Abstract
Traumatic brain injury (TBI) is a major public health issue, and yet medical science has little to offer for the persistent symptoms that prevent many of these individuals from fully re-entering society. Post-traumatic hypopituitarism, and specifically growth hormone deficiency (GHD), has been found in a large percentage of individuals with chronic moderate to severe TBI. Presently, there are no published treatment studies of hormone replacement in this population. In this study, 83 subjects with chronic TBI were screened for hypopituitarism. Forty-two subjects were found to have either GHD or GH insufficiency (GHI), of which 23 agreed to be randomized to either a year of GH replacement or placebo. All subjects completed the study with no untoward side effects from treatment. A battery of neuropsychological tests and functional measures were administered before and after treatment. Improvement was seen on the following tests: Dominant Hand Finger Tapping Test, Wechsler Adult Intelligence Scale III–Information Processing Speed Index, California Verbal Learning Test II, and the Wisconsin Card Sorting Test (executive functioning). The findings of this pilot study provide preliminary evidence suggesting that some of the cognitive impairments observed in persons who are GHD/GHI after TBI may be partially reversible with appropriate GH replacement therapy.
Key words: growth hormone deficiency, hypopituitarism, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is a major health problem. It is the leading killer and disabler of young adults under the age of 35, and the annual cost of acute care and rehabilitation is estimated to be over 10 billion dollars per year (National Institutes of Health, 1998). Although pituitary dysfunction following TBI was reported as early as 90 years ago (Cyran, 1918), the possibility that acute brain injury can cause pituitary dysfunction has only recently been appreciated. Subsequent literature reviews (Benvenga et al., 2000; Edwards and Clark, 1986) demonstrated that hypopituitarism following TBI is not a rare phenomenon. This resulted in numerous cohort studies that demonstrated chronic hypopituitarism in 25–40% of persons with moderate to severe TBI (Bondanelli et al., 2007; Kelly et al., 2006; Lieberman et al., 2001; Schneider et al., 2008). The somatotroph and gonadotroph axes have been consistently shown to be the most vulnerable following TBI (Dusick et al., 2008). Growth hormone deficiency (GHD) is the most common deficiency, affecting approximately 20% of persons with TBI (Agha et al., 2007). The correlation of the severity of the TBI with an increased risk of GH deficiency has not been consistently demonstrated (Bondanelli et al., 2004; Kelly et al., 2000).
TBI results in cognitive impairments in memory, executive functioning, and information processing speed (Draper and Ponsford, 2008; Levin et al., 1992), and few treatments are available for chronic impairments (High et al., 2005, 2006; Landis et al., 2006; Sander et al., 2001; Seale et al., 2002). Most strategies for intervention have focused on acute interventions with neuroprotective agents (Clifton, 2002, 2004; Empey et al., 2006; Narayan et al., 2002). Pharmacological (Levin et al., 1986; Plenger et al., 1996; Poole and Agrawal, 2008), and cognitive interventions for persons with chronic TBI have met with marginal success (Cicerone et al., 2000, 2005; High et al., 1995, 2005; Sohlberg and Mateer, 1987).
Despite the clear evidence that large numbers of persons with TBI have GHD, very few are actually screened for pituitary dysfunction, possibly because the establishment of a linkage between GHD and cognitive impairment after TBI has not been definitively made (Beca et al., 2008). Moreover, the literature on GH replacement after TBI is scant. In this study we examined the effects of 1 year of GH replacement in persons who were GH deficient/insufficient more than 1 year after sustaining a moderate to severe TBI. We administered a comprehensive battery of neuropsychological tests and functional measures before and after GH replacement or placebo therapy, to determine if GH replacement resulted in improvement in cognition, motor speed, and everyday functioning.
Methods
Screening
After informed consent, 83 subjects were screened for GH deficiency at the General Clinical Research Centers (GCRC) of either the University of Texas Medical Branch at Galveston or Baylor College of Medicine. Subjects were all persons with moderate to severe TBI recruited from the Transitional Learning Center in Galveston, Texas, or from The Institute for Rehabilitation and Research in Houston, Texas. All participants were at least 1 year post-injury, with most being several years post-injury. Growth hormone levels can vary over the first year post-injury, but stabilize after 1 year (Aimaretti et al., 2005; Klose et al., 2007).
Subjects arrived fasting in the morning to the GCRC. Blood was drawn for GH, testosterone, luteinizing hormone (LH), follicle-stimulating hormone (FSH), cortisol, free thyroxine (T4), thyroid-stimulating hormone (TSH), prolactin, and insulin-like growth factor (IGF-1). A glucagon stimulation test (GST) was performed after the initial blood draw. The GST was chosen because it has been shown to compare favorably with the insulin tolerance test, which is considered the gold standard for provocative testing for GH deficiency (Littley et al., 1989). The GHRH-arginine stimulation test is not currently available in the United States (Yuen et al., 2009). Glucagon stimulates the release of GH from the pituitary by a mechanism that is not well understood. Glucagon was given (1 mg IM), and blood was sampled at 90, 120, 150, and 180 min for GH. For cortisol deficiency, we performed a cosyntropin stimulation test. Cosyntropin (250 μg IV push) was given and cortisol was measured before and 45 min after injection. If the response was less than 18 ng/mL, then the subject was designated as having cortisol deficiency. The subjects that had a GH response of 3 ng/mL or less to glucagon were designated as GH deficient (GHD). Subjects with a GH response greater than 3 ng/mL but less than 8 ng/mL were designated as GH insufficient (GHI). Subjects were diagnosed with hypothyroidism if the free T4 was below normal, and the TSH was normal or low. Subjects found to be deficient in cortisol or thyroid were replaced with that hormone, but were not studied in this protocol. The GST was performed only once prior to enrollment in the study.
Subjects
Forty-three of the 83 subjects were found to have GH deficiency/insufficiency. Twenty-three of the 43 persons with TBI and GH deficiency/insufficiency agreed to be randomized, evaluated (baseline) with neuropsychological and functional measures, and treated for 1 year with active rhGH (n = 12) or placebo (n = 11). Subjects were consented into the study in two phases: (1) screening for GHD, and (2) enrollment in the replacement trial. More subjects agreed to be screened for GHD than agreed to participation in the treatment trial, which involved a commitment to receiving daily injections for a full year, not knowing if they would be receiving GH or placebo. There was no bias in the subjects who agreed to participate in the trial with respect to age, education, or severity of injury. Both the participants and the evaluators were blinded as to which treatment group the participants were assigned to until after the 1-year evaluations were complete. Table 1 demonstrates that the two groups were comparable with respect to age, education, initial injury severity (initial post-resuscitation Glasgow Coma Scale [GCS] score, or lowest GCS score in the case of patients who deteriorated after admission), level of disability (Disability Rating Scale [DRS]), and level of supervision needed (Supervision Rating Scale [SRS]), at the time of initial evaluation for the study. The subjects were primarily persons with severe TBI. A small percentage of moderate patients were included, but all were severe enough to require inpatient rehabilitation services. There was a statistical trend for the two groups to differ with respect to time between injury (in years) and study enrollment (t = 2.0, df = 21, p < 0.058). Despite randomization, the group treated with active GH had a number of very chronically injured participants (ranging from 1.8–33.9 years post injury), compared to the placebo group (1.9–13.8 years). However, both groups had very chronic injuries, and all subjects were far past the point of any spontaneous recovery.
Table 1.
