction with GPI in similar clinical situations. Similar bleeding and mortality benefits were also observed in patients who had PCI for ST-segment elevation myocardial infarction in the Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction (HORIZON-AMI) trial. [4,8] The reduced rates of bleeding after PCI with bivalirudin in routine clinical practice is an important finding, as multiple studies have reported a strong association between bleeding complications and mortality following PCI. [21–24] In the REPLACE-2 trial, which included elective and lower-risk ACS patients undergoing PCI, [25] there were significantly higher mortality rates at 30 days, 6 months, and 1 year for patients who had bleeding complications compared with those who did not.
Although a non-significant trend towards higher peri-procedural MI rates with bivalirudin is noted in major trials [1, 2, 3], we noted a significantly higher rate of peri-procedural MI with bivalirudin at discharge. A lower proportion of GPI treated patients and the choice of stent use in the bivalirudin group in our study might have contributed to a higher peri-procedural MI in this group.
The 1-year findings of our study presented herein validate the durable efficacy of bivalirudin in routine clinical practice, which extends the observation from selected populations included in randomized controlled trials. Not only was there no excess of MACE rates with bivalirudin therapy, but also death rates trended lower in the bivalirudin group. This study showed a reduced bleeding complication rate observed at 30-days and at 1-year in the bivalirudin group, and there was no significant difference in the subsequent MACE rates both at 30-days and 1-year in the propensity-matched group. The lack of a significant difference in mortality between the bivalirudin and UFH treated patients in this study may have been due to a lack of power due to the sample size following the comprehensive propensity-score matching.
The acute and long-term findings of our study cannot be extrapolated to patient groups that were excluded from this study, principally those undergoing primary PCI for acute myocardial infarction or those with total occlusion. Despite exclusion of these patients, however, 30-day and 1-year MACE rates in our study were similar to those in other recent PCI trials. This study is limited by the inherent bias of an observational cohort study and non-randomized clinical data, as well as by a significant loss to follow up. There were also differences in the characteristics of the patients who followed up for 30-days but who did not participate in 1 year follow up. These results must also be interpreted in light of potential hidden bias of the propensity-score matching, as it may not account for all confounding variables.
Acknowledgments
This study was funded, in part, by grant from National Library of Medicine (NIH R01-LM008142) and the Medicines Company, Parsippany, NJ, United States.
The authors would like to thank Suwada Hinds for her efforts in establishing and maintaining the database.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stone GW, McLaurin BT, Cox DA, Bertrand ME, Lincoff AM, Moses JW, White HD, Pocock SJ, Ware JH, Feit F, Colombo A, Aylward PE, Cequier AR, Darius H, Desmet W, Ebrahimi R, Hamon M, Rasmussen LH, Rupprecht HJ, Hoekstra J, Mehran R, Ohman EM. Bivalirudin for patients with acute coronary syndromes. N Engl J Med. 2006;355:2203–2216. doi: 10.1056/NEJMoa062437. [DOI] [PubMed] [Google Scholar]
- 2.Lincoff AM, Bittl JA, Harrington RA, Feit F, Kleiman NS, Jackman JD, Sarembock IJ, Cohen DJ, Spriggs D, Ebrahimi R, Keren G, Carr J, Cohen EA, Betriu A, Desmet W, Kereiakes DJ, Rutsch W, Wilcox RG, de Feyter PJ, Vahanian A, Topol EJ. Bivalirudin and provisional glycoprotein IIb/IIIa blockade compared with heparin and planned glycoprotein IIb/IIIa blockade during percutaneous coronary intervention: REPLACE-2 randomized trial. JAMA. 2003;289:853–863. doi: 10.1001/jama.289.7.853. [DOI] [PubMed] [Google Scholar]
