Abstract
Background/Aim: Root surface biomodification has been used to treat gingival recession and periodontitis. The principle for this procedure is that removing the smear layer from the root surfaces exposes collagen fibers, which leads to improved healing. Clinical studies generally have failed to find any improvement in clinical parameters when using such procedures, however. The aim of this study was to evaluate and compare the outcome of gingival recession therapy using the subepithelial connective tissue graft (SCTG) with or without Nd:YAG laser application for root surface biomodification. Materials and Methods: Thirty-four teeth in 17 patients with Miller Class 1 and 2 recession were treated with SCTG with (test group) or without (control group) the application of Nd:YAG laser (1 W, 10 Hz, 100 mj, 60 s, 1064 nm). Clinical attachment level (CAL), recession depth (RD), recession width (RW), and probing depth (PD) were measured at baseline and six months postsurgery. Results: Both treatments yielded significant improvements in terms of RD and RW decrease and CAL gain compared to baseline values. For test and control groups, the average root coverage was 33% and 77%, respectively (p < 0.05), and the complete root coverage was 18% and 65%, respectively (p < 0.05). The control group showed a greater reduction in RD and RW compared with the test group (p < 0.05). Conclusions: The use of Nd:YAG laser as a root surface biomodifier negatively affected the outcome of root coverage with the SCTG.
Introduction
Gingival recession, a most common and undesirable condition, is characterized by the displacement of the gingival margin apically from the cemento-enamel junction (CEJ) and the exposure of the root surface to the oral environment. The principal objective in the treatment of gingival recession is to cover the exposed root surfaces to improve esthetics and to reduce hypersensitivity. Additional benefits that result from treating areas of gingival recession may include an increase in the width and thickness of keratinized gingiva. Coverage of denuded roots has become one of the most challenging procedures in periodontal mucogingival surgery.1–4 The search for the appropriate root coverage technique has taken many different approaches. Various surgical options have been developed to achieve the above goals and include the use of subepithelial connective tissue grafts (SCTGs),5 free gingival grafts,6 laterally sliding flaps,7 coronally advanced flaps,8 double papillae flaps,9 guided tissue regeneration,10 and acellular dermal matrix allografts.11 Among these surgical options, variations of subepithelial connective tissue graft (SCTG) procedures demonstrated a high percentage of root coverage with a high predictability and without significant post-surgical complications.12–18 Also, the root coverage gained with SCTG procedures was reported to be stable over the long-term.14 Therefore, SCTG procedures have commonly served as the “gold standard” to evaluate the safety and results of new root-coverage techniques.1–4
So far, a concerted effort has been made in the field of root conditioning to improve the outcome of regenerative periodontal therapies by favoring the attachment of the regenerated periodontal structures. Mechanical instrumentation (scaling and root planing) leaves a smear layer, which inhibits cell re-attachment and can serve as a reservoir for microbial growth.19 Therefore, chemical conditioning of the roots is performed in order to remove the smear layer and to improve their biocompatibility. After the removal of the smear layer, the dentinal collagen is exposed and these collagen fibers supposedly serve as chemo-attractants for periodontal fibroblasts.20 Beside surgical options, various adjunctive agents have been applied to promote healing and further enhance clinical outcomes. These include root conditioners (e.g., citric acid,20–25 tetracycline HCI,26 EDTA,19,27 phosphoric acid,28 and hydrogen peroxide),3 enamel matrix proteins,29 recombinant human growth factors, platelet-rich plasma,30 and dentin bonding conditioner.31 In addition to chemical conditioning, the applicability of different laser systems such as CO2, Nd:YAG, diode and Er:YAG laser in the removal of the smear layer have been demonstrated.32–43
However, until now, no published data have been available concerning the clinical outcomes following root surface biomodification with Nd:YAG laser for the treatment of gingival recession. Therefore, the aim of the present study was to evaluate and compare the outcome of gingival recession therapy using the SCTG with or without Nd:YAG laser application for root surface biomodification.
Materials and Methods
The research protocol and consent forms were initially submitted to the Ethics Committee and The Institutional Internal Review and Ethics Board at the Atatürk University. The Faculty of Dentistry approved the study (AU-IIREB reference code:018). All participants provided written informed consent.
Selection of subjects and test teeth
The study population consisted of 17 patients with esthetic problems due to the exposure of recession-type defects during smiling (9 women and 8 men; age 21–49 years, mean age 31.3 ± 8.4 years) who visited the periodontology department of Atatürk University, Erzurum, Turkey. Teeth with cracked structure, carious lesions, restorations, and non-vital were excluded. All recessions defects (34 teeth) fell into class 1 or class 2 according to the Miller classification, since no loss of interdental soft and hard tissue height was present.44
All patients were non-smokers, systemically and periodontally healthy, with no contraindications for periodontal surgery, and none were taking medications known to interfere with periodontal tissue health or healing.