Demographic and Injury Severity Indicators
| |
Placebo (n = 11) |
Active rhGH (n = 12) |
||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Age (y) | 39.1 | 8.5 | 36.1 | 10.0 |
| Education (y) | 14.1 | 3.0 | 14.8 | 2.9 |
| Initial/lowest GCS score | 6.6 | 3.6 | 5.8 | 3.4 |
| Time since injury (y) | 5.1 | 3.6 | 11.0 | 9.2 |
| Baseline DRS score | 3.1 | 3.1 | 2.3 | 2.8 |
| Baseline SRS score | 4.4 | 3.8 | 3.6 | 3.9 |
The groups were comparable for age, education, initial severity of traumatic brain injury, and level of persisting disability when starting participation in the study. The difference between the groups for time since injury was nearly significant (p < 0.058). The placebo group ranged from 1.9–13.8 years post-injury when they began the study, and the treatment group ranged from 1.8–33.9 years post-injury.
DRS, Disability Rating Scale; SRS, Supervision Rating Scale; SD, standard deviation; GCS, Glasgow Coma Scale; rhGH, recombinant human growth hormone.
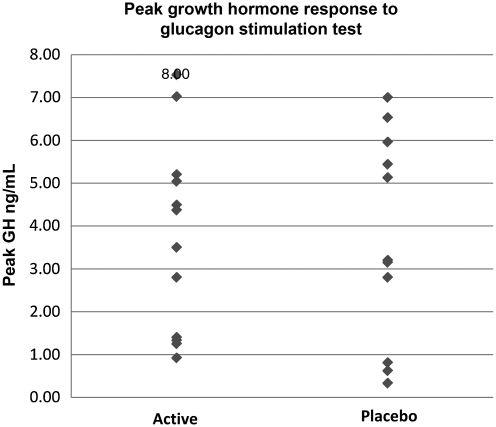
Figure 1 shows that the two groups were very similar with respect to their initial peak GH levels (all subjects were below 8 ng/mL). No statistically significant differences were seen between the groups. Although the mean values of the two groups did not differ, inspection of the distribution of the initial GH levels shows that the placebo group had more of a bimodal distribution, with clusters of subjects in the very low end and at the upper end of the insufficient range. In contrast, the treatment group had a more even distribution of initial GH levels. Given the small sample size, it was decided to covary the initial GH level to ensure that the two groups were as equivalent as possible with respect to initial GH level.
FIG. 1.
Peak growth hormone response to glucagon stimulation test prior to rhGH replacement (rhGH, recombinant human growth hormone; IGF-1, insulin-like growth factor-1).
Procedures
After screening, adults that were GH deficient/insufficient as determine by the GST were randomly assigned to either placebo or rhGH replacement. The rhGH dose was started at 200 μg/d, and then increased by 200 μg every month to 600 μg, at which point an IGF-1 level was drawn. The subject's dose was titrated up if needed to achieve an IGF-1 level in the upper half of the normal range. Those on placebo also had their dose similarly adjusted. The subjects were treated with daily injections of either placebo or rhGH for 1 year.
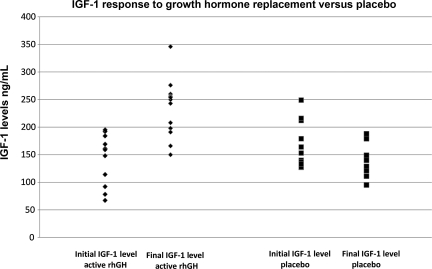
Figure 2 shows the IGF-1 response to GH replacement versus treatment with placebo. Participants treated with rhGH showed substantially improved IGF-1 levels at the end of the trial compared to participants receiving placebo. Subjects receiving placebo showed no improvement in their IGF-1 levels; in fact, IGF-1 levels declined somewhat, but not significantly. Baseline neuropsychological testing was performed before treatment, as was an assessment of physical function, including muscle biopsy, peak oxygen consumption (VO2), muscle strength (1-RM), and dual-energy x-ray absorptiometry (DEXA), to assess lean body mass (LBM) and fat mass. These parameters were re-assessed at 6 months and 1 year. Results of rhGH effects on physical functioning will be reported elsewhere.
FIG. 2.
Initial versus final IGF-1 levels after 1 year of replacement with rhGH or treatment with placebo (IGF-1, insulin-like growth factor-1; rhGH, recombinant human growth hormone).
Neuropsychological tests
The participants were administered measures that assessed neuropsychological and functional abilities. The neuropsychological measures assessed language, visual/spatial functioning, upper extremity motor functioning, information processing efficiency, working memory/attention, learning and memory, executive functioning, intellectual functioning, and emotional functioning. Functional measures included an index of community participation in day-to-day activities (Community Integration Questionnaire, CIQ). The tests included are listed in Table 2.
Table 2.
Neuropsychological Test Battery
| Test name | Cognitive function measured |
|---|---|
| Language functioning | |
| •Controlled Oral Word Association | •Constrained verbal fluency |
| Visual/spatial functioning | |
| •Picture Completion (WAIS-III) | •Visual analysis of visual detail |
| •Block Design (WAIS-III) | •Reproduction of 3-dimensional geometric figures from a 2-dimesional model |
| Motor functioning | |
| •Finger Tapping | •Gross motor speed |
| Attention and memory functioning | |
| •Digit Span–forwards/backwards | •Immediate auditory attention span |
| •California Verbal Learning Test-II (CVLT-II) | •Memorization of a word list |
| Executive functioning | |
| •Controlled Oral Word Association | •Constrained verbal fluency |
| •Trail Making Test-B | •Sequencing and alternation between two stimulus sets |
| •Stroop Color-Word Interference (DKEFS) | •Inhibition and processing efficiency |
| Information processing efficiency | |
| •Digit Symbol (WAIS-III) | •Written transcription of symbols from a key |
| •Trail Making Test-A | •Sequencing of a simple stimulus set |
| •Processing Speed Index (WAIS-III) | •Information processing efficiency |
| Intellectual functioning | |
| WAIS-III | |
| Verbal measures: | |
| •Arithmetic | •Aural arithmetic |
| •Digit Span | •Immediate auditory attention span |
| •Verbal Comprehension Index | •Verbal intellectual functioning |
| Performance measures: | |
| •Picture Completion | •Analysis of visual detail |
| •Digit Symbol | •Written transcription of symbols from a key |
| •Bock Design | •Reproduction of 3-dimensional geometric figures from a 2-dimesional model |
| Emotional functioning | |
| •Beck Depression Inventory-II (BDI-II) | •Self-report of depressive symptoms |
| Quality of life | |
| •Deiner Satisfaction of Life Scale | •Self-report rating of overall quality of life |
| Level of disability | |
| •Disability Rating Scale (DRS) | •Overall rating of level of disability |
| •Supervision Rating Scale (SRS) | •Level of supervision required |
| Community integration: | |
| •Community Integration Questionnaire (CIQ) | •Level of participation in the community |
WAIS-II, Wechsler Adult Intelligence Scale-III; D-KEFS, Delis-Kaplan Executive Function System.
Statistical analysis
Data from each of the above tests were analyzed using a separate mixed repeated-measures analysis of covariance (ANCOVA) with a Greenhouse-Geisser correction. Analyses were conducted between the active rhGH and placebo groups, while the peak GH response to GST was used as a covariate to attempt to equate the two groups with respect to initial GH level. The within-subjects variable was assessment points (baseline, 6 months, and 1 year). Two-way (treatment group by time), and three-way (treatment group by time by initial GH level), interactions were tested. In this analysis, a significant two-way interaction between treatment group (active rhGH or placebo) and time (baseline, 6 months, and 12 months) indicates differences in the effect of the treatments over time. A significant three-way interaction indicates that the effect of treatment type over time also depends on the initial level of GH.
For each analysis with a significant two- or three-way interaction, several post-hoc comparisons were run in order to further understand the source of the interactions. Within each treatment group, paired t-tests were run comparing baseline to performance at 6 and 12 months. Comparisons between the treatment groups were also made at each time point using t-tests. In all comparisons, we expected cognitive improvements for the active rhGH group compared to the placebo group. The number of subjects varied slightly across tests, as not every subject was able to take every test secondary to motor difficulties or the subject having to leave before completion of all of the tests. The missing values did not affect the data in any systematic way. The exact numbers of subjects for the most relevant tests are shown in the figures.
