Abstract
Chronic memory deficits are a major cause of morbidity following traumatic brain injury (TBI). In the rat, the hippocampal theta rhythm is a well-studied correlate of memory function. This study sought to investigate disturbances in hippocampal theta rhythm following lateral fluid percussion injury in the rat. A total of 13 control rats and 12 TBI rats were used. Electrodes were implanted in bilateral hippocampi and an electroencephalogram (EEG) was recorded while the rats explored a new environment, and also while navigating a modified version of the Barnes maze. Theta power and peak theta frequency were significantly attenuated in the injured animals. Further, injured rats were less likely to develop a spatial strategy for Barnes maze navigation compared to control rats. In conclusion, rats sustaining lateral fluid percussion injury demonstrated deficits in hippocampal theta activity. These deficits may contribute to the underlying memory problems seen in chronic TBI.
Key words: electroencephalogram, fluid percussion injury, memory, rat, theta rhythm, traumatic brain injury
Introduction
Long-term cognitive deficits are a major sequela of traumatic brain injury (TBI). Even relatively minor trauma can lead to persistent problems, especially with memory (Kraus and Chu, 2005). The most common complaints and the observed deficits following mild TBI involve impaired working memory (McAllister et al., 2006). Although the cellular and molecular disturbances in the early phases of TBI have been extensively studied, the electrophysiological changes seen following chronic injury remain less well explored. Specifically, disturbances in the patterns of electrophysiological activity and their relationship to memory function require further investigation.
In healthy rats, the hippocampal theta rhythm is a well-known electrophysiological correlate of memory processing, defined as a regular 5- to 12-Hz electroencephalogram (EEG) pattern recorded in the hippocampus, and maintained by activity of interneurons in both CA1 and CA3 (Goutagny et al., 2009; Green and Rawlins, 1979; Winson, 1974). Classically, theta rhythm has been divided into two major types, types I and II. Type I is resistant to atropine and is associated with movement and exploratory activity. Type II is sensitive to atropine, is present under urethane anesthesia, and is associated with a quiet alert state in the behaving animal (Bland, 1986). However, the actual electrophysiological correlates and neurochemical substrates of the theta rhythm are more complex, involving several neurotransmitters, including acetylcholine and GABA, as well as glutamate. Theta is also regulated by various inputs to the hippocampus, mainly from the medial septum, but also from the diagonal band of Broca and the entorhinal cortex (Buzsaki, 2002). Disturbance in theta results in decreased performance on a variety of spatial and working memory tasks (Givens and Olton, 1994; McNaughton et al., 2006; Olton and Markowska, 1994). Several studies have also linked the theta rhythm to both long-term potentiation (LTP) and long-term depression (Diamond et al., 1988; Hyman et al., 2003; Otto et al., 1991), with the firing of hippocampal pyramidal cells shown to be synchronized with phases of the theta rhythm (Brazhnik and Fox, 1999; Manns et al., 2007).
Since the initial studies of the 1970s linking theta rhythm to memory, dozens of investigations have explored the relationship between theta, spatial navigation, and memory (Buzsaki, 2005;Olvera-Cortes et al., 2002, 2004; Wiener et al., 1989). Overall, this dominant hippocampal rhythm appears to be crucial to the oscillatory nature of the hippocampus and its function (Pike et al., 2000). There is also evidence from human studies indicating that hippocampal theta rhythm is associated with spatial memory processing (Basar-Eroglu and Demiralp, 2001; Kahana et al., 1999; Rizzuto et al., 2003).
Lateral fluid percussion (LFP) injury, a widely used rodent model of human TBI, reliably produces deficits in both working and spatial memory (Thompson et al., 2005). Injury affects both hippocampal-dependent place learning, as well as hippocampal-independent cue learning (Bramlett et al., 1997). Electrophysiological studies have also found reduced hippocampal LTP in vivo in rats following TBI (D'Ambrosio et al., 1998; Miyazaki et al., 1992; Sanders et al., 2000). Some studies have documented that such deficits in rodents can persist even months after the initial injury, mirroring the long-term cognitive deficits seen after human TBI (Pierce et al., 1998).
The present study investigated chronic electrophysiological alterations in hippocampal theta activity and spatial memory following LFP injury in rats. We hypothesized that the hippocampal theta rhythm would be fundamentally altered following TBI, and sought to characterize these changes in awake, behaving animals. Specifically, we predicted that theta would be attenuated in tasks involving exploratory activity and spatial memory.
Methods
Subjects
Twenty-five adult 10-week-old male Harlan Sprague-Dawley rats were obtained (Harlan, Indianapolis, IN) and housed at the University of California–Davis vivarium. The animals were housed under 12-hour light-dark cycles with standard lab chow and water available ad libitum. The animals were allowed to acclimate to the laboratory environment for at least 7 days prior to the start of the experiment and were handled daily for 5 min. The animals were divided into two groups: a control group (n = 13) consisting of 8 sham-injury controls plus 5 experimentally-naïve rats, and an LFP injury TBI group (n = 12). Average weight at the beginning of the experiments was 316 g (301–340 g), and did not differ significantly between treatment groups. All procedures conformed to National Institutes of Health guidelines and were approved by the University of California–Davis Institutional Animal Care and Use Committee.
Lateral fluid percussion injury
TBI was produced using the fluid percussion model of brain injury (Dixon et al., 1987), with lateral orientation (McIntosh et al., 1989) as previously described (Folkerts et al., 2007). The rats were anesthetized with 4% isoflurane in a 2:1 N2O:O2 carrier gas. After induction of anesthesia the rats were intubated and mechanically ventilated with 2% isoflurane in 2:1 N2O/O2 for the remainder of the surgical procedures. Core temperature was maintained at 37.5 ± 0.5°C by a feedback temperature controller. A midline incision was made in the scalp and a 4.8-mm craniotomy was performed midway between the lambda and the bregma 3.0 mm to the right of the central suture. A modified Luer-lock connector 2.6 mm in inner diameter was secured to the craniotomy with cyanoacrylate adhesive and dental acrylic. Moderate TBI (2.16 ± 0.02 atm) was produced by rapidly injecting a small volume of saline into the closed cranial cavity (over the right ipsilateral hemisphere) with a fluid percussion device (VCU Biomedical Engineering, Richmond, VA). The animal was removed from the device immediately after injury, and anesthesia was maintained for 5 min after TBI, while the acrylic cap was removed and the incision sutured. Each animal was ventilated on room air without isoflurane until spontaneous breathing resumed, and was closely evaluated for neurological recovery. Duration of suppression of the righting reflex was measured and used as an index of injury magnitude (i.e., longer recovery was associated with more severe injury).
The sham-injury control group received the same surgical procedures as the LFP injury group, including attachment to the fluid percussion device, but without induction of the fluid percussion pulse. The five naïve control animals did not undergo any surgical procedures.