Following examination, all of the teeth received scaling, root planing and crown polishing, and oral hygiene instructions were given four weeks before surgery. All patients demonstrated optimal oral hygiene (Plaque Score ≤15%).45
Clinical Parameters
After the baseline clinical measurements [recession depth (RD), recession width (RW), clinical attachment level (CAL), and probing depth (PD)], the teeth were randomly assigned to the test group or control group. All measurements were performed by means of a Williams periodontal probe (Hu Friedy, Chicago, IL) and 1730-1calipers (Castroviejo, Schwert, Germany).
The following clinical measurements were taken at the facial aspect to the experimental teeth one week before the surgery and at 6 months follow up:
- recession depth (RD), measured from the CEJ to the most apical extension of the gingival margin;
- recession width (RW), measured at the level of the CEJ;
- clinical attachment level (CAL), measured from the CEJ to the bottom of the gingival sulcus;
- probing depth (PD), measured from the gingival margin to the bottom of the gingival sulcus.
The percentage of recession coverage was calculated according to the following formula: Recession coverage =([preoperative recession depth − postoperative recession depth]/preoperative recession depth) × 100.
Examiner Calibration
The investigator charged with clinical assessments was calibrated for intraexaminer repeatability prior to the start of the trial. Twelve patients with a total of 23 teeth with gingival recession were enrolled for this purpose. Duplicate measurements of CAL were collected with an interval of 24 h between the first and the second recording. The intraclass correlation coefficient, as a measure of intraexaminer reproducibility, was 0.99.
Treatment
The exposed root surfaces were planed with curettes to remove edges, grooves, and dental plaque and to reduce the convexity of the root. The area was gently irrigated with sterilized physiological saline solution. Relative isolation of the region was carried out with the aid of a cotton roll and the drying of the buccal surface with gauze. Lasing was performed in the test group, and the exposed root surface was conditioned with Nd:YAG laser, two times.
Lasing was performed using a Smarty A10 laser machine (Smarty A10, DEKA, Italy). Root surfaces in the test group were radiated by a laser beam of 1 W, 10 Hz, 60 s, 1064 nm with sweeping motion and without any coolants. In addition, the distance between the end of the fiber optic (diameter: 300 μm) hand piece and tooth surface was adjusted at about 2 mm. When the lasers were in use, protective eyewear of appropriate optical density was worn by the investigator and patients.
Before surgery, extraoral antisepsis was performed with 10% Povidone-Iodine solution (Glividon®, Bikar Drug Ltd., Istanbul, Turkey) and intraoral antisepsis with 0.12% chlorhexidine rinse (Kloroben®, Drogsan Drug Ltd., Istanbul, Turkey). All defects were treated by using the Langer and Langer technique.5 A local anesthetic (Ultracaine DS Forte®, Hoechst Roussel, Frankfurt, Germany) was administered to donor and recipient sites to achieve anesthesia. A partial thickness flap was created with two vertical incisions placed at least one-half to one tooth wider mesiodistally than the area of gingival recession. The coronal margin of the flap was started with a horizontal sulcular incision to preserve all existing facial gingiva. The proximal papillae were left intact. Care was taken to extend the flap to the mucobuccal fold without perforations that could affect the blood supply. The area was irrigated with sterile saline solution.
The connective tissue graft was harvested from the palate using the “trap-door” approach described by Harris.15 A connective tissue graft in an adequate size of 2 mm thickness was harvested, and pressure was applied to the donor area with gauze soaked in saline after the graft was taken. The donor area was closed with silk 4-0 sling sutures. The graft was trimmed with a sharp surgical blade, if necessary, and the SCTG was introduced to the recipient site, where the flap was pulled over a major portion of the SCTG. The recipient flap was then sutured directly over the graft with silk 5-0 sutures. The overlying flap covered the donor tissue as much as possible to provide more blood supply to the graft. The vertical incisions were also closed with silk 5-0 sutures. A mild compress with gauze soaked in saline was applied for 5 min. Dry foil was applied to the recipient area, and then a non-eugenol periodontal dressing (Coe-Pak, GC America, Alsip, IL) was placed over the dry foil to stabilize and protect the donor site for 14 days postsurgery. Techniques were performed with an interval of six weeks between surgeries.
Postsurgical Care
All patients were instructed to take antibiotics [amoxicillin (Remoxil®, I.E. Ulagay Drug Ltd., Istanbul, Turkey) 500 mg, every 8 h, 7 days] and analgesic medication [Naproxen (Apranax®, Abdi İbrahim Drug Ltd., Istanbul, Turkey) 550 mg, every 12 h, 5 days], to discontinue tooth-brushing around the surgical sites for the first 14 days after surgery, and an extraoral cold compress and bland diet were advised. During this period, plaque control was provided by rinsing with 0.12% chlorhexidine digluconate (Kloroben®, Drogsan Drug Ltd., Istanbul, Turkey) solution twice a day for 1 min. After this period, the sutures were removed. Plaque control in the surgically treated area was maintained by 0.12% chlorhexidine rinses for an additional 2 weeks, and gentle tooth-brushing with a soft-filamented toothbrush was permitted twice daily. The patients were instructed to perform a non-traumatic brushing technique (roll technique) using a standardized soft toothbrush and toothpaste during the trial.
