Abstract
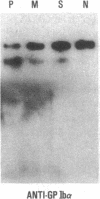
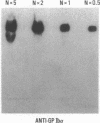
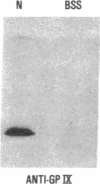
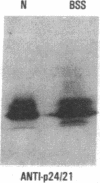
We have studied a patient with a congenital bleeding disorder and phenotypic manifestations typical of Bernard-Soulier syndrome, including giant platelets with absent ristocetin-induced von Willebrand factor binding. Two monoclonal antibodies reacting with distinct epitopes in the amino-terminal domain of the alpha-chain of glycoprotein (GP) Ib were used to estimate the number of GP Ib molecules on the platelet membrane. In the patient, binding of one antibody (LJ-Ib10) was approximately 50% of normal, while binding of the other (LJ-Ib1) was absent. Binding of both antibodies was reduced to approximately 50% of normal in the mother and one sister of the propositus, and their platelets exhibited approximately 70% of normal von Willebrand factor binding. Immunoblotting studies confirmed the presence of GP Ib alpha, as well as GP IX, in patient platelets. Antibody LJ-Ib10, but not LJ-Ib1, could immunoprecipitate the patient's GP Ib alpha from surface-labeled proteins. Thus, platelets from the propositus contained a structurally and functionally altered GP Ib-IX complex lacking a specific antibody epitope and the ability to bind von Willebrand factor. In contrast, the binding of human alpha-thrombin to the patient's platelets was normal, and three classes of binding sites with high, intermediate, and low affinity could be detected. These studies define a distinct variant form of Bernard-Soulier syndrome and provide evidence, based on a naturally occurring mutant molecule, that the amino-terminal region of GP Ib alpha contains a von Willebrand factor-binding domain distinct from the high affinity thrombin-binding site. Use of different monoclonal antibodies with distinct epitope specificities appears to be essential for a correct identification of variant Bernard-Soulier syndrome.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruck C., Portetelle D., Glineur C., Bollen A. One-step purification of mouse monoclonal antibodies from ascitic fluid by DEAE Affi-Gel blue chromatography. J Immunol Methods. 1982 Sep 30;53(3):313–319. doi: 10.1016/0022-1759(82)90178-8. [DOI] [PubMed] [Google Scholar]
- Clemetson K. J., Lüscher E. F. Membrane glycoprotein abnormalities in pathological platelets. Biochim Biophys Acta. 1988 Feb 24;947(1):53–73. doi: 10.1016/0304-4157(88)90019-6. [DOI] [PubMed] [Google Scholar]
- Clemetson K. J., McGregor J. L., James E., Dechavanne M., Lüscher E. F. Characterization of the platelet membrane glycoprotein abnormalities in Bernard-Soulier syndrome and comparison with normal by surface-labeling techniques and high-resolution two-dimensional gel electrophoresis. J Clin Invest. 1982 Aug;70(2):304–311. doi: 10.1172/JCI110618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marco L., Fabris F., Casonato A., Fabris P., Dal Ben M. G., Barbato A., Girolami A. Bernard-Soulier syndrome: diagnosis by an ELISA method using monoclonal antibodies in 2 new unrelated patients. Acta Haematol. 1986;75(4):203–208. doi: 10.1159/000206125. [DOI] [PubMed] [Google Scholar]
- De Marco L., Girolami A., Russell S., Ruggeri Z. M. Interaction of asialo von Willebrand factor with glycoprotein Ib induces fibrinogen binding to the glycoprotein IIb/IIIa complex and mediates platelet aggregation. J Clin Invest. 1985 Apr;75(4):1198–1203. doi: 10.1172/JCI111816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marco L., Shapiro S. S. Properties of human asialo-factor VIII. A ristocetin-independent platelet-aggregating agent. J Clin Invest. 1981 Aug;68(2):321–328. doi: 10.1172/JCI110259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X., Beutler L., Ruan C., Castaldi P. A., Berndt M. C. Glycoprotein Ib and glycoprotein IX are fully complexed in the intact platelet membrane. Blood. 1987 May;69(5):1524–1527. [PubMed] [Google Scholar]
- Duperray A., Troesch A., Berthier R., Chagnon E., Frachet P., Uzan G., Marguerie G. Biosynthesis and assembly of platelet GPIIb-IIIa in human megakaryocytes: evidence that assembly between pro-GPIIb and GPIIIa is a prerequisite for expression of the complex on the cell surface. Blood. 1989 Oct;74(5):1603–1611. [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Fox J. E., Aggerbeck L. P., Berndt M. C. Structure of the glycoprotein Ib.IX complex from platelet membranes. J Biol Chem. 1988 Apr 5;263(10):4882–4890. [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Ganguly P. Binding of thrombin to functionally defective platelets: a hypothesis on the nature of the thrombin receptor. Br J Haematol. 1977 Sep;37(1):47–51. [PubMed] [Google Scholar]
- Handa M., Titani K., Holland L. Z., Roberts J. R., Ruggeri Z. M. The von Willebrand factor-binding domain of platelet membrane glycoprotein Ib. Characterization by monoclonal antibodies and partial amino acid sequence analysis of proteolytic fragments. J Biol Chem. 1986 Sep 25;261(27):12579–12585. [PubMed] [Google Scholar]