Results
The results of the analyses for all neuropsychological tests and outcome measures are shown in Table 3. There were no differences between the groups on most of the measures. However, differential improvement between the treatment and placebo groups was observed on four of the measures. There were insufficient numbers of subjects to analyze the effects rhGH replacement separately for persons who were GH-insufficient versus those who were GH-deficient. In general, the effect of rhGH replacement was greater for persons who were deficient.
Table 3.
Neuropsychological Test Results at Baseline, 6 Months, and 12 Months
| |
|
Baseline |
6 months |
12 months |
|
|
|||
|---|---|---|---|---|---|---|---|---|---|
| Active | Placebo | Active | Placebo | Active | Placebo | Results summary | Post-hoc summary | ||
| Visual Naming | M | 50.05 | 51.14 | 50.71 | 52.24 | 52.27 | 53.68 | ns | None |
| SE | (2.45) | (2.69) | (2.45) | (2.68) | (2.06) | (2.25) | |||
| COWA | M | 40.54 | 28.51 | 43.14 | 34.55 | 46.19 | 36.39 | Time | None |
| SE | (5.18) | (5.43) | (4.90) | (5.14) | (4.36) | (4.58) | |||
| Token Test | M | 65.15 | 55.17 | 69.19 | 64.67 | 74.06 | 57.04 | ns | None |
| SE | (10.73) | (11.31) | (6.95) | (7.32) | (7.97) | (8.41) | |||
| Trails A (seconds) | M | 67.40 | 88.84 | 60.35 | 87.99 | 64.42 | 95.18 | ns | None |
| SE | (26.33) | (27.50) | (21.09) | (22.02) | (23.83) | (24.89) | |||
| Trails B (seconds) | M | 127.00 | 170.45 | 110.51 | 159.49 | 100.18 | 150.73 | ns | None |
| SE | (31.50) | (31.50) | (26.30) | (26.30) | (27.87) | (27.87) | |||
| WCST (total correct) | M | 68.57 | 67.02 | 67.55 | 67.84 | 72.13 | 63.34 | Time Time × GH level Time × group × GH level | Active >placebo at 12 months* |
| SE | (4.43) | (4.86) | (4.21) | (4.62) | (4.46) | (4.89) | |||
| WAIS-III (scaled scores) | |||||||||
| Vocabulary | M | 9.18 | 9.28 | 9.56 | 8.63 | 9.38 | 9.34 | Time × GH Level | None |
| SE | (1.02) | (1.12) | (1.05) | (1.15) | (1.06) | (1.17) | |||
| Similarities | M | 10.36 | 8.57 | 10.88 | 9.64 | 11.54 | 10.56 | Time | None |
| SE | (0.92) | (1.01) | (1.18) | (1.29) | (1.14) | (1.25) | |||
| Arithmetic | M | 8.48 | 8.52 | 9.38 | 9.15 | 10.23 | 9.43 | ns | None |
| SE | (0.98) | (1.08) | (1.02) | (1.12) | (1.05) | (1.15) | |||
| Digit Span | M | 9.53 | 8.66 | 9.11 | 9.57 | 10.43 | 8.88 | ns | None |
| SE | (1.03) | (1.13) | (0.93) | (1.02) | (1.05) | (1.16) | |||
| Information | M | 9.10 | 9.38 | 9.33 | 9.51 | 9.51 | 10.18 | ns | None |
| SE | (0.93) | (1.02) | (1.05) | (1.15) | (1.07) | (1.17) | |||
| Letter-Number Seq. | M | 8.23 | 7.93 | 8.55 | 8.24 | 8.63 | 8.35 | ns | None |
| SE | (1.05) | (1.15) | (0.87) | (0.95) | (1.22) | (1.34) | |||
| Picture Completion | M | 8.59 | 7.09 | 8.75 | 8.45 | 10.17 | 9.27 | ns | None |
| SE | (1.20) | (1.25) | (1.07) | (1.12) | (1.31) | (1.37) | |||
| Digit Symbol Coding | M | 5.58 | 4.55 | 5.92 | 5.00 | 6.59 | 5.18 | Time | None |
| SE | (0.94) | (0.99) | (0.85) | (0.88) | (0.92) | (0.96) | |||
| Block Design | M | 10.25 | 8.27 | 10.09 | 9.18 | 9.92 | 8.63 | ns | None |
| SE | (1.02) | (1.06) | (1.17) | (1.22) | (1.08) | (1.13) | |||
| Matrix Reasoning | M | 9.25 | 9.72 | 10.25 | 9.82 | 10.50 | 9.91 | ns | None |
| SE | (1.07) | (1.12) | (1.12) | (1.17) | (1.03) | (1.08) | |||
| Symbol Search | M | 7.40 | 7.46 | 7.21 | 6.67 | 8.39 | 6.38 | ns | None |
| SE | (1.33) | (1.39) | (1.07) | (1.12) | (1.01) | (1.06) | |||
| VIQ | M | 95.76 | 92.59 | 97.54 | 96.05 | 101.43 | 97.78 | Time | None |
| SE | (5.42) | (5.94) | (5.71) | (6.26) | (6.17) | (6.76) | |||
| PIQ | M | 90.10 | 83.17 | 92.60 | 88.44 | 97.85 | 90.35 | Time | None |
| SE | (5.87) | (6.13) | (5.85) | (6.11) | (6.72) | (7.02) | |||
| FSIQ | M | 92.64 | 88.43 | 95.19 | 93.17 | 99.80 | 95.04 | Time | None |
| SE | (5.44) | (5.96) | (5.84) | (6.40) | (6.35) | (6.96) | |||
| VCI | M | 97.18 | 94.79 | 98.76 | 96.09 | 101.28 | 100.47 | Time | None |
| SE | (5.06) | (5.54) | (5.50) | (6.03) | (5.86) | (6.42) | |||
| POI | M | 96.52 | 89.35 | 98.52 | 94.62 | 101.52 | 95.71 | ns | None |
| SE | (5.83) | (6.09) | (6.04) | (6.30) | (6.47) | (6.76) | |||
| WMI | M | 92.15 | 90.02 | 88.80 | 93.54 | 96.95 | 92.76 | ns | None |
| SE | (5.62) | (6.16) | (7.09) | (7.77) | (5.79) | (6.34) | |||
| PSI | M | 82.84 | 76.58 | 83.59 | 79.06 | 88.02 | 78.68 | Time × GH level Time × group × GH level | Active group: improvement from baseline to 1 year Placebo group: improvement from baseline to 6 months only |
| SE | (5.11) | (5.35) | (4.72) | (4.95) | (4.78) | (5.01) | |||
| ROCFT Copy (raw) | M | 31.24 | 27.88 | 31.95 | 28.91 | 31.65 | 29.04 | ns | None |
| SE | (2.57) | (2.70) | (2.19) | (2.29) | (2.24) | (2.34) | |||
| ROCFT Immediate | M | 13.58 | 14.40 | 16.07 | 15.25 | 15.73 | 16.89 | ns | None |
| Recall (raw) | SE | (2.53) | (2.79) | (2.62) | (2.90) | (2.36) | (2.61) | ||
| ROCFT Delayed | M | 14.19 | 14.59 | 15.23 | 14.60 | 16.10 | 17.50 | ns | None |
| Recall (raw) | SE | (2.31) | (2.42) | (2.32) | (2.43) | (2.57) | (2.70) | ||
| Logical Memory I | M | 7.95 | 7.76 | 8.29 | 7.66 | 8.28 | 8.26 | ns | None |
| (standard score) | SE | (0.88) | (0.97) | (1.02) | (1.12) | (1.05) | (1.15) | ||
| Logical Memory II | M | 6.75 | 7.31 | 7.12 | 7.16 | 7.39 | 7.73 | ns | None |