All patients were recalled for prophylaxis 1 and 3 weeks after suture removal and, subsequently, monthly until the end of the study period. All participants completed the study and reported 100% compliance.
The initial therapy, the laser application, and the surgical treatments were performed by one investigator and the clinical measurements were assessed by another investigator.
Statistical analysis
The data thus collected were assessed using SPSS 11.0 statistical software (SPSS, Inc., Chicago, IL). The Wilcoxon's signed ranks test was chosen to compare test and control group differences in RD, RW, CAL, and PD. The differences in mean RD, RW, CAL, and PD values between baseline and six months were evaluated using Wilcoxon's signed ranks test. In addition, the Wilcoxon's signed ranks test was used to compare recession coverage (percentages and mm) between groups.
Results
The 34 experimental non-molar teeth were identified and randomly balanced into two groups. Seventeen test group teeth, including six incisors, four canines, seven premolars, and 17 control group teeth, including seven incisors, five canines, and five premolars, were subjected to one treatment modality in each group (Table 1).
Table 1.
Frequency Distributions of the Two Groups
|
Evaluation group |
Anterior absolute frequency |
Premolar absolute frequency |
||
|---|---|---|---|---|
| Gingival recession | Class 1 | Class 2 | Class 1 | Class 2 |
| Test group | 4 | 6 | 3 | 4 |
| Control group | 7 | 5 | 3 | 2 |
Following the initial oral hygiene phase as well as at the post-treatment examinations, all subjects showed low frequencies of plaque harboring tooth surfaces (PI < 20%) and bleeding gingival units (GI < 15%), indicating a good standard of supragingival plaque control during the study period.
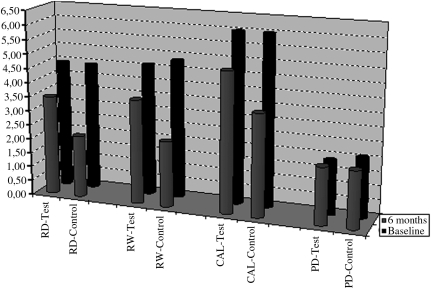
The statistical analyses for the clinical parameters at baseline and after six months for both groups are presented in Table 2 and Fig. 1.
Table 2.
Clinical Parameters (in mm) at Baseline and 6 Months Postoperatively
| Parameters | Test group | Control group | p-Value |
|---|---|---|---|
| Recession depth (RD) | |||
| baseline | 3.29 ± 1.18 | 3.42 ± 1.04 | >0.05 |
| six months | 2.25 ± 1.23* | 0.83 ± 1.34** | <0.05 |
| Recession width (RW) | |||
| baseline | 3.42 ± 1.19 | 3.58 ± 1.26 | >0.05 |
| six months | 2.33 ± 1.31** | 0.92 ± 1.38** | <0.05 |
| Clinical attachment Level (CAL) | |||
| baseline | 4.67 ± 1.33 | 4.88 ± 1.12 | >0.05 |
| six months | 3.75 ± 1.16** | 2.33 ± 1.25** | <0.01 |
| Probing depth (PD) | |||
| baseline | 1.38 ± 0.51 | 1.46 ± 0.63 | >0.05 |
| six months | 1.50 ± 0.50 | 1.50 ± 0.50 | >0.05 |
p < 0.05, **p < 0.01, significant differences between baseline and six months.
FIG. 1.
Clinical Parameters (in mm) at baseline and six months postoperatively.
No statistically significant differences between groups were observed for any of the clinical parameters at baseline (p > 0.05). In comparison, at 6 months, statistically significant differences were found between the test and control groups for RD, RW, and CAL. A mean RD of 2.25 ± 1.23 mm was calculated for cases in the test group, and the corresponding value was 0.83 ± 1.34 mm for cases in the control group (p < 0.05). A mean RW of 2.33 ± 1.31 mm was calculated for cases in the test group, and the corresponding value was 0.92 ± 1.38 mm for cases in the control group (p < 0.05). A mean CAL of 3.75 ± 1.16 mm was calculated for cases in the test group, and the corresponding value was 2.33 ±1.25 mm for cases in the control group (p < 0.01).
In the test group, statistically significant changes from baseline were found for RD, RW, and CAL. RD decreased from 3.29 ± 1.18 to 2.25 ± 1.23 mm (p < 0.05), RW decreased from 3.42 ± 1.19 to 2.33 ± 1.31 mm (p < 0.01), and CAL decreased from 4.67 ± 1.33 to 3.75 ± 1.16 mm (p < 0.01).
In the control group, statistically significant changes from baseline were found for RD, RW, and CAL. RD decreased from 3.42 ± 1.04 to 0.83 ± 1.34 mm, RW decreased from 3.58 ± 1.26 to 0.92 ± 1.38 mm, and CAL decreased from 4.88 ± 1.12 to 2.33 ± 1.25 mm (p < 0.01).