Electrode implantation
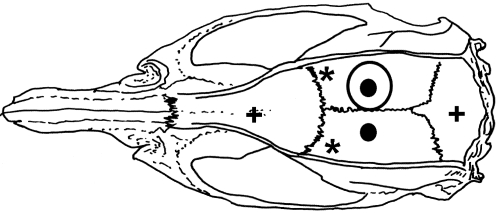
All 25 animals underwent additional surgery for bilateral implantation of bipolar recording electrodes in the dorsal hippocampus. A minimum of 1 month of post-surgical recovery elapsed between the TBI or sham procedures and subsequent electrode placement. The animals were anesthetized, intubated, and mechanically ventilated as described above. Using a stereotaxic device (David Kopf Instruments, Tujunga, CA), the rats had bipolar recording electrodes implanted into bilateral dorsal hippocampi. The electrodes were 0.23-mm bipolar, twisted wire electrodes (MS333/2/3; Plastic Products, Roanoke, VA), with one tip 1.0 mm shorter than the other, and a tip separation of 0.5 mm. The electrodes were positioned so that the shorter tip was located in the CA1 subregion (i.e., superficial electrode), and the longer tip (i.e., deep electrode) was positioned in the molecular layer of the dentate gyrus. Thus the electrode tips spanned the hippocampal fissure to maximize the amplitude of the theta signal, as previously described (McNaughton et al., 2006). The stereotaxic coordinates used for electrode implantation were 4.0 mm posterior to the bregma, 2.5 mm lateral to the midline, and 3.0 mm and 4.0 mm ventral to the skull surface, based on a stereotaxic atlas (Paxinos and Watson, 1998). Each bipolar electrode was grounded to the dural surface in its respective hemisphere by a stainless steel ground screw secured into the skull. The electrode assembly was attached to the skull surface with dental acrylic and 2 metal skull screws. The electrode and reference configurations are shown in Figure 1.
FIG. 1.
Positions of electrodes on the rat's skull. The large open circle shows the craniectomy defect for the lateral fluid percussion injury. The small dark circles show where the recording electrodes where implanted. The stars represent the dural reference leads. The plus signs illustrate where small screws where placed to fix the electrodes and acrylic to the skull.
EEG recordings
After a minimum of 2 weeks of post-surgical recovery from electrode placement, each animal was removed from its home cage and placed in an unfamiliar plastic bin. The bin was an opaque plastic box 25 × 45 cm at the base and 50 cm in height. It is referred to as the “New Environment.” The rat was left there for 6 min for initial acclimation, and then returned to its home cage. The plastic bin was thoroughly cleaned with 70% ethyl alcohol spray between and after each use. After 10 min in its home cage it was placed back in the plastic bin for another 6 min, during which time a continuous EEG was recorded (model 7P5; Grass Instrument Co., Quincy, MA), and digitized at 120 Hz. EEG data were filtered between 0.3 and 30 Hz and stored on a computer (PolyView software; Grass Instruments). Baseline electrophysiological activity was recorded from each of the four tips of the recording electrodes within the hippocampus. Specifically, recordings were obtained from the ipsilateral and contralateral CA1 and dentate gyrus and referenced to ground. Digital video recordings of the behavior of the rats in the plastic bin were made while theta activity was simultaneously recorded in the hippocampal EEG. After each recording session the animal was returned to its home cage.
After a minimum of 1 week, the animals were again placed in the plastic bin under dark conditions (i.e., with all room lights turned off). EEG and video recordings were made as the rats explored the plastic bin in the dark for 4 min. This “Dark Environment” condition was used so that hippocampal EEG activity could be recorded in the dark, when maximal exploratory activity occurs.
Barnes maze
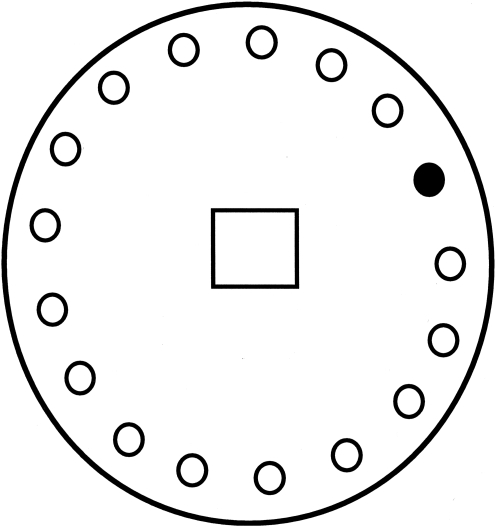
An average of 12 weeks (range: 10–19 weeks) after TBI or sham-injury, the rats were tested for spatial memory performance on a modified version of the Barnes maze (Barnes, 1979). The maze consisted of a flat, white circular platform (122 cm diameter) with 18 circular holes (9.2 cm diameter) equally spaced along the periphery. The platform was elevated 1 meter off of the floor. A dark escape compartment was located underneath one of the small circular openings. An overhead light (150 watts) brightly illuminated the surface of the platform in order to give greater incentive for escape into the dark compartment. Each animal was removed from its home cage, placed in the center of the maze, and initially covered by a black box for 60 sec. The box was then lifted and the time to find the escape hole and enter the dark chamber below was measured (Fig. 2). The animals were given a maximum of 5 min to find the correct opening leading to the escape compartment, after which they were placed inside the escape compartment by the experimenter for 30 sec. Two training trials were run each day, and the animals were placed back in their home cages for 2 min between trials. The position of the escape compartment was kept constant for the first 3 days. On the fourth day, the escape compartment was rotated 135 degrees from its original place for both trials on that day. Continuous EEG activity and video recordings of maze performance were recorded for all trials.
FIG. 2.
The modified Barnes maze. The animals were placed under the start box in the middle of the table. The peripheral circles represent holes in the table. The black circle represents the location of the escape compartment.
In addition to measuring escape latency from the Barnes maze, the search strategies used by the animals were also evaluated based on off-line analyses of the video recordings. The video reviewer was blinded to group identity (injured versus sham). Three distinct escape strategies were observed and used to characterize Barnes maze performance: (1) random: animals moved back and forth across the platform in no discernible pattern until the escape compartment was found; (2) peripheral: animals sequentially checked each opening at the periphery of the maze until the correct one was found; and (3) spatial: animals directly traveled to and escaped through the circular opening leading into the escape compartment, consistent with the use of a spatial strategy. These strategies were described in a previous TBI and Barnes maze study on mice (Fox et al., 1998).
EEG analysis
Spectral power in the theta frequency range (6–10 Hz) was measured using standard Fast Fourier Transform (FFT) analysis. The raw EEG data were divided into 1-sec epochs and the FFT for each epoch was calculated. The FFT epochs were then averaged together to give an overall spectral power waveform under the various test conditions. From these FTT waveforms, theta power was defined as the total power between 6 and 10 Hz. For each animal, theta power measurements were made for EEG signals recorded from each of the four electrodes under each experimental condition.
For the New Environment condition, in which animals were placed in an unfamiliar plastic bin, the rats spent some time exploring, but they were also immobile part of the time. Given that theta power is greatest in the hippocampus with active exploration, theta was measured as a difference in average theta power during epochs when the animal was engaged in active exploration, minus theta power during epochs when the animal was inactive. Active exploration was defined as rearing behavior along the sides of the plastic bin, and not locomotion from one end of the bin to the other. For the Dark Environment condition as well as during all Barnes maze trials, behavior and EEG power were analyzed over the entire time period since the animals were continually active in the dark. Under these conditions, the entire time period was divided into 1-sec epochs and these were averaged to give a single spectral waveform. All activity, including rearing and locomotion, was therefore included.
The peak theta frequency for each animal was also calculated, and was defined as the frequency (between 6 and 10 Hz) at which the greatest power occurred in the averaged spectral waveforms. The peak frequencies for each of the four recording electrodes were then averaged to give one average peak frequency for each animal for all three test conditions.