| (standard score) | SE | (1.15) | (1.26) | (1.19) | (1.31) | (1.28) | (1.41) | ||
| CVLT-II Trials 1–5(T score) | M | 42.40 | 42.06 | 44.27 | 40.60 | 47.61 | 41.93 | ns | Active group: Scores improve across time |
| SE | (4.25) | (4.46) | (4.72) | (4.95) | (5.78) | (6.07) | |||
| CVLT-II Short Delay | M | −1.60 | −1.59 | −1.27 | −1.40 | −0.99 | −1.46 | ns | None |
| Free Recall (z score) | SE | (0.42) | (0.44) | (0.51) | (0.53) | (0.45) | (0.47) | ||
| CVLT-II Long Delay | M | −1.38 | −1.43 | −1.47 | −1.58 | −1.26 | −1.53 | ns | None |
| Free Recall (z score) | SE | (0.52) | (0.55) | (0.52) | (0.49) | (0.55) | (0.51) | ||
| Finger Tapping Dominant Hand (# taps) | M | 39.11 | 43.19 | 43.64 | 39.70 | 44.99 | 41.45 | Time × group | Active group: Improvement from baseline to 1 year |
| SE | (3.15) | (3.64) | (3.37) | (3.90) | (3.29) | (3.81) | |||
| Finger Tapping Nondominant Hand (# taps) | M | 31.42 | 39.10 | 34.52 | 38.88 | 35.71 | 39.45 | ns | None |
| SE | (2.67) | (2.92) | (3.28) | (3.60) | (2.83) | (3.10) | |||
| Grooved Pegboard | M | 96.51 | 115.27 | 95.71 | 113.92 | 93.96 | 106.16 | ns | None |
| Dominant Hand (sec) | SE | (13.98) | (15.47) | (15.24) | (16.86) | (11.80) | (13.05) | ||
| Grooved Pegboard Nondominant Hand (sec) | M | 150.87 | 161.45 | 146.90 | 162.72 | 143.29 | 149.45 | ns | None |
| SE | (26.36) | (28.88) | (25.66) | (28.12) | (23.83) | (26.11) | |||
| Disability Rating Scale | M | 1.90 | 2.51 | 2.26 | 2.32 | 1.53 | 2.21 | ns | None |
| SE | (0.82) | (0.86) | (0.95) | (1.00) | (0.69) | (0.72) | |||
| Supervision Rating Scale | M | 3.59 | 3.80 | 3.07 | 3.32 | 2.83 | 3.21 | ns | None |
| SE | (1.05) | (1.15) | (0.89) | (0.98) | (0.82) | (0.90) | |||
| Community Integration Questionnaire | M | 14.59 | 13.80 | 13.99 | 13.41 | 15.95 | 13.76 | ns | None |
| SE | (1.81) | (1.99) | (1.90) | (2.08) | (1.60) | (1.75) | |||
| Beck Depression Inventory | M | 7.12 | 9.88 | 5.72 | 11.38 | 7.17 | 12.23 | ns | None |
| SE | (2.18) | (2.18) | (2.32) | (2.32) | (2.38) | (3.38) | |||
Not significant at p < 0.05.
Mean values are adjusted for initial GH levels before replacement.
COWA, Controlled Oral Word Association; WCST, Wisconsin Card Sorting Test; WAIS-III, Wechsler Adult Intelligence Scale-III; VIQ, verbal intelligence quotient; ns, not significant; GH, growth hormone; PIQ, Performance Intelligence Quotient; FSIQ, Full-Scale Intelligence Quotient; VCI, Verbal Comprehension Index; POI, Perceptual Orientation Inventory; WMI, Working Memory Index; PSI, Processing Speed Index; ROCFT, Rey-Osterrieth Complex Figure Test; CVLT, California Verbal Learning Test; m, mean; SE, standard error.
Finger tapping
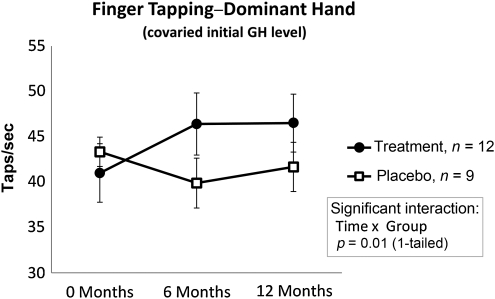
Improvements were observed in simple upper extremity motor speed in the dominant hand (Fig. 3). The interaction (tested in the ANCOVA model) between treatment group and time tested was significant (F(2,32) = 5.45, p < 0.01), indicating differential improvement in performance of the treatment group versus the placebo group. Post-hoc paired t-tests showed significant improvement in motor speed from baseline to 1 year for the active rhGH group (t = −3.31, p < 0.01), but not for the placebo group (t = 1.05, p > 0.05). The differential improvement was seen for the dominant hand only. A case-by-case analysis revealed that virtually every person in the rhGH group improved in their dominant-hand performance.
FIG. 3.
Finger Tapping–Dominant Hand. Significant improvements were observed for participants treated with active rhGH, but not for participants receiving placebo. The interaction between treatment group and time tested was significant, indicating differential improvement in performance of the treatment group versus the placebo group. Post-hoc analysis showed significant improvement in motor speed from baseline to 1 year for the active rhGH group, but not for the placebo group (rhGH, recombinant human growth hormone; GH, growth hormone).
WAIS-III: Processing Speed Index
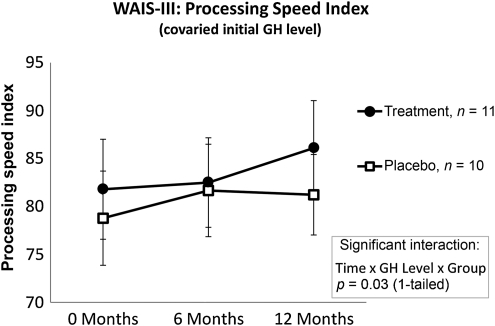
Similar results were seen for cognitive processing speed on the Processing Speed Index from the Wechsler Adult Intelligence Scale-III (WAIS III; Fig. 4). Although the interaction between time and group failed to reach significance (F(2,32) = 1.25, p > 0.05), there was a three-way interaction between time, group, and the peak GH response to GST (F(4,36) = 2.80, p < 0.05). Pairwise comparisons using a Bonferroni correction indicated that the active rhGH group demonstrated statistically significant improvement from baseline to 1 year (p < 0.05). While the placebo group showed some improvement from baseline to 6 months (p < 0.01), the pairwise comparison from baseline to 1 year failed to reach significance (p > 0.05).
FIG. 4.
Processing Speed Index. Significant improvements were observed for participants treated with active rhGH. While a significant improvement was seen for the placebo group from baseline to 6 months, the improvements from baseline to 12 months, and from 6 months to 12 months, were not significant. Pairwise comparisons indicated that the active rhGH group demonstrated improvement from baseline to 1 year. The placebo group showed some improvement from baseline to 6 months (rhGH, recombinant human growth hormone; GH, growth hormone).