Probing depths did not show a statistically significant change after treatment in both groups (p > 0.05). PD values were 1.38 ± 0.51 mm, 1.46 ± 0.63 mm at baseline, and 1.50 ±0.50 and 1.50 ± 0.50 mm at 6 months in the test and control groups, respectively.
The recession coverage at six months postoperatively for the test and the control groups are shown in Table 3.
Table 3.
Recession Coverage at 6 Months Postoperatively
| Test group | Control group | p-Value | |
|---|---|---|---|
| Recession coverage (mm) | 1.04 ± 1.03 | 2.58 ± 1.50 | <0.05 |
| Recession coverage (%) | 32.49 ± 36.01 | 76.67 ± 35.85 | <0.05 |
The mean recession coverage value for the test group was 1.04 ± 1.03 mm and 32.49% ± 36.01%. The corresponding value for the control group was 2.58 ± 1.50 mm and 76.67% ± 35.85%. These values were statistically different (p < 0.05).
Complete recession coverage was accomplished in 17.6% (3 of 17) of the treated cases in the test group and in 64.7% (11 of 17) of the treated cases in the control group (Table 4).
Table 4.
Frequency of Recession Coverage in Test and Control Groups
| Treatment | 100% | 99–50% | 49–0% |
| Test group | 3 (17.6%) | 5 (29.4%) | 9 (52.9%) |
| Control group | 11 (64.7%) | 3 (17.6%) | 3 (17.6%) |
Discussion
The aim of this randomized-controlled, split-mouth, double-blind designed clinical trial was to evaluate and compare the outcome of gingival recession therapy using the SCTG with or without Nd:YAG laser application for root surface biomodification. Data from the present study indicated that Nd:YAG laser use negatively affected recession coverage with SCTG. The test group had lower percentages of recession coverage (33%) and complete root coverage (18%) compared with the control group (77% and 65%, respectively). Both of these results compare well with those of others. Chambrone et al.,1 Oates et al.,3 and Roccuzzo et al.4 have reported that an average mean root coverage in 64.7–97.3% and complete root coverage in 18.1–96.1% of the studies when SCTG was used to obtain root coverage.
Patients with gingival recessions who complain of esthetic concerns and hypersensitivity are possible candidates for root coverage procedures. Before performing periodontal plastic surgery, the clinicians should select the most predictable way to achieve successful root coverage. Although the more accepted techniques were free gingival grafts and various pedicle grafts during the 1960s and 1970s,6–9 SCTGs began to be used to enhance covering of localized areas of root exposure in the early 1980s.5 Different flap procedures further modified this technique.16–18 Longitudinal observations and case studies have shown a high success rate and predictability.1–4 To fulfill patients' esthetic requirements and to obtain successful root coverage, surgical techniques with SCTG continued to be improved.11–18 In the present study, SCTG was therefore chosen for treatment of gingival recessions.
Complete root coverage is considered the true goal of treatment because only complete coverage assures recovery from the hypersensitivity and esthetic defects associated with recession areas.1–4 Previous studies have tried to improve the percentages of complete coverage with root surface biomodification. Root surface conditioning has been introduced, using a variety of agents, in order to detoxify, decontaminate, and demineralize the root surface, thereby removing the smear layer and exposing the collagenous matrix of dentin and cement.19–28 However, the literature is controversial with respect to root conditioning. The results of some studies have demonstrated that the conditioned root surfaces had a higher percentage of complete root coverage compared with sites not treated with root conditioning agents.21–23 Conversely, however, the results of other studies have shown no significant clinical benefit from root conditioning in conjunction with root coverage procedures.24,26,27 The results of the present study for the control group (non-conditioned root surfaces) are in agreement with the results of the latter studies. Our study showed no clinical benefit from root conditioning with Nd:YAG laser.