- 3.Kastrati A, Neumann FJ, Mehilli J, Byrne RA, Iijima R, Buttner HJ, Khattab AA, Schulz S, Blankenship JC, Pache J, Minners J, Seyfarth M, Graf I, Skelding KA, Dirschinger J, Richardt G, Berger PB, Schomig A. Bivalirudin versus unfractionated heparin during percutaneous coronary intervention. N Engl J Med. 2008;359:688–696. doi: 10.1056/NEJMoa0802944. [DOI] [PubMed] [Google Scholar]
- 4.Stone GW, Witzenbichler B, Guagliumi G, Peruga JZ, Brodie BR, Dudek D, Kornowski R, Hartmann F, Gersh BJ, Pocock SJ, Dangas G, Wong SC, Kirtane AJ, Parise H, Mehran R. Bivalirudin during primary PCI in acute myocardial infarction. N Engl J Med. 2008;358:2218–2230. doi: 10.1056/NEJMoa0708191. [DOI] [PubMed] [Google Scholar]
- 5.White HD, Ohman EM, Lincoff AM, Bertrand ME, Colombo A, McLaurin BT, Cox DA, Pocock SJ, Ware JA, Manoukian SV, Lansky AJ, Mehran R, Moses JW, Stone GW. Safety and efficacy of bivalirudin with and without glycoprotein IIb/IIIa inhibitors in patients with acute coronary syndromes undergoing percutaneous coronary intervention 1-year results from the ACUITY (Acute Catheterization and Urgent Intervention Triage strategY) trial. J Am Coll Cardiol. 2008;52:807–814. doi: 10.1016/j.jacc.2008.05.036. [DOI] [PubMed] [Google Scholar]
- 6.Lincoff AM, Kleiman NS, Kereiakes DJ, Feit F, Bittl JA, Jackman JD, Sarembock IJ, Cohen DJ, Spriggs D, Ebrahimi R, Keren G, Carr J, Cohen EA, Betriu A, Desmet W, Rutsch W, Wilcox RG, de Feyter PJ, Vahanian A, Topol EJ. Long-term efficacy of bivalirudin and provisional glycoprotein IIb/IIIa blockade vs heparin and planned glycoprotein IIb/IIIa blockade during percutaneous coronary revascularization: REPLACE-2 randomized trial. JAMA. 2004;292:696–703. doi: 10.1001/jama.292.6.696. [DOI] [PubMed] [Google Scholar]
- 7.Schulz S, Mehilli J, Ndrepepa G, Neumann FJ, Birkmeier KA, Kufner S, Richardt G, Berger PB, Schomig A, Kastrati A. Bivalirudin vs. unfractionated heparin during percutaneous coronary interventions in patients with stable and unstable angina pectoris: 1-year results of the ISAR-REACT 3 trial. Eur Heart J. 2010;31:582–587. doi: 10.1093/eurheartj/ehq008. [DOI] [PubMed] [Google Scholar]
- 8.Mehran R, Lansky AJ, Witzenbichler B, Guagliumi G, Peruga JZ, Brodie BR, Dudek D, Kornowski R, Hartmann F, Gersh BJ, Pocock SJ, Wong SC, Nikolsky E, Gambone L, Vandertie L, Parise H, Dangas GD, Stone GW. Bivalirudin in patients undergoing primary angioplasty for acute myocardial infarction (HORIZONS-AMI): 1-year results of a randomised controlled trial. Lancet. 2009;374:1149–1159. doi: 10.1016/S0140-6736(09)61484-7. [DOI] [PubMed] [Google Scholar]
- 9.Cannon CP, Battler A, Brindis RG, Cox JL, Ellis SG, Every NR, Flaherty JT, Harrington RA, Krumholz HM, Simoons ML, Van De Werf FJ, Weintraub WS, Mitchell KR, Morrisson SL, Anderson HV, Cannom DS, Chitwood WR, Cigarroa JE, Collins-Nakai RL, Gibbons RJ, Grover FL, Heidenreich PA, Khandheria BK, Knoebel SB, Krumholz HL, Malenka DJ, Mark DB, McKay CR, Passamani ER, Radford MJ, Riner RN, Schwartz JB, Shaw RE, Shemin RJ, Van Fossen DB, Verrier ED, Watkins MW, Phoubandith DR, Furnelli T. American College of Cardiology key data elements and definitions for measuring the clinical management and outcomes of patients with acute coronary syndromes. A report of the American College of Cardiology Task Force on Clinical Data Standards (Acute Coronary Syndromes Writing Committee) J Am Coll Cardiol. 2001;38:2114–2130. doi: 10.1016/s0735-1097(01)01702-8. [DOI] [PubMed] [Google Scholar]
- 10.American College of Cardiology. [Accessed March 17, 2010];National Cardiovascular Data Registry. Available from http://wwwaccncdrcom/WebNCDR/ELEMENTSASPX/
- 11.Smith SC, Jr, Feldman TE, Hirshfeld JW, Jr, Jacobs AK, Kern MJ, King SB, 3rd, Morrison DA, O’Neill WW, Schaff HV, Whitlow PL, Williams DO, Antman EM, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Hunt SA, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention-Summary Article: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/SCAI Writing Committee to Update the 2001 Guidelines for Percutaneous Coronary Intervention) J Am Coll Cardiol. 2006;47:216–235. doi: 10.1016/j.jacc.