Histology
At the conclusion of the behavioral testing and EEG recording, all rats were euthanized (pentobarbital 100 mg/kg IP) and perfused transcardially with 200 mL of ice cold 0.1 M phosphate-buffered saline (pH 7.4), followed by 400 mL of 4% paraformaldehyde. The brains were removed and cryoprotected in 30% sucrose solution before being frozen in powdered dry ice and stored at −80°C. The hippocampus was then sectioned into 45-μm-thick slices using a microtome (model #869; American Optical Corporation, Buffalo, NY).
The tissue was stained using cresyl violet acetate stain. The tissue was dehydrated in a series of alcohol baths (50%, 70%, 95%, and 100% ethanol), for 2–4 min at each concentration. The tissue was then cleared in xylene for 15 min, followed by re-hydration using a series of alcohols and water (100%, 95%, and 70% ethanol to distilled water), with 1 min in each. Once re-hydrated, the tissue was put into cresyl violet acetate for 2–10 min, rinsed briefly with water, and differentiated for 2–10 min using 95% ethanol with acetic acid (150 μL/100 mL ethanol). Finally, the tissue was dehydrated again using 95% and 100% ethanol for 1 min each, put into xylene for 10 min, and cover-slipped using slide mounting medium.
Hippocampal damage was estimated by calculating the total hippocampal volume using the Cavalieri method. The region of interest was the dorsal hippocampus, not including the fimbria, ranging from −1.60 mm bregma rostrally to −4.30 mm bregma caudally. Every fifth slice within this region, for a total of 10 slices, was chosen from the hippocampus to estimate the total volume. The region of interest was traced at 4× power on a microscope while using stereology software (Stereo Investigator software, version 8; Microbrightfield Inc., Williston, VT). Using a grid and placing a mark in each square, with two different markers used to distinguish between the ipsilateral and contralateral sides of the hippocampus, an estimate of the contoured area was made. After the area was accurately estimated, the volume was determined using the Cavalieri estimator feature of the stereology program. The volume of each brain slice was calculated using the area of the contour multiplied by the measured tissue thickness. Finally, the total hippocampal volume was estimated by multiplying the measured volume of each contoured slice by a factor of five to account for our tissue section sampling method (i.e., every fifth slice was analyzed).
The location of each electrode tip was identified from examination of the tissue sections as shown in Figure 3. Recordings from electrodes found to be in post-injury cysts or far from the hippocampus were excluded from further analysis.
FIG. 3.
Electrode placement. A diagram of coronal sections of the rat hippocampus where the distal recoding electrodes were found on histology. They are arranged rostral to caudal (1–9). This illustrates all of the electrodes included in the analysis for all rats.
Statistical analysis
Theta power differences between the injured and control groups were analyzed using univariate analysis of variance (ANOVA). Because there were no statistically significant differences between the sham-injured and naïve control groups in EEG activity, data from these two groups were combined into one control group for all statistical analyses. Group differences in theta power combined across hippocampal hemispheres were compared using independent t-tests. A 2 × 2 ANOVA was used to test for significant interactions between groups (TBI versus controls), and hemispheres (ipsilateral to injury versus contralateral), as well as between groups, and electrode depth (superficial versus deep). Another 2 × 2 ANOVA (group × hemisphere) was used to test for differences in hippocampal volumes. For differences in righting time and peak theta frequency, a t-test was used to compare groups. To test for differences in Barnes maze performance, a repeated-measures ANOVA was employed (group × trial). Finally, correlations between Barnes maze performance and righting times after surgery were calculated using Spearman's correlations.
Three animals (one control and two TBI) lost their acrylic electrode cap before or during running of the Barnes maze. They were therefore excluded from all Barnes analyses as well as the hippocampal volume measurements. All statistical analyses were performed using SPSS software, and the alpha level for type I error was set at 0.05 for rejecting the null hypothesis. All data are expressed as means ± standard error of the mean.
Results
The average LFP injury severity was 2.15 atm (range 2.10–2.20 atm). Average return of the righting reflex was 5.38 (SEM = 0.43) min in the sham control animals, and 16.92 (SEM = 2.31) min in the injured animals.
Electrophysiology
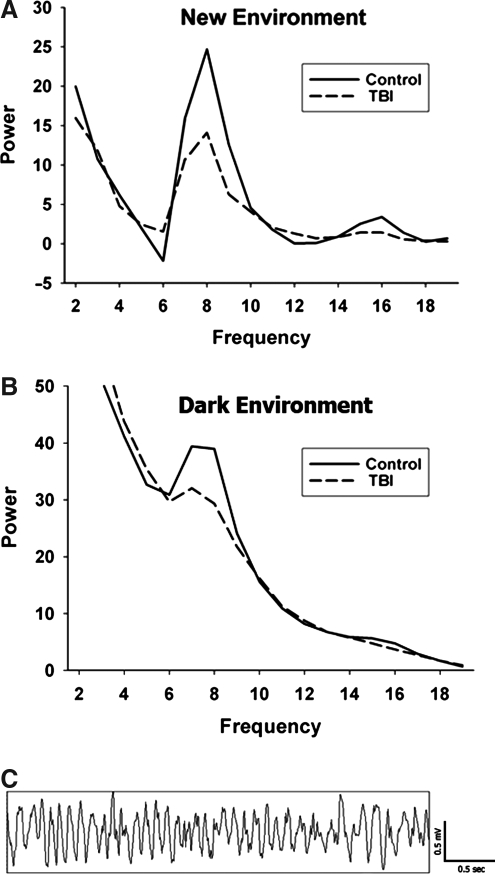
Spectral power functions calculated from the recorded EEGs and averaged over all four hippocampal electrodes are shown in Figure 4, under the New Environment (4A) and Dark Environment conditions (4B). Figure 4C shows a sample raw EEG tracing with theta activity. Under the New Environment condition, the spectral power waveform is a “difference” waveform, obtained by subtracting the spectral power distribution during quiet, still behavior, from the spectral power distribution during active rearing behavior. As such, this served as a measure of theta activation, representing the differences in hippocampal spectral power between non-active and active periods of exploratory behavior.
FIG. 4.
Spectral power waveforms. The average spectral power waveforms for control and lateral fluid percussion injured rats in (A) the New Environment condition, and (B) the Dark Environment condition. (C) A sample raw electroencephalographic recording of theta rhythm (TBI, traumatic brain injury).
Under the Dark Environment condition, the spectral power waveform represents the average hippocampal spectral power throughout the entire recording period. Due to the general increase in exploratory activity in a darkened environment, measurements made under the Dark Environment condition reflected mostly active behavior, including rearing and all locomotion. Out of the 4 min of recording time, both the control and TBI rats spent, on average, only 13 sec being still and non-active.