So far, no published data are available concerning the clinical outcomes following root surface biomodification with Nd:YAG laser for the treatment of gingival recession, whereas the use of lasers has often been propagated for this indication. In previous clinical studies, it has been demonstrated that lasers are an effective tool in the field of root conditioning to improve the outcome of regenerative periodontal therapies by favoring the attachment of the regenerated periodontal structures.32–43
The Nd:YAG laser has been reported to remove the smear layer, uncovering dentinal tubules and exposing collagen fibers on root surfaces.37,38 The Nd:YAG laser, with non-contact delivery mode and short irradiation time, has caused no damage to or alteration of root surfaces.37 In addition, Tewfik et al.39 reported effects of Nd:YAG laser on root cementum topography and fibroblastic attachment, and suggested that the modification of cementum surfaces depends on the energy level of the laser irradiation. Similar results have also been found with CO2 and Er:YAG laser.32–34,40–43
In contrast to the favorable effects of laser application found in the above studies, several studies have reported negative effects of the Nd:YAG laser when used directly on root surfaces due to carbonization and melting effects. The Nd:YAG laser application reported by some investigators exhibited, under SEM analysis, surface alterations such as charring, crater formation, and cement meltdown.38,46–54 Studies with Nd:YAG laser using different parameters47,48 have shown that mean power settings ranging between 1.25–3.00 W promote a root surface change leading to fusion and resolidification of the cement mineral portion alongside crack and fissure formation. Thomas et al.46 reported that the Nd:YAG laser denatures root surface proteins, and they suggest that the complement of the conventional mechanical treatment of the root surface would be important on the reduction of these irregularities aiming for a more biocompatible root surface. Meanwhile, Trylovich et al.49 found that Nd:YAG laser application modifies biocompatibility of root surfaces and reduces the number of attached fibroblasts in comparison with untreated controls. Their study suggested that Nd:YAG laser could alter the biocompatibility of the cementum surface, making it unfavorable for fibroblast attachment. The laser parameters used in the present study (1 W, 10 Hz, 100 mJ) were based on some literature studies,47,48 which demonstrated that the Nd:YAG laser, when used with mean power up to 2 W, promoted minimal root surface hazard effects.
The results of the present study demonstrate that root surface conditioning with Nd:YAG laser has no additional clinical benefit when compared with root planing alone with regard to the CAL, RD, and RW. In the control group (non-conditioning with laser), the mean CAL, RD, and RW values showed decreases from baseline to six months, and these changes were statistically significant. Caffessse et al.24 and Bouchard et al.25 compared the SCTG in conjunction with citric acid to the SCTG where no root conditioning was performed, and reported that root conditioning did not affect the clinical outcome of the surgical technique. Our findings are in accord with these reports. However, there are differences between the results seen in the present study, which found no improvements after Nd:YAG laser irradiation, and other studies with opposite results. Perhaps this was due to the Nd:YAG laser's ability to alter the biocompatibility of the root surface, which might inhibit cell migration, making it unfavorable for fibroblast attachment, leading to changes in the dentin's protein structure. In addition, Nd:YAG laser irradiation might have a negative influence on the proliferation of periodontal ligament fibroblasts and subsequently on periodontal wound healing.
The laser parameters affecting the amount of energy applied to a given surface include power level (W), exposure time (seconds), pulsed versus continuous wave energy, energy density (J/cm2), distance from the surface, and the angle between the target tissue and the fiber tip. As a laser beam strikes a target tissue surface, the light energy can be affected in four ways: it can be reflected, transmitted, absorbed, or scattered, and the changes seen in target tissues are largely due to the absorbed energy.54 Therefore, the most important issue in laser therapy is to determine the correct parameters to use to achieve satisfactory results, without inducing detrimental thermal effects in the pulp, or causing fracturing or carbonization.
Conclusions