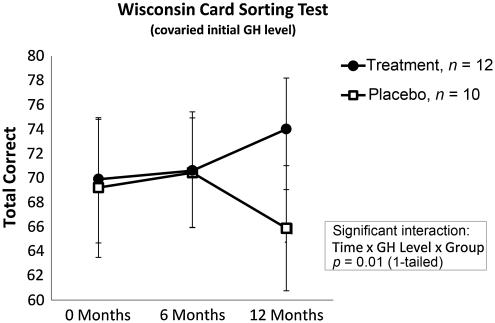
Wisconsin Card Sorting Test: Total Correct
A three-way interaction for time by group by the peak GH response to GST was also seen for the Wisconsin Card Sorting Test–Total Correct (F(2,38) = 3.71, p < 0.01). Post-hoc pairwise comparisons, covaried for the peak GH response to GST, indicated a trend in which the active rhGH group showed greater scores than the placebo group at 12 months, although this difference failed to reach significance (p = 0.2; Fig. 5).
FIG. 5.
Wisconsin Card Sorting Test–Total Correct. A three-way interaction for time by group by the peak GH response to GST was observed. Post-hoc pairwise comparisons indicated a trend in which the active rhGH group showed significantly greater scores than the placebo group at 12 months, although this difference failed to reach statistical significance (rhGH, recombinant human growth hormone; GH, growth hormone; GST, glucagon stimulation test).
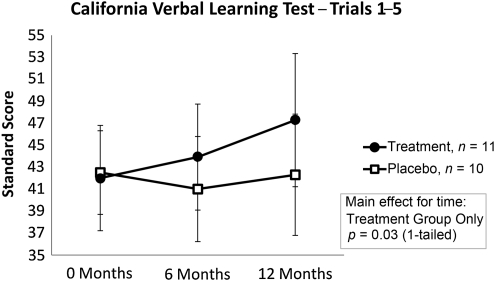
California Verbal Learning Test-II: Learning over trials 1–5
The interactions between time and treatment group were not statistically significant using multivariate ANCOVA. However, a significant improvement over time for learning and memory was demonstrated on the California Verbal Learning Test-II (CVLT-II) for the rhGH group, but not for the placebo group (F(2,18) = 4.21, p < 0.05; Fig. 6).
FIG. 6.
California Verbal Learning Test-II–Trials 1–5 (CVLT-II). The interactions between time and treatment group were statistically significant using multivariate analysis of covariance. A significant improvement over time for learning and memory was demonstrated on the CVLT-II for the rhGH group, but not for the placebo group (rhGH, recombinant human growth hormone).
Discussion
The mechanism for the effect of GH on cognition in humans is not well-understood. GH receptors are located throughout the brain. From the animal literature, it is clear that GH and IGF-1 play a role in modulating the N-methyl-d-aspartate (NMDA) receptor. GH influences the NMDA receptor system in the hippocampus, an essential component of long-term potentiation, which is highly involved in memory acquisition (Le Greves et al., 2006; Mahmoud and Grover, 2006). Furthermore, there may be a relationship between the NMDA receptor subunit mRNA expression levels and learning ability. Learning is improved by GH replacement in rats that have had their pituitaries removed (Le Greves et al., 2006). Additionally, following CNS injury, IGF-1 has also been found to increase progenitor cell proliferation and numbers of new neurons, oligodendrocytes, and blood vessels in the dentate gyrus of the hippocampus (Aberg et al., 2006). In contrast, deficiency in GH and IGF-I decreases survival of dentate granule neurons (Lichtenwalner et al., 2006).
The effects of GH deficiency and replacement on cognition from causes other than TBI have been relatively well-studied in both childhood-onset and adult-onset GHD. Both have been associated with cognitive impairments in memory, attention/concentration (working memory), and information processing speed. (Baum et al., 1998; Deijen et al., 1996; Lijffijt et al., 2003; Peace et al., 1998; van Dam et al., 2005). Replacement of GH in persons with childhood-onset GHD has shown improvement in these cognitive domains (Arwert et al., 2005). Adults with GHD due to reasons other than TBI show similar improvements in impaired cognition with replacement therapy (Almqvist et al., 1986; Deijen et al., 1998; Oertel et al., 2004; Sartorio et al., 1995). GH replacement has also been associated with improved quality of life (Bengtsson et al., 1993; Binnerts et al., 1992; McGauley, 1989). Falleti and colleagues (2006) conducted a meta-analysis of 340 GH-deficient patients (non-TBI) across 14 studies, which demonstrated the link between GH and cognitive performance, which improved with GH replacement.
A recent study by Kelly and colleagues (2006) failed to find significant differences in persons who were GH-deficient after TBI on measures of memory and attention/concentration compared to persons with TBI who were GH-sufficient. The study did find a greater incidence of depression and decreased quality of life for the GH-deficient group. However, the study included only 8 persons who were GH-deficient. The deficient group performed more poorly on every cognitive measure reported, but the results did not reach statistical significance. It is likely that the study lacked sufficient power to detect group differences. Indeed, in a very recent study, Leon-Carrion and co-workers (2007), with a slightly larger group of persons GH-deficient after TBI (n = 11), were able to show GH-related cognitive impairment in attention, executive functioning, memory, and emotion, compared to persons with TBI without GH deficiency.
The effects of GH replacement in persons deficient following TBI have only recently been reported. Hatton and associates (2006) reported on the effects of replacing IGF-1 and GH to improve metabolic and nutritional end-points in the acute phase of recovery from moderate to severe TBI. Kreitschmann-Andermahr and colleagues (2008) reported improved quality of life following GH replacement after TBI, similar to that seen in persons with GHD from other causes. Tanriverdi and co-workers (2010) reported substantial improvements in body composition parameters, lipid profiles, and quality of life scores, in two retired boxers with GHD. Bhagia and associates (2010) reported a case study of GH replacement in a woman who was deficient after mild TBI. Improvement was observed in single muscle fiber, body composition, lower extremity strength, and oxygen consumption. Cognitive test performance did not improve significantly, but there were improvements in motor dexterity and speed.
The present pilot study is the first report of a double-blind, placebo-controlled design examining the impact of GH replacement on cognition for persons with GHD following moderate to severe TBI. The data need to be interpreted cautiously due to the small sample size and the large number of comparisons made. A large number of neuropsychological tests were used for this initial study to ensure that we were sampling from a large enough domain of cognitive measures that we would be able to detect the effect of rhGH treatment. The other major limitation of the small data set is that we are unable to fully examine the differential effect of rhGH treatment for persons who are GH-insufficient versus those who are GH-deficient. The significant three-way interactions would indicate that it may be an important factor. Our sample was too small to make any reliable comparisons, and we were forced to co-vary for initial GH levels using covariance. While inspection of individual cases tended to support the idea that replacement had the greatest impact on those with more severe injuries, it was also seen to have substantial impact on those with less severe injuries. Larger studies will need to systematically examine this issue. Another limitation of the study is that although both groups were chronically injured (by the design of the study), the actively-treated group had a number of individuals with more lengthy time between post-injury and study enrollment, despite the randomization process. However, it seems implausible to attribute the results to this discrepancy. Although both groups were far beyond any spontaneous recovery, the active group was more chronically injured, making it even harder for that group to show any spontaneous gains in function.
While the number of comparisons in the current study is large, the findings are extremely intriguing because they are consistent with the much larger body of literature on the effects of GH deficiency and GH replacement on cognition for etiologies other than TBI (Maruff et al. 2005; Faletti et al. 2006). The results indicate improvement in simple motor speed, information-processing speed, executive functioning, and memory. Regardless of etiology, GH replacement in persons who are deficient results in improvements in information-processing speed and the storage of new information (episodic memory). Our data also indicate that the effects of GH replacement for GH deficiency after TBI may be even more widespread, as additional improvements were also seen in simple motor speed and in mental flexibility. The findings for finger tapping were especially intriguing. A clear effect for GH treatment was seen for improved motor speed for the dominant (more frequently used) hand, but not for the non-dominant hand. While this may be a chance finding, it suggests that the effect of GH replacement may be partially use-dependent. While there are examples of use-dependent therapies in stroke (Wolf et al., 2007), this has not been described in the GHD literature.