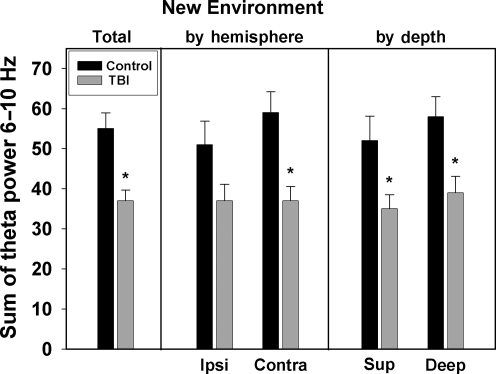
Group comparisons of theta power summed from 6–10 Hz for the New Environment and Dark Environment conditions are shown in Figures 5 and 6, respectively. Figure 5 demonstrates an average of total theta power over all four recording electrodes in the New Environment condition. The TBI group had significantly lower total theta power in the New Environment condition compared to uninjured controls (54 ± 6.1 versus 34 ± 4.0; p = 0.013). For the New Environment condition, a 2 × 2 ANOVA did not show any significant group × hemisphere interaction [F(1,46) = 0.391, p = 0.535)]. Post-hoc analysis of theta power using t-test comparisons for each hemisphere (Fig. 5) showed a significant group difference in theta power in the contralateral hemisphere (p = 0.01). A trend toward lower theta power in the TBI group was observed in the ipsilateral hemisphere (p = 0.078). Similarly, a 2 × 2 ANOVA did not show a significant group × electrode depth interaction [F(1,46) = 0.105; p = 0.748]. When theta power was analyzed separately for superficial and deep electrodes across both hemispheres (Fig. 5), there was significantly lower theta power in TBI animals for both electrode depths.
FIG. 5.
Theta power in the New Environment condition. The left panel summarizes total theta power summed from 6–10 Hz for control and TBI rats for all electrodes. The center panel summarizes theta power by hemisphere: ipsilateral (Ipsi) and contralateral (Contra). The right panel summarizes theta power by electrode depth: superficial (Sup) and deep (*p < 0.05 compared to control animals; TBI, traumatic brain injury).
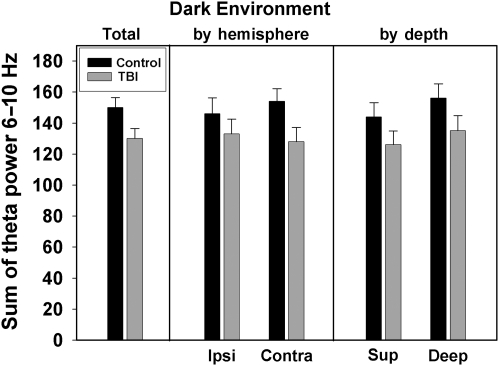
FIG. 6.
Theta power in the Dark Environment condition. The left panel summarizes total theta power summed from 6–10 Hz for control and TBI rats for all electrodes. The center panel summarizes theta power by hemisphere: ipsilateral (Ipsi) and contralateral (Contra). The right panel summarizes theta power by electrode depth: superficial (Sup) and deep (TBI, traumatic brain injury).
Under the Dark Environment condition there was a trend toward lower total theta power for the TBI group (149 ± 6.7 versus 128 ± 9.9; p = 0.083). There were no significant group × hemisphere or group × depth interactions observed with 2 × 2 ANOVA analysis [F(1,46) = 0.051, p = 0.822, and F(1,46) = 0.029, p = 0.866 for hemisphere and depth effects, respectively]. Post-hoc analysis with t-tests showed no significant differences in theta power between the control and TBI groups when analyzed separately for each hemisphere, or when analyzed separately for electrode depth (Fig. 6).
The average peak spectral power frequency under the New Environment condition was 7.8 ± 0.13 Hz in controls, and 7.4 ± 0.18 Hz in injured rats, and this difference approached, but did not reach, statistical significance (p = 0.069). For the Dark Environment condition, the average peak frequency was 7.2 ± 0.18 Hz and 6.7 ± 0.11 Hz in control and TBI rats respectively, and this difference was statistically significant (p = 0.027).
The Barnes mean theta power was averaged for all electrodes in each rat and analyzed across trials with repeated-measures ANOVA. No significant differences were detected between the TBI and control groups across all trials [F(1,19) = 0.838, p = 0.371]. The mean theta power across all trials for controls was 115.0 ± 6.91, and for TBI animals was 109.1 ± 7.04.
Behavioral data
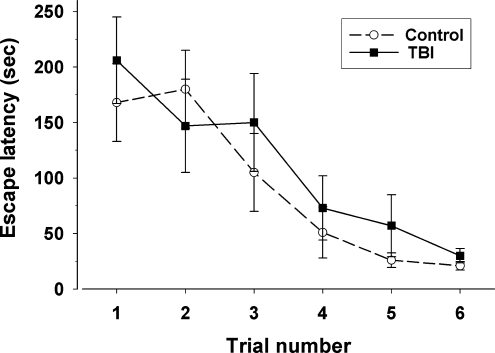
The time to correctly complete the modified Barnes maze for trials 1 through 6 is shown in Figure 7. When the first two trials (the first day) of testing are excluded from analysis, as the rats had not yet become habituated to the maze, a repeated-measures ANOVA analysis showed a trend toward better performance in the control group [F(1,20) = 1.924, p = 0.181].
FIG. 7.
Barnes maze performance. Data points are the average escape latencies for injured and control rats over subsequent Barnes maze trials (TBI, traumatic brain injury).
Injured rats also made more total errors during all Barnes maze trials than control rats, 11 versus 3, respectively. An error was defined as the rat committing to entering into an incorrect hole in the maze.
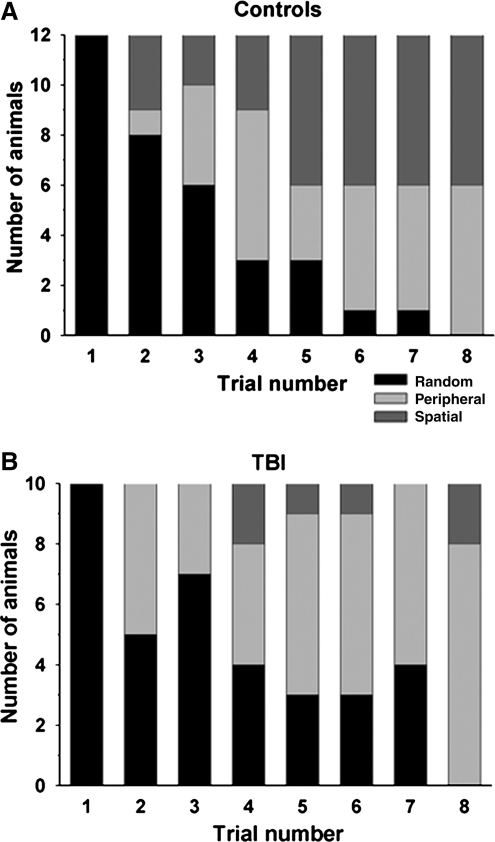
An analysis comparing the search strategies used by control and injured rats was carried out. Three general search patterns emerged across the training trials: random, peripheral, and spatial. Under the random search strategy, the rats did not show any apparent pattern in their search across the maze platform for the escape compartment. Under the peripheral search strategy, the rats moved around the periphery of the maze, often sequentially searching each hole for the escape compartment. Rats that were able to head directly to the escape compartment location appeared to attend to the spatial cues in the environment, and thus their search strategy was defined as spatial. As shown in Figure 8A, the search patterns of the animals in the control group changed over the eight training trials, beginning with a predominantly random search pattern in the early trials (trials 1 and 2), but gradually evolving so that by the end of the training period, half of the rats used a peripheral strategy, and the remaining half used the spatial strategy. The search patterns used by the TBI group shown in Figure 8B were quite different across the eight training trials. Specifically, only 20% of the animals in the LFP-injured group developed the spatial search strategy, while the rest continued to use either a random or peripheral search strategy. The different search patterns analyzed by chi-square analysis, collapsing data across all trials, revealed a significant difference in the distribution of search patterns between the TBI and control groups [χ2 (df = 2) = 13.503, p < 0.001].