The present study suggests that surgical treatment of gingival recessions with SCTG result in satisfactory root coverage and attachment gain. The most significant and interesting finding of the present study is that the use of Nd:YAG laser as a root surface biomodifier material negatively affected the outcome of root coverage with the SCTG. Further comparative studies are needed in order to clarify this issue and to evaluate the long-term effects of this type of therapy.
Acknowledgments
The investigation was supported by Grant (PN-2005/243) from Atatürk University, Turkey. We would like to thank Dr Erkan Oktay for statistical assistance.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Chambrone L. Chambrone D. Pustiglioni F.E. Chambrone L.A. Lima L.A. Can subepithelial connective tissue grafts be considered the gold standard procedure in the treatment of Miller Class I and II recession-type defects? J. Dent. 2008;36:659–671. doi: 10.1016/j.jdent.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 2.Mariotti A. Efficacy of chemical root surface modifiers in the treatment of periodontal disease. A systematic review. Ann. Periodontol. 2003;8:205–226. doi: 10.1902/annals.2003.8.1.205. [DOI] [PubMed] [Google Scholar]
- 3.Oates T.W. Robinson M. Gunsolley J.C. Surgical therapies for the treatment of gingival recession A systematic review. Ann. Periodontol. 2003;8:303–320. doi: 10.1902/annals.2003.8.1.303. [DOI] [PubMed] [Google Scholar]
- 4.Roccuzzo M. Bunino M. Needleman I. Sanz M. Periodontal plastic surgery for treatment of localized gingival recessions: a systematic review. J. Clin. Periodontol. 2002;29:178–194. doi: 10.1034/j.1600-051x.29.s3.11.x. [DOI] [PubMed] [Google Scholar]
- 5.Langer B. Langer L. Subepithelial connective tissue graft technique for root coverage. J. Periodontol. 1985;56:715–720. doi: 10.1902/jop.1985.56.12.715. [DOI] [PubMed] [Google Scholar]
- 6.Miller P.D., Jr. Root coverage using a free soft tissue autograft following citric acid application Part 1: Technique. Int. J. Periodontics Restorative Dent. 1982;2:65–70. [PubMed] [Google Scholar]
- 7.Grupe H. Warren R.J. Repair of gingival defects by a sliding flap operation. J. Periodontol. 1956;27:290–295. [Google Scholar]
- 8.Bernimoulin J.P. Luscher B. Muhlemann H.R. Coronally repositioned periodontal Flap. J. Clin. Periodontol. 1968;39:65–67. doi: 10.1111/j.1600-051x.1975.tb01721.x. [DOI] [PubMed] [Google Scholar]
- 9.Cohen D.W. Ross S.E. The double papillae repositioned flap in periodontal therapy. J. Periodontol. 1968;39:65–70. doi: 10.1902/jop.1968.39.2.65. [DOI] [PubMed] [Google Scholar]
- 10.Pini Prato G. Tinti C. Vincenzi G. Magnani C. Cortellini P. Clauser C. Guided tissue regeneration versus mucogingival surgery in the treatment of human buccal gingival recession. J. Periodontol. 1992;63:919–928. doi: 10.1902/jop.1992.63.11.919. [DOI] [PubMed] [Google Scholar]
- 11.Tal H. Moses O. Zohar R. Meir H. Nemcovsky C. Root coverage of advanced gingival recession: A comparative study between acellular dermal matrix allograft and subepithelial connective tissue grafts. J. Periodontol. 2002;73:1405–1411. doi: 10.1902/jop.2002.73.12.1405. [DOI] [PubMed] [Google Scholar]
- 12.Han J.S. John V. Blanchard S.B. Kowolik M.J. Eckert G.J. Changes in gingival dimensions following connective tissue grafts for root coverage: comparison of two procedures. J. Periodontol. 2008;79:1346–1354. doi: 10.1902/jop.2008.070472. [DOI] [PubMed] [Google Scholar]
- 13.Griffin T.J. Cheung W.S. Zavras A.I. Damoulis P.D. Postoperative complications following gingival augmentation procedures. J. Periodontol. 2006;77:2070–2079. doi: 10.1902/jop.2006.050296. [DOI] [PubMed] [Google Scholar]
- 14.Harris R.J. Root coverage with connective tissue grafts: An evaluation of short- and long-term results. J. Periodontol. 2002;73:1054–1059. doi: 10.1902/jop.2002.73.9.1054. [DOI] [PubMed] [Google Scholar]
- 15.Harris R. The connective tissue and partial thickness double pedicle graft: a predictable method of obtaining root coverage. J. Periodontol. 1992;63:477–486. doi: 10.1902/jop.1992.63.5.477. [DOI] [PubMed] [Google Scholar]
- 16.Allen A.L. Use of the supraperiosteal envelope in soft tissue grafting for root coverage I. Rationale and technique. Int. J. Periodontics Restorative Dent. 1994;14:216–227. [PubMed] [Google Scholar]
- 17.Raetzke P.B. Covering localized areas of root exposure employing the “envelope” technique. J. Periodontol. 1985;56:397–402. doi: 10.1902/jop.1985.56.7.397. [DOI] [PubMed] [Google Scholar]