The findings in this study examined only the effects of GH on cognitive and upper extremity motor performance. It is possible that the effects of GH replacement could be enhanced by simultaneous physical, occupational, and cognitive therapies. The effect of GH replacement on cognitive flexibility (Wisconsin Card Sorting Test) was also very encouraging, as problems with executive functioning are especially debilitating after TBI, and treatment of this problem is most difficult (Cicerone et al., 2005).
Unlike the literature on GH replacement for etiologies other than TBI, we did not see improvements in our quality of life measure, the Community Integration Questionnaire (CIQ). The CIQ was chosen for the study based on its sensitivity in showing improvement due to treatment in post-acute rehabilitation (High et al., 2006). Some improvement was seen in the scores for the group receiving rhGH. The effects of treatment on quality of life will need to be tested in a larger sample.
Conclusion
For many years, investigators have assumed that all of the cognitive impairments in executive functioning, information-processing speed, and memory, were due solely to diffuse axonal injury and structural focal injuries to the frontal and temporal lobes. The findings from this study and from other recent studies indicate that in a significant proportion of persons with moderate to severe TBI, some of the observed cognitive impairments may actually be the result of GH deficiency, and could potentially be partially reversible with GH replacement therapy. Furthermore, there is the intriguing possibility that the effects of GH replacement may be partially use-dependent, raising the possibility that the effects of GH replacement may be maximized in the context of vigorous rehabilitation.
Acknowledgments
This work was partially funded by the generous support of the Moody Endowment and Pfizer, Inc. Recombinant growth hormone was supplied by Pfizer, Inc. This study was conducted in the General Clinical Research Center (GCRC) at the University of Texas Medical Branch at Galveston, funded by grant M01RR00073, and on the GCRC at Baylor University, Houston, Texas, funded by grant M01RR00188 from the National Center for Research Resources, National Institutes of Health, U.S. Public Health Service.
The authors thank Dr. Gerard Francisco, University of Texas Health Science Center–Houston for assistance with the screening of participants for GHD, and to Jordan Harp, M.S. for statistical and technical assistance.
Author Disclosure Statement
No competing financial interests exist.
Dr. Brent Masel has served as a consultant to Pfizer, Inc. and NovoNordisk.
References
- Aberg N.D. Brywe K.G. Isgaard J. Aspects of growth hormone and insulin-like growth factor-I related to neuroprotectin, regeneration, and functional plasticity in the adult brain. Scientific World J. 2006;6:53–80. doi: 10.1100/tsw.2006.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agha A. Phillips J. Thompson C.J. Hypopituitarism following traumatic brain injury (TBI) Br. J. Neurosurg. 2007;21:210–216. doi: 10.1080/02688690701253331. [DOI] [PubMed] [Google Scholar]
- Almqvist O. Thoren M. Saaf M. Eriksson O. Effects of growth hormone substitution on mental performance in adults with growth hormone deficiency: A pilot study. Psychoneuroendocrinology. 1986;11:347–352. doi: 10.1016/0306-4530(86)90020-x. [DOI] [PubMed] [Google Scholar]
- Aimaretti G. Ambrosio M.R. Di Somma C. Gasperi M. Cannavo S. Scaroni C. Fusco A. Del Monte P. De Menis E. Faustini-Fustini M. Grimaldi F. Logoluso F. Razzore P. Rovere S. Benvenga S. Delgli Umberti E.C. De Marinis L. Lombardi G. Mantero F. Martino E. Giordano G. Ghigo E. Residual pituitary function after brain injury-induced hypopituitarism: A prospective 12-month study. J. Clin. Endocrinol. Metab. 2005;90:6085–6092. doi: 10.1210/jc.2005-0504. [DOI] [PubMed] [Google Scholar]
- Arwert L.I. Deijen J.B. Drent M.L. The relation between insulin-like growth factor I levels and cognition in healthy elderly: A meta-analysis. Growth Horm. IGF Res. 2005;15:416–422. doi: 10.1016/j.ghir.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Baum H.B.A. Katznelson L. Sherman J.C. Biller B.M. Hayden D.L. Schoenfeld D.A. Cannistraro K.E. Klibanski A. Effects of physiological growth hormone (GH) therapy on cognition and quality of life in patients with adult onset GH deficiency. J. Clin. Endocrinol. Metab. 1998;83:3184–3189. doi: 10.1210/jcem.83.9.5112. [DOI] [PubMed] [Google Scholar]
- Beca S.G. High W.M. Masel B.E. Mossberg K.A. Urban R.J. What are critical outcome measures for patients receiving pituitary replacement following brain injury? Pituitary. 2008. [DOI] [PubMed]
- Bengtsson B.A. Eden S. Lonn L. Kvist H. Stokland A. Lindstedt G. Bosaeus E. Tolli J. Sjostrom L. Isaksson O.G. Treatment of adults with growth hormone (GH) deficiency with recombinant human GH. J. Clin. Endocrinol. Metab. 1993;76:309–317. doi: 10.1210/jcem.76.2.8432773. [DOI] [PubMed] [Google Scholar]
- Benvenga S. Campenni A. Ruggeri R.M. Trimarchi F. Hypopituitarism secondary to head trauma. J. Clin. Endocrinol. Metab. 2000;85:1353–1361. doi: 10.1210/jcem.85.4.6506. [DOI] [PubMed] [Google Scholar]
- Bhagia V. Gilkison C. Fitts R.H. Zgaljardic D.J. High W.M. Masel B.E. Urban R.J. Mossberg K.A. Effect of recombinant growth hormone replacement in a growth hormone deficient subject recovering from mild traumatic brain injury: A case report. Brain Inj. 2010;24:560–567. doi: 10.3109/02699051003601705. [DOI] [PubMed] [Google Scholar]
- Binnerts A. Swart G.R. Wilson J.H. Hoogerbrugge N. Pols H.A. Birkenhager J.C. Lamberts S.W. The effect of growth hormone administration in growth hormone deficient adults on bone, protein, carbohydrate and lipid homeostasis, as well as on body composition. Clin. Endocrinol. 1992;37:79–87. doi: 10.1111/j.1365-2265.1992.tb02287.x. [DOI] [PubMed] [Google Scholar]
- Bondanelli M. Ambrosio M.R. Cavazzini L. Bertocchi A. Zatelli M.C. Carli A. Valle D. Basaglia N. Uberti E.C. Anterior pituitary function may predict functional and cognitive outcome in patients with traumatic brain injury undergoing rehabilitation. J. Neurotrauma. 2007;24:1687–1697. doi: 10.1089/neu.2007.0343. [DOI] [PubMed] [Google Scholar]
- Bondanelli M. De Marinis L. Ambrosio M.R. Monesi M. Valle D. Zatelli M.C. Fusco A. Bianchi A. Farneti M. degli Umberti E.C. Occurrence of pituitary dysfunction following traumatic brain injury. J. Neurotrauma. 2004;21:685–696. doi: 10.1089/0897715041269713. [DOI] [PubMed] [Google Scholar]