FIG. 8.
Barnes maze search strategies. Shown are the numbers of control (A) or TBI (B) animals for each Barnes maze trial using random, peripheral, and spatial search strategies. Chi-square analysis collapsing data across all trials revealed a significant difference in the distribution of search patterns between the TBI and control groups (p < 0.001; TBI, traumatic brain injury).
Histology
Hippocampal volume was analyzed with a 2 × 2 ANOVA and used as a general measure of cumulative damage. Ipsilateral volumes were 10.39 ± 0.268 and 9.25 ± 0.331 mm3 for the control and TBI groups, respectively. Contralateral volumes were 10.86 ± 0.226 and 10.53 ± 0.225 mm3 for the control and TBI groups, respectively. Total hippocampal volume (both hemispheres) for the TBI group was significantly smaller than that of controls [F(1,46) = 7.212, p = 0.01]. A significant group × hemisphere interaction [F(1,46) = 4.043, p = 0.05], showed that volume loss in the ipsilateral hemisphere compared to that in the contralateral hemisphere was significantly greater in the TBI group than in controls. Post-hoc comparisons revealed a significantly smaller volume (p < 0.05) in the ipsilateral hemisphere of the TBI group.
Correlations
Injury severity as measured by the time to recovery of the righting reflex after TBI (i.e., righting time) was significantly correlated with worse performance in the Barnes maze, as measured by the average maze completion time over trials 3 through 6 (Pearson correlation = 0.656; p = 0.021).
Discussion
Behavior and hippocampal EEG were measured chronically after lateral fluid percussion TBI in rats. TBI produced a significant reduction in hippocampal theta power and a significant reduction in peak theta frequency compared to uninjured controls. EEG spectral power differences between groups were specific to the theta range, which is consistent with alterations in hippocampal-specific electrophysiological activity. Changes in theta activity in this study were evaluated for up to 3 months after injury, in contrast to previous studies that have generally focused on more immediate responses to injury, without special focus on the hippocampus (West et al., 1982). Behavioral deficits in the Barnes maze for TBI rats were characterized by changes in the search pattern strategy compared to uninjured controls. Specifically, TBI rats failed to develop the spatial search strategy exhibited by uninjured controls during Barnes maze acquisition.
The hippocampal theta activity analyzed in the present study was recorded while the rats were mobile, actively exploring either a new environment or navigating the Barnes maze. The theta measurements therefore more likely reflected the classic type I, movement-associated theta, which is less dependent on cholinergic input (Winson, 1974). However, some studies have shown that even movement-associated theta is altered by cholinergic blockade, and type I and II theta are known to co-occur in behaving animals (Buzsaki, 2002). Thus discriminating between theta types in the present experiments is difficult.
Given the various neurotransmitters and pathways necessary for establishing theta rhythm, different potential biochemical explanations for the observed attenuation of hippocampal theta exist. One such possibility is cholinergic hypofunction after TBI. Chronic cholinergic dysfunction in traumatic brain injury is well documented, as is its association with persistent memory deficits (Lyeth, 2001). Studies have shown that pharmacologic blockade of cholinergic activity with scopolamine in both TBI rats and in chronic TBI patients leads to decreased performance on cognitive tasks (Dixon et al., 1994). Furthermore, studies have demonstrated a decrease in basal forebrain choline acetyltransferase in several rodent models of brain injury, including lateral fluid percussion (Leonard et al., 1994), supporting the possibility that reduced cholinergic input to the hippocampus contributes to the decrease in theta power. Structural damage to the septo-hippocampal pathway following fluid percussion injury is also well known (Leonard et al., 1997). Given that experimental lesioning of the fimbria/fornix abolishes theta rhythm, structural damage to these pathways following injury may contribute to reduced theta power.
The hippocampus also contains the intrinsic neural circuitry for theta oscillations, and this circuitry appears to be modulated by glutamate and GABA, and not by acetylcholine (Goutagny et al., 2009). GABAergic input from the septum to the hippocampus could also affect hippocampal theta rhythms. Indeed, Wu and colleagues have shown that cholinergic excitation of the septal nuclei leads to increased GABA activity within hippocampal target cells (Wu et al., 2000). Given that GABA activity between the hippocampal interneurons forms the basis of the background theta rhythm (Green and Rawlins, 1979), TBI-induced changes in hippocampal GABA function, either due to disrupted input from the septum or intrinsic interneuron dysfunction or direct damage, offer a plausible theory as well.
Finally, glutamatergic input from the entorhinal cortex plays an important role in atropine-resistant theta (Buzsaki, 2002). Disruption of these inputs, whether biochemical or through structural alterations, may certainly decrease theta strength.
Besides affecting theta power, fluid percussion injury also affects theta frequency. The peak theta frequency was, on average, lower in the TBI group than in the control animals. Previous studies have linked decreased theta frequency to impaired spatial learning (Pan and McNaughton, 1997), and locomotor speed has also been shown to be correlated with theta frequency (Oddie and Bland, 1998). If the shift in theta frequency contributes to cognitive loss following TBI, it might be possible to improve behavioral performance through techniques to alter theta frequency. Since activity in the supramammillary nucleus contributes to theta frequency (Vertes, 1982), this structure could be a target for techniques such as deep brain stimulation to re-establish normal theta patterns.
One goal of this study was to measure chronic changes in spatial memory deficits following LFP in rats. Chronic memory deficits measured by escape latency in the Barnes maze were not significantly different between the TBI and control groups, although there was a trend toward worse performance in the TBI rats compared to controls. In addition, no significant differences between treatment groups were observed in hippocampal theta activity during Barnes maze testing. However, the measured theta power in both groups was lower at approximately 10–12 weeks post-TBI when Barnes maze testing was carried out compared to the earlier recording sessions (i.e., the New Environment and the Dark Environment), which were made 6–8 weeks post-TBI. There could have been deterioration of electrode efficiency with chronic electrode recordings. Thus, the long delay between TBI and theta recording during memory testing may not have been ideal for evaluating the relationships between memory performance and theta activity.
There were differences, however, in the search strategies employed by the TBI and control groups, which is consistent with previous studies of spatial navigation deficits in TBI mice (Brody and Holtzman, 2006; Fox et al., 1998). Based on an analysis of search patterns in the Barnes maze, most injured rats used a non-spatial trial-and-error approach to find the escape compartment, while control animals appeared to use a spatial strategy. Interestingly, in a 1995 study by Bach and associates, transgenic mice with a mutation in the CaMKII receptor, who had lost the ability for LTP generation at the theta frequency, also had specific spatial learning impairments in the Barnes maze (Bach et al., 1995). Previous studies using a Morris water maze paradigm have shown that theta activity does not appear to be associated with trial-and-error learning as much as with spatial learning (Olvera-Cortes et al., 2002, 2004). In a more recent study, rats trained using a spatial learning strategy in an open-field maze had increased theta activity in the seconds before and after exploration of incorrect openings, whereas those trained with a non-spatial trial-and-error approach did not, indicating the involvement of theta activity in the formation of spatial reference memory (Woldeit and Korz, 2010). Therefore, one explanation for impaired spatial search strategies in injured rats may be a deficit in theta-specific memory functions of the hippocampus.