- 18.Blanes R.J. Allen E.P. The bilateral pedicle flap-tunnel technique: a new approach to cover connective tissue grafts. Int. J. Periodontics Restorative Dent. 1999;19:471–479. [PubMed] [Google Scholar]
- 19.Blomlöf J.P. Blomlöf L.B. Lindskog S.F. Smear removal and collagen exposure after non-surgical root planing followed by etching with an EDTA gel preparation. J. Periodontol. 1996;67:841–845. doi: 10.1902/jop.1996.67.9.841. [DOI] [PubMed] [Google Scholar]
- 20.Polson A.M. Frederick G.T. Ladenheim S. Hanes P.J. The production of a root surface smear layer by instrumentation and its removal by citric acid. J. Periodontol. 1984;55:443–446. doi: 10.1902/jop.1984.55.8.443. [DOI] [PubMed] [Google Scholar]
- 21.Common J. McFall W.T., Jr. The effects of citric acid on attachment of laterally positioned flaps. J. Periodontol. 1983;54:9–18. doi: 10.1902/jop.1983.54.1.9. [DOI] [PubMed] [Google Scholar]
- 22.Miller P.D., Jr. Root coverage using the free soft tissue autograft following citric acid application III. A successful and predictable procedure in areas of deep-wide recession. Int. J. Periodontics Restorative Dent. 1985;5:14–37. [PubMed] [Google Scholar]
- 23.Tolmie P.N. Rubins R.P. Buck G.S. Vagianos V. Lanz J.C. The predictability of root coverage by way of free gingival autografts and citric acid application: an evaluation by multiple clinicians. Int. J. Periodontics Restorative Dent. 1991;11:261–271. [PubMed] [Google Scholar]
- 24.Caffesse R.G. De LaRosa M. Garza M. Munne-Travers A. Mondragon J.C. Weltman R. Citric acid demineralization and subepithelial connective tissue grafts. J. Periodontol. 2000;71:568–572. doi: 10.1902/jop.2000.71.4.568. [DOI] [PubMed] [Google Scholar]
- 25.Bouchard P. Etienne D. Ouhayoun J.P. Nilvéus R. Subepithelial connective tissue grafts in the treatment of gingival recessions A comparative study of 2 procedures. J. Periodontol. 1994;65:929–936. doi: 10.1902/jop.1994.65.10.929. [DOI] [PubMed] [Google Scholar]
- 26.Bouchard P. Nilveus R. Etienne D. Clinical evaluation of tetracycline HCl conditioning in the treatment of gingival recessions A comparative study. J. Periodontol. 1997;68:262–269. doi: 10.1902/jop.1997.68.3.262. [DOI] [PubMed] [Google Scholar]
- 27.Bittencourt S. Ribeiro E.P. Sallum E.A. Sallum A.W. Nociti F.H., Jr Casati M.Z. Root surface biomodification with EDTA for the treatment of gingival recession with a semilunar coronally repositioned flap. J. Periodontol. 2007;78:1695–1701. doi: 10.1902/jop.2007.060507. [DOI] [PubMed] [Google Scholar]
- 28.Héritier M. Effects of phosphoric acid on root dentin surface A scanning and transmission electron microscopic study. J. Periodontal. Res. 1984;19:168–176. doi: 10.1111/j.1600-0765.1984.tb00805.x. [DOI] [PubMed] [Google Scholar]
- 29.Sallum E.A. Casati M.Z. Caffesse R.G. Funis L.P. Nociti J.F.H. Sallum A.W. Coronally positioned flap with or without enamel matrix protein derivative for the treatment of gingival recessions. Am. J. Dent. 2003;16:287–291. [PubMed] [Google Scholar]
- 30.Lacoste E. Martineau I. Gagnon G. Platelet concentrates: effects of calcium and thrombin on endothelial cell proliferation and growth factor release. J. Periodontol. 2003;74:1498–1507. doi: 10.1902/jop.2003.74.10.1498. [DOI] [PubMed] [Google Scholar]
- 31.Abitbol T. Settembrini L. Santi E. Scherer W. Root surface biomodification using a dentin bonding conditioner. Periodontal Clin. Investig. 1996;18:27–30. [PubMed] [Google Scholar]
- 32.Misra V. Mehrotra K.K. Dixit J. Maitra S.C. Effect of a carbon dioxide laser on periodontally involved root surfaces. J. Periodontol. 1999;70:1046–1052. doi: 10.1902/jop.1999.70.9.1046. [DOI] [PubMed] [Google Scholar]
- 33.Crespi R. Barone A. Covani U. Ciaglia R.N. Romanos G.E. Effects of CO2 laser treatment on fibroblast attachment to root surfaces A scanning electron microscopy analysis. J. Periodontol. 2002;73:1308–1312. doi: 10.1902/jop.2002.73.11.1308. [DOI] [PubMed] [Google Scholar]
- 34.Barone A. Covani U. Crespi R. Romanos G.E. Root surface morphological changes after focused versus defocused CO2 laser irradiation: a scanning electron microscopy analysis. J. Periodontol. 2002;73:370–373. doi: 10.1902/jop.2002.73.4.370. [DOI] [PubMed] [Google Scholar]
- 35.Pant V. Dixit J. Agrawal A.K. Seth P.K. Pant A.B. Behavior of human periodontal ligament cells on CO2 laser irradiated dentinal root surfaces: an in vitro study. J. Periodontal Res. 2004;39:373–379. doi: 10.1111/j.1600-0765.2004.00751.x. [DOI] [PubMed] [Google Scholar]
- 36.Israel M. Cobb C.M. Rossmann J.A. Spencer P. The effects of CO2, Nd:YAG and Er:YAG lasers with and without surface coolant on tooth root surfaces. An in vitro study. J. Clin. Periodontol. 1997;24:595–602. doi: 10.1111/j.1600-051x.1997.tb00235.x. [DOI] [PubMed] [Google Scholar]