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 12.King SB, 3rd, Smith SC, Jr, Hirshfeld JW, Jr, Jacobs AK, Morrison DA, Williams DO, Feldman TE, Kern MJ, O’Neill WW, Schaff HV, Whitlow PL, Adams CD, Anderson JL, Buller CE, Creager MA, Ettinger SM, Halperin JL, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura R, Page RL, Riegel B, Tarkington LG, Yancy CW. 2007 focused update of the ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice guidelines. J Am Coll Cardiol. 2008;51:172–209. doi: 10.1016/j.jacc.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Kushner FG, Hand M, Smith SC, Jr, King SB, 3rd, Anderson JL, Antman EM, Bailey SR, Bates ER, Blankenship JC, Casey DE, Jr, Green LA, Hochman JS, Jacobs AK, Krumholz HM, Morrison DA, Ornato JP, Pearle DL, Peterson ED, Sloan MA, Whitlow PL, Williams DO. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update) a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2009;54:2205–2241. doi: 10.1016/j.jacc.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 14.Rosenbaum PRD. The central role of propensity score in observational studies for casual effects. Biometrika. 1983;70:41–45. [Google Scholar]
- 15.Rubin D. Using propensity score to help design observational studies: application to the tobacco litigation. Health Serv Outcomes Res Methodol. 2001;2:169–188. [Google Scholar]
- 16.Ahmed A, Husain A, Love TE, Gambassi G, Dell’Italia LJ, Francis GS, Gheorghiade M, Allman RM, Meleth S, Bourge RC. Heart failure, chronic diuretic use, and increase in mortality and hospitalization: an observational study using propensity score methods. Eur Heart J. 2006;27:1431–1439. doi: 10.1093/eurheartj/ehi890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Austin PC. Some methods of propensity-score matching had superior performance to others: results of an empirical investigation and Monte Carlo simulations. Biom J. 2009;51:171–184. doi: 10.1002/bimj.200810488. [DOI] [PubMed] [Google Scholar]
- 18.Austin PC, Grootendorst P, Anderson GM. A comparison of the ability of different propensity score models to balance measured variables between treated and untreated subjects: a Monte Carlo study. Stat Med. 2007;26:734–753. doi: 10.1002/sim.2580. [DOI] [PubMed] [Google Scholar]
- 19.Ayanian JZ, Landrum MB, Guadagnoli E, Gaccione P. Specialty of ambulatory care physicians and mortality among elderly patients after myocardial infarction. N Engl J Med. 2002;347:1678–1686. doi: 10.1056/NEJMsa020080. [DOI] [PubMed] [Google Scholar]
- 20.Ndrepepa G, Schulz S, Keta D, Mehilli J, Birkmeier A, Massberg S, Laugwitz KL, Neumann FJ, Seyfarth M, Berger PB, Schomig A, Kastrati A. Bleeding after percutaneous coronary intervention with Bivalirudin or unfractionated Heparin and one-year mortality. Am J Cardiol. 2010;105:163–167. doi: 10.1016/j.amjcard.2009.08.668. [DOI] [PubMed] [Google Scholar]
- 21.Nikolsky E, Mehran R, Dangas G, Fahy M, Na Y, Pocock SJ, Lincoff AM, Stone GW. Development and validation of a prognostic risk score for major bleeding in patients undergoing percutaneous coronary intervention via the femoral approach. Eur Heart J. 2007;28:1936–1945. doi: 10.1093/eurheartj/ehm194. [DOI] [PubMed] [Google Scholar]
- 22.Eikelboom JW, Mehta SR, Anand SS, Xie C, Fox KA, Yusuf S. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation. 2006;114:774–782. doi: 10.1161/CIRCULATIONAHA.106.612812. [DOI] [PubMed] [Google Scholar]
- 23.Rao SV, O’Grady K, Pieper KS, Granger CB, Newby LK, Van de Werf F, Mahaffey KW, Califf RM, Harrington RA. Impact of bleeding severity on clinical outcomes among patients with acute coronary syndromes. Am J Cardiol. 2005;96:1200–1206. doi: 10.1016/j.amjcard.2005.06.056. [DOI] [PubMed] [Google Scholar]
- 24.Kinnaird TD, Stabile E, Mintz GS, Lee CW, Canos DA, Gevorkian N, Pinnow EE, Kent KM, Pichard AD, Satler LF, Weissman NJ, Lindsay J, Fuchs S. Incidence, predictors, and prognostic implications of bleeding and blood transfusion following percutaneous coronary interventions. Am J Cardiol. 2003;92:930–935. doi: 10.1016/s0002-9149(03)00972-x. [DOI] [PubMed] [Google Scholar]
- 25.Feit F, Voeltz MD, Attubato MJ, Lincoff AM, Chew DP, Bittl JA, Topol EJ, Manoukian SV. Predictors and impact of major hemorrhage on mortality following percutaneous coronary intervention from the REPLACE-2 Trial. Am J Cardiol. 2007;100:1364–1369. doi: 10.1016/j.amjcard.2007.06.026. [DOI] [PubMed] [Google Scholar]