The present study, however, showed no differences in Barnes maze theta power between control and injured rats, despite their differing learning strategies. Discovering correlations between theta power and search strategies may require more sophisticated analysis, in which specific behavior is synchronized with EEG activity, as was shown by Woldeit and Korz (2010). The limitations of our electrophysiological recording equipment and video camera did not allow us to precisely synchronize the EEG with specific exploratory behaviors that take place in a fraction of a second. Therefore it is not surprising that general correlations between theta power and search strategy did not yield significant results in this study. No differences were found in deep versus superficial electrodes, and reduced theta power was seen in both the injured and uninjured hemispheres. Lateral fluid percussion TBI typically results in focal pathology in the ipsilateral hemisphere. However, based on the use of Fluoro-jade to label degenerating neurons, there is also clear evidence of neuronal injury in the contralateral hemisphere after LFP injury (Hallam et al., 2004). Thus it is not surprising that bilateral theta dysfunction was observed in the present study, consistent with bilateral neuronal injury without significant tissue loss in the contralateral hemisphere. However, an additional explanation for global decreases in theta power arises from injury-induced dysfunction in the modulators of theta, such as the medial septal nucleus (discussed above), in addition to focal hippocampal injury.
Separate analysis of theta power in the ipsilateral and contralateral hemispheres showed a decrease in power in both hemispheres that was statistically significant for the contralateral hemisphere, and approached significance for the ipsilateral hemisphere (p = 0.078). The lack of a statistically significant group difference in theta power for the ipsilateral hemisphere may have been due to a small decrease in theta power for the sham control group, possibly a consequence of the larger craniectomy performed over the ipsilateral hemisphere. Given that theta rhythm dysfunction was not evident in the Barnes maze, it is possible that some recovery of theta rhythm in the contralateral hippocampus may have occurred, at least partially restoring patterns of theta activity. This is consistent with previous studies of lateral fluid percussion TBI, in which both physiological and histological signs of injury were seen in both the ipsilateral and the contralateral hemisphere 7 days after injury, but were absent in the contralateral hemisphere by 30 days (Tran et al., 2006).
Theta power analysis during the Dark Environment condition also showed a trend toward decreased power in the injured group that was not statistically significant. One possible explanation is that the theta power in the Dark Environment was averaged across the entire 4-min recording period, whereas in the New Environment theta power was calculated as a difference in power between periods of activity and inactivity. Under the Dark Environment, the rats did not spend enough time motionless for one to calculate such a difference waveform. Differences in theta strength may be specific to certain behaviors, and it may be theta activation (i.e., the difference in baseline theta and theta associated with exploration) that is impaired in the injured rats. Calculating theta power over all behaviors may have obscured this effect. In the present experiments we were unable to determine whether theta disruption was the result of damage to theta dipoles in the CA1 or CA3/dentate gyrus following TBI, or to damage to both. These two regions are believed to generate theta independently, and are thought to be responsible for distinct memory functions (i.e., encoding and retrieval, respectively; Villarreal et al., 2007; Vinogradova, 2001). A finer subregional analysis of theta activity following TBI will probably require the use techniques that can better localize the source of theta activity, such as multi-electrode arrays.
The observed disruption of hippocampal theta activity after fluid percussion injury poses an interesting explanation for the chronic LTP dysfunction seen in LFP-injured rats (Sanders et al., 2000). Several cellular mechanisms have been suggested to explain the impairment in LTP, such as changes in glutamate metabolism, or alterations in dendritic spine architecture or calcium signaling (Schwarzbach et al., 2006). However, hippocampal theta activity is an important modulator of LTP (Hyman et al., 2003). Therefore TBI may impair hippocampal function at the electrophysiological level, including LTP, by altering theta activity. Experimental lesions in the septo-hippocampal pathway are known to disrupt both LTP and hippocampal theta activity (Li et al., 2005). This raises the possibility that restoration or normalization of theta rhythm after injury by using pharmacological treatment or synchronized electrical stimulation could improve LTP induction, and help alleviate post-traumatic memory deficits. Although Schwarzbach and colleagues showed that in hippocampal slice preparations from fluid percussion-injured mice, LTP is not enhanced by stimulation in the theta frequency, it is still conceivable that LTP may be improved by restoring hippocampal theta rhythm in live animals (Schwarzbach et al., 2006).
There have been recent advances and promising outcomes in deep brain stimulation for the treatment of TBI. The targets have been deep thalamic relay nuclei which are involved in cortical arousal pathways (Schiff et al., 2007; Shirvalkar et al., 2006). One potential treatment option for memory and attentional deficits might be to restore or normalize theta activity, and thus improve memory processing by the hippocampus. Previous studies have shown the feasibility of improving Morris water maze performance in rats by experimentally restoring theta rhythm via electrical brain stimulation of the septal input to the hippocampus (McNaughton et al., 2006). Earlier studies have shown that septal stimulation in aged rats leads to improved performance in an open-field maze (Jiang et al., 1997). Transcranial magnetoencephalography studies in humans have shown that stimulation in the theta frequency range can enhance performance in cognitively-demanding tasks (Luber et al., 2007). Current brain stimulation protocols do not routinely consider intrinsic background rhythms generated from deep brain nuclei. It would be important to investigate whether stimulation delivered in phase with these intrinsic rhythms would be of even greater benefit. Whether such extrinsic stimulation can help restore theta rhythm after TBI, and more importantly whether restoring theta rhythm will prove to be beneficial for memory processing, remains to be seen.
In conclusion, in this study we demonstrated that hippocampal theta power and frequency are attenuated following lateral fluid percussion injury in the rat. Injured rats performed more poorly in a modified Barnes maze designed to test memory performance. They also developed search strategies that appeared to be less dependent on hippocampal processing strategies than uninjured control rats. Disruption of hippocampal-dependent theta rhythms may contribute to decreased cognitive performance following traumatic brain injury, and provide a target for the development of treatment strategies to improve cognition.
Acknowledgments
Expert technical assistance was provided by Christina Kopriva, Maria Dynin, Damoon Rejaei, and Ken Van. This research was supported in part by National Institutes of Health grant NS29995.
Author Disclosure Statement
No competing financial interests exist.