- 37.Ito K. Nishikata J. Murai S. Effects of Nd:YAG laser radiation on removal of a root surface smear layer after root planing: a scanning electron microscopic study. J. Periodontol. 1993;64:547–552. doi: 10.1902/jop.1993.64.6.547. [DOI] [PubMed] [Google Scholar]
- 38.Wilder-Smith P. Arrastia A.M. Schell M.J. Liaw L.H. Grill G. Berns M.W. Effect of ND:YAG laser irradiation and root planing on the root surface: structural and thermal effects. J. Periodontol. 1995;66:1032–1039. doi: 10.1902/jop.1995.66.12.1032. [DOI] [PubMed] [Google Scholar]
- 39.Tewfik H.M. Garnick J.J. Schuster G.S. Sharawy M.M. Structural and functional changes of cementum surface following exposure to a modified Nd:YAG laser. J. Periodontol. 1994;65:297–302. doi: 10.1902/jop.1994.65.4.297. [DOI] [PubMed] [Google Scholar]
- 40.Schwarz F. Aoki A. Sculean A. Georg T. Scherbaum W. Becker J. In vivo effects of an Er:YAG laser, an ultrasonic system and scaling and root planing on the biocompatibility of periodontally diseased root surfaces in cultures of human PDL fibroblasts. Lasers Surg. Med. 2003;33:140–147. doi: 10.1002/lsm.10201. [DOI] [PubMed] [Google Scholar]
- 41.Schwarz F. Sculean A. Berakdar M. Szathmari L. Georg T. Becker J. In vivo and in vitro effects of an Er:YAG laser, a GaAlAs diode laser, and scaling and root planing on periodontally diseased root surfaces: a comparative histologic study. Lasers Surg. Med. 2003;32:359–366. doi: 10.1002/lsm.10179. [DOI] [PubMed] [Google Scholar]
- 42.Folwaczny M. Mehl A. Haffner C. Benz C. Hickel R. Root substance removal with Er:YAG laser radiation at different parameters using a new delivery system. J. Periodontol. 2000;71:147–155. doi: 10.1902/jop.2000.71.2.147. [DOI] [PubMed] [Google Scholar]
- 43.Yamaguchi H. Kobayashi K. Osada R., et al. Effects of irradiation of an erbium:YAG laser on root surfaces. J. Periodontol. 1997;68:1151–1155. doi: 10.1902/jop.1997.68.12.1151. [DOI] [PubMed] [Google Scholar]
- 44.Miller P.D., Jr. A classification of marginal tissue recession. Int. J. Periodontics Restorative Dent. 1985;5:8–13. [PubMed] [Google Scholar]
- 45.O'Leary T.J. Drake R.B. Naylor J.E. The plaque control record. J. Periodontol. 1972;43:38. doi: 10.1902/jop.1972.43.1.38. [DOI] [PubMed] [Google Scholar]
- 46.Thomas D. Rapley J. Cobb C. Spencer P. Killoy W. Effects of the Nd:YAG laser and combined treatments on in vitro fibroblast attachment to root surfaces. J. Clin. Periodontol. 1994;21:38–44. doi: 10.1111/j.1600-051x.1994.tb00274.x. [DOI] [PubMed] [Google Scholar]
- 47.Morlock B.J. Pippin D.J. Cobb C.M. Killoy W.J. Rapley J.W. The effect of Nd:YAG laser exposure on root surfaces when used as an adjunct to root planing: an in vitro study. J. Periodontol. 1992;63:637–641. doi: 10.1902/jop.1992.63.7.637. [DOI] [PubMed] [Google Scholar]
- 48.Cobb C.M. McCawley T.K. Killoy W.J. A preliminary study on the effects of the Nd:YAG laser on root surfaces and subgingival microflora in vivo. J. Periodontol. 1992;63:701–707. doi: 10.1902/jop.1992.63.8.701. [DOI] [PubMed] [Google Scholar]
- 49.Trylovich D.J. Cobb C.M. Pippin D.J. Spencer P. Killoy W.J. The effects of the Nd:YAG laser on in vitro fibroblast attachment to endotoxin-treated root surfaces. J. Periodontol. 1992;63:626–632. doi: 10.1902/jop.1992.63.7.626. [DOI] [PubMed] [Google Scholar]
- 50.Naylor F. Aranha A.C. Eduardo Cde.P. Arana-Chavez V.E. Sobral M.A. Micromorphological analysis of dentinal structure after irradiation with Nd:YAG laser and immersion in acidic beverages. Photomed. Laser Surg. 2006;24:745–752. doi: 10.1089/pho.2006.24.745. [DOI] [PubMed] [Google Scholar]
- 51.Aranha A.C. Domingues F.B. Franco V.O. Gutknecht N. Eduardo Cde.P. Effects of Er:YAG and Nd:YAG lasers on dentin permeability in root surfaces: a preliminary in vitro study. Photomed. Laser Surg. 2005;23:504–508. doi: 10.1089/pho.2005.23.504. [DOI] [PubMed] [Google Scholar]
- 52.de Magalhães M.F. Matson E. de Rossi W. Alves J.B. A morphological in vitro study of the effects of Nd:YAG laser on irradiated cervical dentin. Photomed. Laser Surg. 2004;22:527–532. doi: 10.1089/pho.2004.22.527. [DOI] [PubMed] [Google Scholar]
- 53.Lee B.S. Lin C.P. Lin F.H. Lan W.H. Ultrastructural changes of human dentin after irradiation by Nd:YAG laser. Lasers Surg. Med. 2002;30:246–252. doi: 10.1002/lsm.10038. [DOI] [PubMed] [Google Scholar]
- 54.Kimura Y. Wilder-Smith P. Yonaga K. Matsumoto K. Treatment of dentine hypersensitivity by lasers: a review. J. Clin. Periodontol. 2000;27:715–721. doi: 10.1034/j.1600-051x.2000.027010715.x. [DOI] [PubMed] [Google Scholar]