- Cicerone K.D. Dahlberg C. Kalmar K. Langenbahn D.M. Malec J.F. Bergquist T.F. Felicetti T. Giacino J.T. Harley J.P. Harrington D.E. Herzog J. Kneipp S. Laatsch L. Morse P.A. Evidence-based cognitive rehabilitation: Recommendations for clinical practice. Arch. Phys. Med. Rehabil. 2000;81:1596–1615. doi: 10.1053/apmr.2000.19240. [DOI] [PubMed] [Google Scholar]
- Cicerone K.D. Dahlberg C. Malec J.F. Langenbahn D.M. Felicetti T. Kneipp S. Ellmo W. Kalmar K. Giacino J.T. Harley J.P. Laatsch L. Morse P.A. Catanese J. Evidence-based cognitive rehabilitation: Updated review of the literature from 1998 through 2002. Arch. Phys. Med. Rehabil. 2005;86:1681–1692. doi: 10.1016/j.apmr.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Clifton G.L. Is keeping cool still hot? An update on hypothermia in brain injury. Curr. Opin. Crit. Care. 2004;10:116–119. doi: 10.1097/00075198-200404000-00007. [DOI] [PubMed] [Google Scholar]
- Clifton G.L. Miller E.R. Choi S.C. Levin H.S. McCauley S. Smith K.R., Jr. Muizelaar J.P. Marion D.W. Luerssen T.G. Hypothermia on admission in patients with severe brain injury. J. Neurotrauma. 2002;19:293–301. doi: 10.1089/089771502753594864. [DOI] [PubMed] [Google Scholar]
- Cyran E. Hypophysenschadigung durch Schadelbasisfraktur. Dtsch. Med. Wschr. 1918;44:1261. [Google Scholar]
- Deijen J.B. de Boer H. van der Veen A. Cognitive changes during growth hormone replacement in adult men. Psychoneuroendocrinology. 1998;23:45–55. doi: 10.1016/s0306-4530(97)00092-9. [DOI] [PubMed] [Google Scholar]
- Deijen J.B. de Boer H. Blok G.J. van der Veen E.A. Cognitive impairments and mood disturbances in growth hormone deficient men. Psychoneuroendocrinology. 1996;21:313–322. doi: 10.1016/0306-4530(95)00050-x. [DOI] [PubMed] [Google Scholar]
- Draper K. Ponsford J. Cognitive functioning ten years following traumatic brain injury and rehabilitation. Neuropsychology. 2008;22:618–625. doi: 10.1037/0894-4105.22.5.618. [DOI] [PubMed] [Google Scholar]
- Dusick J.R. Wang C. Cohan P. Swerdloff R. Kelly D.F. Chapter 1: Pathophysiology of hypopituitarism in the setting of brain injury. Pituitary. 2008. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- Edwards O.M. Clark J.D. Post-traumatic hypopituitarism. Six cases and a review of the literature. Medicine (Baltimore) 1986;65:281–290. [PubMed] [Google Scholar]
- Empey P.E. McNamara P.J. Young B. Rosbolt M.B. Hatton J. Cyclosporin A disposition following acute traumatic brain injury. J. Neurotrauma. 2006;23:109–116. doi: 10.1089/neu.2006.23.109. [DOI] [PubMed] [Google Scholar]
- Falleti M.G. Maruff P. Burman P. Harris A. The effects of growth hormone (GH) deficiency and GH replacement on cognitive performance in adults: A meta-analysis of the current literature. Psychoneuroendocrinology. 2006;31:681–691. doi: 10.1016/j.psyneuen.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Hatton J. Kryscio R. Ryan M. Ott L. Young B. Systemic metabolic effects of combined insulin-like growth factor-I and growth hormone therapy in patients who have sustained acute traumatic brain injury. J. Neurosurg. 2006;105:843–852. doi: 10.3171/jns.2006.105.6.843. [DOI] [PubMed] [Google Scholar]
- High W.M., Jr. Boake C. Lehmkuhl L.D. Critical analysis of studies evaluating the effectiveness of rehabilitation after traumatic brain injury. J. Head Trauma Rehabil. 1995;10:14–26. [Google Scholar]
- High W.M., Jr. Effectiveness of TBI rehabilitation programs. In: High W.M. Jr., editor; Sander A.M., editor; Struchen M.A., editor; Hart K.A., editor. Rehabilitation for Traumatic Brain Injury. Oxford University Press; New York: 2005. [Google Scholar]
- High W.M., Jr. Roebuck-Spencer T. Sander A.M. Struchen M.A. Sherer M. Early versus later admission to postacute rehabilitation: Impact on functional outcome after traumatic brain injury. Arch. Phys. Med. Rehabil. 2006;87:334–342. doi: 10.1016/j.apmr.2005.11.028. [DOI] [PubMed] [Google Scholar]
- High W.M., Jr. Sander A.M. Struchen M.A. Hart K.A. Rehabilitation for Traumatic Brain Injury. Oxford University Press; New York: 2005. [Google Scholar]
- Kelly D.F. Gonzalo I.T. Cohan P. Berman N. Swerdloff R. Wang C. Hypopituitarism following traumatic brain injury and aneurismal subarachnoid hemorrhage: A preliminary report. J. Neurosurg. 2000;93:743–752. doi: 10.3171/jns.2000.93.5.0743. [DOI] [PubMed] [Google Scholar]
- Kelly D.F. McArthur D.L. Levin H. Swimmer S. Dusick J.R. Cohan P. Wang C. Swerdloff R. Neurobehavioral and quality of life changes associated with growth hormone insufficiency after complicated mild, moderate, or severe traumatic brain injury. J. Neurotrauma. 2006;23:928–942. doi: 10.1089/neu.2006.23.928. [DOI] [PubMed] [Google Scholar]
- Klose M. Juul A. Struck J. Morgenthaler N.G. Kosteljanetz M. Feldt-Rasmussen U. Acute and long-term pituitary insufficiency in traumatic brain injury: A prospective single-centre study. Clin. Endocrinol. 2007;67:598–606. doi: 10.1111/j.1365-2265.2007.02931.x. [DOI] [PubMed] [Google Scholar]
- Kreitschmann-Andermahr I. Poll E.M. Reineke A. Gilsbach J.M. Brabant G. Buchfelder M. Fassbender W. Faust M. Kann P.H. Wallaschofski H. Growth hormone deficient patients after traumatic brain injury—baseline characteristics and benefits after growth hormone replacement–an analysis of the German KIMS database. Growth Horm. IGF Res. 2008;18:472–478. doi: 10.1016/j.ghir.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Landis J. Hanten G. Levin H.S. Li X. Ewing-Cobbs L. Duron J. High W.M., Jr. Evaluation of the errorless learning technique in children with traumatic brain injury. Arch. Phys. Med. Rehabil. 2006;87:799–805. doi: 10.1016/j.apmr.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Le Greves M. Zhou Q. Berg M. Le Greves P. Fholenhag K. Meyerson B. Nyberg F. Growth hormone replacement in hypophysectomized rats affects spatial performance and hippocampal levels of NMDA receptor subunit and PSD-95 gene transcript levels. Exp. Brain Res. 2006;173:267–273. doi: 10.1007/s00221-006-0438-2. [DOI] [PubMed] [Google Scholar]
- Leon-Carrion J. Leal-Cerro A. Cabezas F.M. Atutxa A.M. Gomez S.G. Cordero J.M. Moreno A.S. Ferrari M. Dominguez-Morales M.R. Cognitive deterioration due to GH deficiency in patients with traumatic brain injury: A preliminary report. Brain Inj. 2007;21:871–875. doi: 10.1080/02699050701484849. [DOI] [PubMed] [Google Scholar]
- Levin H.S. Peters B.H. Kalisky Z. High W.M., Jr. Von Laufen A. Eisenberg H.M. Morrison D.P. Gary H.E., Jr. Effects of oral physostigmine and lecithin on memory and attention in closed head injured patients. Central Nerv. Sys. Trauma. 1986;3:333–342. doi: 10.1089/cns.1986.3.333. [DOI] [PubMed] [Google Scholar]