References
- Bach M.E. Hawkins R.D. Osman M. Kandel E.R. Mayford M. Impairment of spatial but not contextual memory in CaMKII mutant mice with a selective loss of hippocampal LTP in the range of the theta frequency. Cell. 1995;81:905–915. doi: 10.1016/0092-8674(95)90010-1. [DOI] [PubMed] [Google Scholar]
- Barnes C. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J. Compar. Physiological Psychol. 1979;93:74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- Basar-Eroglu C. Demiralp T. Event-related theta oscillations: an integrative and comparative approach in the human and animal brain. Int. J. Psychophysiol. 2001;39:167–195. doi: 10.1016/s0167-8760(00)00140-9. [DOI] [PubMed] [Google Scholar]
- Bland B.H. The physiology and pharmacology of hippocampal formation theta rhythms. Prog. Neurobiol. 1986;26:1–54. doi: 10.1016/0301-0082(86)90019-5. [DOI] [PubMed] [Google Scholar]
- Bramlett H.M. Green E.J. Dietrich W.D. Hippocampally dependent and independent chronic spatial navigational deficits following parasagittal fluid percussion brain injury in the rat. Brain Res. 1997;762:195–202. doi: 10.1016/s0006-8993(97)00387-9. [DOI] [PubMed] [Google Scholar]
- Brazhnik E. Fox S.E. Action potentials and relations to the theta rhythm of medial septal neurons in vivo. Exp. Brain Res. 1999;127:244–258. doi: 10.1007/s002210050794. [DOI] [PubMed] [Google Scholar]
- Brody D.L. Holtzman D.M. Morris water maze search strategy analysis in PDAPP mice before and after experimental traumatic brain injury. Exp. Neurol. 2006;197:330–340. doi: 10.1016/j.expneurol.2005.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Theta rhythm of navigation: link between path integration and landmark navigation, episodic and semantic memory. Hippocampus. 2005;15:827–840. doi: 10.1002/hipo.20113. [DOI] [PubMed] [Google Scholar]
- D'Ambrosio R. Maris D.O. Grady M.S. Winn H.R. Janigro D. Selective loss of hippocampal long-term potentiation, but not depression, following fluid percussion injury. Brain Res. 1998;786:64–79. doi: 10.1016/s0006-8993(97)01412-1. [DOI] [PubMed] [Google Scholar]
- Diamond D.M. Dunwiddie T.V. Rose G.M. Characteristics of hippocampal primed burst potentiation in vitro and in the awake rat. J. Neurosci. 1988;8:4079–4088. doi: 10.1523/JNEUROSCI.08-11-04079.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon C.E. Hamm R.J. Taft W.C. Hayes R.L. Increased anticholinergic sensitivity following closed skull impact and controlled cortical impact traumatic brain injury in the rat. J. Neurotrauma. 1994;11:275–287. doi: 10.1089/neu.1994.11.275. [DOI] [PubMed] [Google Scholar]
- Dixon C.E. Lyeth B.G. Povlishock J.T. Findling R.L. Hamm R.J. Marmarou A. Young H.F. Hayes R.L. A fluid percussion model of experimental brain injury in the rat. J. Neurosurg. 1987;67:110–119. doi: 10.3171/jns.1987.67.1.0110. [DOI] [PubMed] [Google Scholar]
- Folkerts M.M. Parks E.A. Dedman J.R. Kaetzel M.A. Lyeth B.G. Berman R.F. Phosphorylation of calcium calmodulin-dependent protein kinase II following lateral fluid percussion brain injury in rats. J. Neurotrauma. 2007;24:638–650. doi: 10.1089/neu.2006.0188. [DOI] [PubMed] [Google Scholar]
- Fox G.B. Fan L. LeVasseur R.A. Faden A.I. Effect of traumatic brain injury on mouse spatial and nonspatial learning in the Barnes circular maze. J. Neurotrauma. 1998;15:1037–1046. doi: 10.1089/neu.1998.15.1037. [DOI] [PubMed] [Google Scholar]
- Givens B. Olton D.S. Local modulation of basal forebrain: effects on working and reference memory. J. Neurosci. 1994;14:3578–3587. doi: 10.1523/JNEUROSCI.14-06-03578.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goutagny R. Jackson J. Williams S. Self-generated theta oscillations in the hippocampus. Nat. Neurosci. 2009;12:1491–1493. doi: 10.1038/nn.2440. [DOI] [PubMed] [Google Scholar]
- Green K.F. Rawlins J.N. Hippocampal theta in rats under urethane: generators and phase relations. Electroencephalogr. Clin. Neurophysiol. 1979;47:420–429. doi: 10.1016/0013-4694(79)90158-5. [DOI] [PubMed] [Google Scholar]
- Hallam T.M. Floyd C.L. Folkerts M.M. Lee L.L. Gong Q.Z. Lyeth B.G. Muizelaar J.P. Berman R.F. Comparison of behavioral deficits and acute neuronal degeneration in rat lateral fluid percussion and weight-drop brain injury models. J. Neurotrauma. 2004;21:521–539. doi: 10.1089/089771504774129865. [DOI] [PubMed] [Google Scholar]
- Hyman J.M. Wyble B.P. Goyal V. Rossi C.A. Hasselmo M.E. Stimulation in hippocampal region CA1 in behaving rats yields long-term potentiation when delivered to the peak of theta and long-term depression when delivered to the trough. J. Neurosci. 2003;23:11725–11731. doi: 10.1523/JNEUROSCI.23-37-11725.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang F. Racine R. Turnbull J. Electrical stimulation of the septal region of aged rats improves performance in an open-field maze. Physiol. Behav. 1997;62:1279–1282. doi: 10.1016/s0031-9384(97)00306-5. [DOI] [PubMed] [Google Scholar]
- Kahana M.J. Sekuler R. Caplan J.B. Kirschen M. Madsen J.R. Human theta oscillations exhibit task dependence during virtual maze navigation. Nature. 1999;399:781–784. doi: 10.1038/21645. [DOI] [PubMed] [Google Scholar]
- Kraus J.P. Chu L.D. Epidemiology, in: Neuropsychiatry of Traumatic Brain Injury. In: Silver J.S., editor; McAllister T.W., editor; Yudofsky S.C., editor. American Psychiatric Press; Washington: 2005. pp. 3–26. [Google Scholar]
- Leonard J.R. Grady M.S. Lee M.E. Paz J.C. Westrum L.E. Fluid percussion injury causes disruption of the septohippocampal pathway in the rat. Exp. Neurol. 1997;143:177–187. doi: 10.1006/exnr.1996.6366. [DOI] [PubMed] [Google Scholar]
- Leonard J.R. Maris D.O. Grady M.S. Fluid percussion injury causes loss of forebrain choline acetyltransferase and nerve growth factor receptor immunoreactive cells in the rat. J. Neurotrauma. 1994;11:379–392. doi: 10.1089/neu.1994.11.379. [DOI] [PubMed] [Google Scholar]
- Li C. Maier D.L. Cross B. Doherty J.J. Christian E.P. Fimbria-fornix lesions compromise the induction of long-term potentiation at the Schaffer collateral-CA1 synapse in the rat in vivo. J. Neurophysiol. 2005;93:3001–3006. doi: 10.1152/jn.00546.2004. [DOI] [PubMed] [Google Scholar]
- Luber B. Kinnunen L.H. Rakitin B.C. Ellsasser R. Stern Y. Lisanby S.H. Facilitation of performance in a working memory task with rTMS stimulation of the precuneus: frequency- and time-dependent effects. Brain Res. 2007;1128:120–129. doi: 10.1016/j.brainres.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Lyeth B.G. Cholinergic receptors in head trauma. In: Miller L.P., editor; Hayes R.L., editor. Head Trauma: Basic, Preclinical, and Clinical Directions. Wiley-Liss; New York: 2001. pp. 115–140. [Google Scholar]
- Manns J. Zilli E.A. Ong K.C. Hasselmo M.E. Eichenbaum H. Hippocampal CA1 spiking during encoding and retrieval: relation to theta phase. Neurobiol. Learn. Mem. 2007;87:9–20. doi: 10.1016/j.nlm.2006.05.007. [DOI] [PubMed] [Google Scholar]