- Harmon J. T., Jamieson G. A. The glycocalicin portion of platelet glycoprotein Ib expresses both high and moderate affinity receptor sites for thrombin. A soluble radioreceptor assay for the interaction of thrombin with platelets. J Biol Chem. 1986 Oct 5;261(28):13224–13229. [PubMed] [Google Scholar]
- Hickey M. J., Williams S. A., Roth G. J. Human platelet glycoprotein IX: an adhesive prototype of leucine-rich glycoproteins with flank-center-flank structures. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6773–6777. doi: 10.1073/pnas.86.17.6773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson G. A., Okumura T. Reduced thrombin binding and aggregation in Bernard-Soulier platelets. J Clin Invest. 1978 Mar;61(3):861–864. doi: 10.1172/JCI109000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lopez J. A., Chung D. W., Fujikawa K., Hagen F. S., Davie E. W., Roth G. J. The alpha and beta chains of human platelet glycoprotein Ib are both transmembrane proteins containing a leucine-rich amino acid sequence. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2135–2139. doi: 10.1073/pnas.85.7.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez J. A., Chung D. W., Fujikawa K., Hagen F. S., Papayannopoulou T., Roth G. J. Cloning of the alpha chain of human platelet glycoprotein Ib: a transmembrane protein with homology to leucine-rich alpha 2-glycoprotein. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5615–5619. doi: 10.1073/pnas.84.16.5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery R. R., Kunicki T. J., Taves C., Pidard D., Corcoran M. Diagnosis of Bernard-Soulier syndrome and Glanzmann's thrombasthenia with a monoclonal assay on whole blood. J Clin Invest. 1983 Feb;71(2):385–389. doi: 10.1172/JCI110780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Nurden A. T., Didry D., Rosa J. P. Molecular defects of platelets in Bernard-Soulier syndrome. Blood Cells. 1983;9(2):333–358. [PubMed] [Google Scholar]
- O'Toole T. E., Loftus J. C., Plow E. F., Glass A. A., Harper J. R., Ginsberg M. H. Efficient surface expression of platelet GPIIb-IIIa requires both subunits. Blood. 1989 Jul;74(1):14–18. [PubMed] [Google Scholar]
- Okumura T., Jamieson G. A. Platelet glycocalicin: a single receptor for platelet aggregation induced by thrombin or ristocetin. Thromb Res. 1976 May;8(5):701–706. doi: 10.1016/0049-3848(76)90250-4. [DOI] [PubMed] [Google Scholar]
- Phillips D. R., Agin P. P. Platelet plasma membrane glycoproteins. Evidence for the presence of nonequivalent disulfide bonds using nonreduced-reduced two-dimensional gel electrophoresis. J Biol Chem. 1977 Mar 25;252(6):2121–2126. [PubMed] [Google Scholar]
- Rosa J. P., McEver R. P. Processing and assembly of the integrin, glycoprotein IIb-IIIa, in HEL cells. J Biol Chem. 1989 Jul 25;264(21):12596–12603. [PubMed] [Google Scholar]
- Ruggeri Z. M., De Marco L., Gatti L., Bader R., Montgomery R. R. Platelets have more than one binding site for von Willebrand factor. J Clin Invest. 1983 Jul;72(1):1–12. doi: 10.1172/JCI110946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligsohn U., Coller B. S., Zivelin A., Plow E. F., Ginsberg M. H. Immunoblot analysis of platelet glycoprotein IIb in patients with Glanzmann thrombasthenia in Israel. Br J Haematol. 1989 Jul;72(3):415–423. doi: 10.1111/j.1365-2141.1989.tb07725.x. [DOI] [PubMed] [Google Scholar]
- Solum N. O., Hagen I., Peterka M., Gjemdal T. Absence of the 145,000 molecular weight, soluble platelet membrane glycoprotein--lack of platelet agglutination. Thromb Haemost. 1980 Feb 29;42(5):1626–1629. [PubMed] [Google Scholar]
- Titani K., Takio K., Handa M., Ruggeri Z. M. Amino acid sequence of the von Willebrand factor-binding domain of platelet membrane glycoprotein Ib. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5610–5614. doi: 10.1073/pnas.84.16.5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente V., Houghten R. A., Ruggeri Z. M. Identification of a site in the alpha chain of platelet glycoprotein Ib that participates in von Willebrand factor binding. J Biol Chem. 1990 Jan 5;265(1):274–280. [PubMed] [Google Scholar]
- Vicente V., Kostel P. J., Ruggeri Z. M. Isolation and functional characterization of the von Willebrand factor-binding domain located between residues His1-Arg293 of the alpha-chain of glycoprotein Ib. J Biol Chem. 1988 Dec 5;263(34):18473–18479. [PubMed] [Google Scholar]
- Weiss H. J., Tschopp T. B., Baumgartner H. R., Sussman I. I., Johnson M. M., Egan J. J. Decreased adhesion of giant (Bernard-Soulier) platelets to subendothelium. Further implications on the role of the von Willebrand factor in hemostasis. Am J Med. 1974 Dec;57(6):920–925. doi: 10.1016/0002-9343(74)90170-3. [DOI] [PubMed] [Google Scholar]