- Levin H.S. Williams D.H. Eisenberg H.M. High W.M., Jr. Guinto F.C., Jr Serial magnetic resonance imaging and neurobehavioral findings after mild to moderate closed head injury. J. Neurol. Neurosurg. Psychiatry. 1992;55:255–262. doi: 10.1136/jnnp.55.4.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenwalner R.J. Forbes M.E. Sonntag W.E. Riddle D.R. Adult-onset deficiency in growth hormone and insulin-like growth factor-I decreases survival of dentate granule neurons: Insights into the regulation of adult hippocampal neurogenesis. J. Neurosci. Res. 2006;83:199–210. doi: 10.1002/jnr.20719. [DOI] [PubMed] [Google Scholar]
- Lieberman S.A. Oberoi A.L. Gilkison C.R. Masel B.E. Urban R.J. Prevalence of neuroendocrine dysfunction in patients recovering from traumatic brain injury. J. Clin. Endocrinol. Metab. 2001;86:2752–2756. doi: 10.1210/jcem.86.6.7592. [DOI] [PubMed] [Google Scholar]
- Lijffijt M. van Dam P.S. Kenemans J.L. Koppeschaar H.P. de Vries W.R. Drent M.L. Wittenberg A. Kemner C. Somatotropic-axis deficiency affects brain substrates of selective attention in childhood-onset growth hormone deficient patients. Neurosci. Lett. 2003;353:123–126. doi: 10.1016/j.neulet.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Littley M.D. Gibson S. White A. Shalet S.M. Comparison of the ACTH and cortisol response to provocative testing with glucagon and insulin hypoglycemia in normal subjects. Clin. Endocrinol. (Oxf.) 1989;31:527–533. doi: 10.1111/j.1365-2265.1989.tb01276.x. [DOI] [PubMed] [Google Scholar]
- Mahmoud G.S. Grover L.M. Growth hormone enhances excitatory synaptic transmission in area CA1 of rat hippocampus. J. Neurophysiol. 2006;95:2962–2974. doi: 10.1152/jn.00947.2005. [DOI] [PubMed] [Google Scholar]
- Maruff P. Faletti M. Cognitive function in growth hormone deficiency and growth hormone replacement. Horm. Res. 2005;64(Suppl. 3):100–108. doi: 10.1159/000089325. [DOI] [PubMed] [Google Scholar]
- McGauley G.A. Quality of life assessment before and after growth hormone treatment in adults with growth hormone deficiency. Acta Paediatrica Scandinavica. 1989;356:70–72. doi: 10.1111/j.1651-2227.1989.tb11249.x. [DOI] [PubMed] [Google Scholar]
- Narayan R.K. Michel M.E. Ansell B. Baethmann A. Biegon A. Bracken M.B. Bullock M.R. Choi S.C. Clifton G.L. Contant C.F. Coplin W.M. Dietrich W.D. Ghajar J. Grady S.M. Grossman R.G. Hall E.D. Heetderks W. Hovda D.A. Jallo J. Katz R.L. Knoller N. Kochanek P.M. Maas A.I. Majde J. Marion D.W. Marmarou A. Marshall L.F. McIntosh T.K. Miller E. Mohberg N. Muizelaar J.P. Pitts L.H. Quinn P. Riesenfeld G. Robertson C.S. Strauss K.I. Teasdale G. Temkin N. Tuma R. Wade C. Walker M.D. Weinrich M. Whyte J. Wilberger J. Young A.B. Yurkewicz L. Clinical trials in head injury. J. Neurotrauma. 2002;19:503–557. doi: 10.1089/089771502753754037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Health. Rehabilitation of persons with traumatic brain injury. NIH Consensus Statement. 1998;16:1–41. (1998, October 26–28) [PubMed] [Google Scholar]
- Oertel H. Schneider H.J. Stalla G.K. Holsboer F. Zihl J. The effect of growth hormone substitution on cognitive performance in adult patients with hypopituitarism. Psychoneuroendocrinology. 2004;29:839–850. doi: 10.1016/S0306-4530(03)00151-3. [DOI] [PubMed] [Google Scholar]
- Peace K.A. Orme S.M. Padayatty S.J. Godfrey H.P.D. Belchetz P.E. Cognitive dysfunction in patients with pituitary tumour who have been treated with transfrontal or transsphenoidal surgery or medication. Clin. Endocrinol. 1998;49:391–396. doi: 10.1046/j.1365-2265.1998.00543.x. [DOI] [PubMed] [Google Scholar]
- Plenger P.M. Dixon C.E. Castillo R.M. Frankowski R.F. Yablon S.A. Levin H.S. Subacute methylphenidate treatment for moderate to moderately severe traumatic brain injury: A preliminary double-blind placebo-controlled study. Arch. Phys. Med. Rehabil. 1996;77:536–540. doi: 10.1016/s0003-9993(96)90291-9. [DOI] [PubMed] [Google Scholar]
- Poole N.A. Agrawal N. Cholinomimetic agents and neurocognitive impairment following head injury: A systematic review. Brain Inj. 2008;22:519–534. doi: 10.1080/02699050802132495. [DOI] [PubMed] [Google Scholar]
- Sander A.M. Roebuck T.M. Struchen M.A. Sherer M. High W.M., Jr. Long-term maintenance of gains obtained in post acute rehabilitation by persons with traumatic brain injury. J. Head Trauma Rehabil. 2001;16:356–373. doi: 10.1097/00001199-200108000-00006. [DOI] [PubMed] [Google Scholar]
- Sartorio A. Molinari E. Riva G. Conti A. Morabito F. Faglia G. Growth hormone treatment in adults with childhood onset growth hormone deficiency: Effects on psychological capabilities. Hormone Res. 1995;44:6–11. doi: 10.1159/000184582. [DOI] [PubMed] [Google Scholar]
- Schneider M. Schneider H.J. Yassouridis A. Saller B. von Rosen F. Stalla G.K. Predictors of anterior pituitary insufficiency after traumatic brain injury. Clin. Endocrinol. 2008;68:206–212. doi: 10.1111/j.1365-2265.2007.03020.x. [DOI] [PubMed] [Google Scholar]
- Seale G.S. Caroselli J.S. High W.M., Jr. Becker C.L. Neese L.E. Scheibel R. Use of the Community Integration Questionnaire (CIQ) to characterize changes in functioning for individuals with traumatic brain injury who participated in a post-acute rehabilitation programme. Brain Inj. 2002;16:955–967. doi: 10.1080/02699050210155258. [DOI] [PubMed] [Google Scholar]
- Sohlberg M.M. Mateer C.A. Effectiveness of an attention-training program. J. Clin. Exper. Neuropsychol. 1987;9:117–130. doi: 10.1080/01688638708405352. [DOI] [PubMed] [Google Scholar]
- Tanriverdi F. Unluhizarci K. Karaca Z. Casanueva F.F. Kelestimur F. Hypopituitarism due to sports related head trauma and the effects of growth hormone replacement in retired amateur boxers. Pituitary. 2010;13:111–114. doi: 10.1007/s11102-009-0204-0. [DOI] [PubMed] [Google Scholar]
- Yuen K. Biller B. Molitch M. Cook D. Is lack of recombinant GH-releasing hormone in the United States a setback or time to consider glucagon testing for adult growth hormone deficiency? J. Clin. Endocrinol. Metabo. 2009;94:2702–2707. doi: 10.1210/jc.2009-0299. [DOI] [PubMed] [Google Scholar]
- van Dam P.S. de Winter C.F. de Vries R. van der Grond J. Drent M.L. Lijffijt M. Kenemans J.L. Aleman A. de Haan E.H. Koppeschaar H.P. Childhood-onset growth hormone deficiency, cognitive function and brain N-acetylaspartate. Psychoneuroendocrinology. 2005;30:357–363. doi: 10.1016/j.psyneuen.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Wolf S.L. Newton H. Maddy D. Blanton S. Zhang Q. Winstein C.J. Morris D.M. Light K. The Excite Trial: relationship of intensity of constraint induced movement therapy to improvement in the Wolf motor function test. Restor. Neurol. Neurosci. 2007;25:549–562. [PubMed] [Google Scholar]