- McAllister T.W. Flashman L.A. McDonald B.C. Saykin A.J. Mechanisms of working memory dysfunction after mild and moderate TBI: evidence from functional MRI and neurogenetics. J. Neurotrauma. 2006;23:1450–1467. doi: 10.1089/neu.2006.23.1450. [DOI] [PubMed] [Google Scholar]
- McIntosh T.K. Vink R. Noble L. Yamakami I. Fernyak S. Soares H. Faden A.L. Traumatic brain injury in the rat: characterization of a lateral fluid-percussion model. Neuroscience. 1989;28:233–244. doi: 10.1016/0306-4522(89)90247-9. [DOI] [PubMed] [Google Scholar]
- McNaughton N. Ruan M. Woodnorth M.A. Restoring theta-like rhythmicity in rats restores initial learning in the Morris water maze. Hippocampus. 2006;16:1102–1110. doi: 10.1002/hipo.20235. [DOI] [PubMed] [Google Scholar]
- Miyazaki S. Katayama Y. Lyeth B.G. Jenkins L.W. DeWitt D.S. Goldberg S.J. Newlon P.G. Hayes R.L. Enduring suppression of hippocampal long-term potentiation following traumatic brain injury in rat. Brain Res. 1992;585:335–339. doi: 10.1016/0006-8993(92)91232-4. [DOI] [PubMed] [Google Scholar]
- Oddie S.D. Bland B.H. Hippocampal formation theta activity and movement selection. Neurosci. Biobehav. Rev. 1998;22:221–231. doi: 10.1016/s0149-7634(97)00003-1. [DOI] [PubMed] [Google Scholar]
- Olton D.S. Markowska A.L. Memory and hippocampal function as targets for neurotoxic substances. Neurotoxicology. 1994;15:439–443. [PubMed] [Google Scholar]
- Olvera-Cortes E. Cervantes M. Gonzalez-Burgos I. Place-learning, but not cue-learning training, modifies the hippocampal theta rhythm in rats. Brain Res. Bull. 2002;58:261–270. doi: 10.1016/s0361-9230(02)00769-4. [DOI] [PubMed] [Google Scholar]
- Olvera-Cortes E. Guevara M.A. Gonzalez-Burgos I. Increase of the hippocampal theta activity in the Morris water maze reflects learning rather than motor activity. Brain Res. Bull. 2004;62:379–384. doi: 10.1016/j.brainresbull.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Otto T. Eichenbaum H. Wiener S.I. Wible C.G. Learning-related patterns of CA1 spike trains parallel stimulation parameters optimal for inducing hippocampal long-term potentiation. Hippocampus. 1991;1:181–192. doi: 10.1002/hipo.450010206. [DOI] [PubMed] [Google Scholar]
- Pan W.X. McNaughton N. The medial supramammillary nucleus, spatial learning and the frequency of hippocampal theta activity. Brain Res. 1997;764:101–108. doi: 10.1016/s0006-8993(97)00431-9. [DOI] [PubMed] [Google Scholar]
- Paxinos G. Watson C. The Rat Brain in Stereotaxic Coordinates. 4th. Academic Press; San Diego: 1998. [Google Scholar]
- Pierce J.E. Smith D.H. Trojanowski J.Q. McIntosh T.K. Enduring cognitive, neurobehavioral and histopathological changes persist for up to one year following severe experimental brain injury in rats. Neuroscience. 1998;87:359–369. doi: 10.1016/s0306-4522(98)00142-0. [DOI] [PubMed] [Google Scholar]
- Pike F.G. Goddard R.S. Suckling J.M. Ganter P. Kasthuri N. Paulsen O. Distinct frequency preferences of different types of rat hippocampal neurones in response to oscillatory input currents. J. Physiol. 2000;529:205–213. doi: 10.1111/j.1469-7793.2000.00205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto D.S. Madsen J.R. Bromfield E.B. Schulze-Bonhage A. Seelig D. Aschenbrenner-Scheibe R. Kahana M.J. Reset of human neocortical oscillations during a working memory task. Proc. Natl. Acad. Sci. USA. 2003;100:7931–7936. doi: 10.1073/pnas.0732061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders M.J. Sick T.J. Perez-Pinzon M.A. Dietrich W.D. Green E.J. Chronic failure in the maintenance of long-term potentiation following fluid percussion injury in the rat. Brain Res. 2000;861:69–76. doi: 10.1016/s0006-8993(00)01986-7. [DOI] [PubMed] [Google Scholar]
- Schiff N.D. Giacino J.T. Kalmar K. Victor J.D. Baker K. Gerber M. Fritz B. Eisenberg B. Biondi T. O'Connor J. Kobylarz E.J. Farris S. Machado A. McCagg C. Plum F. Fins J.J. Rezai A.R. Behavioural improvements with thalamic stimulation after severe traumatic brain injury. Nature. 2007;448:600–603. doi: 10.1038/nature06041. [DOI] [PubMed] [Google Scholar]
- Schwarzbach E. Bonislawski D.P. Xiong G. Cohen A.S. Mechanisms underlying the inability to induce area CA1 LTP in the mouse after traumatic brain injury. Hippocampus. 2006;16:541–550. doi: 10.1002/hipo.20183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirvalkar P. Seth M. Schiff N.D. Herrera D.G. Cognitive enhancement with central thalamic electrical stimulation. Proc. Natl. Acad. Sci, USA. 2006;103:17007–17012. doi: 10.1073/pnas.0604811103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson H.J. Lifshitz J. Marklund N. Grady M.S. Graham D.I. Hovda D.A. McIntosh T.K. Lateral fluid percussion brain injury: a 15-year review and evaluation. J. Neurotrauma. 2005;22:42–75. doi: 10.1089/neu.2005.22.42. [DOI] [PubMed] [Google Scholar]
- Tran L.D. Lifshitz J. Witgen B.M. Schwarzbach E. Cohen A.S. Grady M.S. Response of the contralateral hippocampus to lateral fluid percussion brain injury. J. Neurotrauma. 2006;23:1330–1342. doi: 10.1089/neu.2006.23.1330. [DOI] [PubMed] [Google Scholar]
- Vertes R.P. Brain stem generation of the hippocampal EEG. Prog. Neurobiol. 1982;19:159–186. doi: 10.1016/0301-0082(82)90005-3. [DOI] [PubMed] [Google Scholar]
- Villarreal D.M. Gross A.L. Derrick B.E. Modulation of CA3 afferent inputs by novelty and theta rhythm. J. Neurosci. 2007;27:13457–13467. doi: 10.1523/JNEUROSCI.3702-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradova O.S. Hippocampus as comparator: role of the two input and two output systems of the hippocampus in selection and registration of information. Hippocampus. 2001;11:578–598. doi: 10.1002/hipo.1073. [DOI] [PubMed] [Google Scholar]
- West M. Parkinson D. Havlicek V. Spectral analysis of the electroencephalographic response in experimental concussion in the rat. Electroencephalogr. Clin. Neurophysiol. 1982;53:192–200. doi: 10.1016/0013-4694(82)90023-2. [DOI] [PubMed] [Google Scholar]
- Wiener S.I. Paul C.A. Eichenbaum H. Spatial and behavioral correlates of hippocampal neuronal activity. J. Neurosci. 1989;9:2737–2763. doi: 10.1523/JNEUROSCI.09-08-02737.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winson J. Patterns of hippocampal theta rhythm in the freely moving rat. Electroencephalogr. Clin. Neurophysiol. 1974;36:291–301. doi: 10.1016/0013-4694(74)90171-0. [DOI] [PubMed] [Google Scholar]
- Woldeit M.L. Korz V. Theta oscillations during holeboard training in rats: different learning strategies entail different context-dependent modulations in the hippocampus. Neuroscience. 2010;165:642–653. doi: 10.1016/j.neuroscience.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Wu M. Shanabrough M. Leranth C. Alreja M. Cholinergic excitation of septohippocampal GABA but not cholinergic neurons: implications for learning and memory. J. Neurosci. 2000;20:3900–3908. doi: 10.1523/JNEUROSCI.20-10-03900.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]