Abstract
For patients with profound hearing loss, a cochlear implant is the only treatment available today. The function of a cochlear implant depends in part on the function and survival of spiral ganglion neurons. Following deafferentation, glial cell-derived neurotrophic factor (GDNF) is known to affect spiral ganglion neuron survival. The purpose of this study was to assess delayed GDNF treatment after deafening, the effects of cessation of GDNF treatment, and the effects of subsequent antioxidants on responsiveness and survival of the spiral ganglion neurons. Three-week deafened (by local neomycin administration) guinea pigs were implanted in the scala tympani with a combined cochlear implant electrode and cannula. GDNF (1 μg/mL) or artificial perilymph was then delivered for 4 weeks, following which the animals received systemic ascorbic acid + Trolox® or saline for an additional 4 weeks. Thresholds for electrically-evoked auditory brain stem responses (eABRs) were significantly elevated at 3 weeks with deafness, stabilized with GDNF, and showed no change with GDNF cessation and treatment with antioxidants or saline. The populations of spiral ganglion neurons were reduced with deafness (by 40% at 3 weeks and 70% at 11 weeks), and rescued from cell death by GDNF with no further reduction at 8 weeks following 4 weeks of cessation of GDNF treatment equally in both the antioxidant- and saline-treated groups. Local growth factor treatment of the deaf ear may prevent deterioration in electrical responsiveness and rescue auditory nerve cells from death; these effects outlast the period of treatment, and may enhance the benefits of cochlear implant therapy for the deaf.
Key words: auditory function, cochlear implant, glial cell-derived neurotrophic factor, spiral ganglion neuron
Introduction
In most cases of hearing loss, the sensory receptors (hair cells) are damaged or missing, and as a result sound perception is severely impaired. However, using by a cochlear implant it is possible to electrically stimulate the subsequent functional unit, the spiral ganglion, and thus activate the auditory pathway in a nearly physiological manner, resulting in the sensation of hearing despite the missing sensory cells. The efficacy of a cochlear implant is thought to be related to the number and functional state of the spiral ganglion neurons within the cochlea (Incesulu and Nadol, 1998; Nadol et al., 1989). This notion is supported by experimental studies demonstrating a correlation between the electrical responsiveness of the cochlea, as tested by an electrode inserted into scala tympani, and the density of the remaining auditory neurons (Agterberg et al., 2009; Fransson et al., 2009; Maruyama et al., 2007, 2008; Yamagata et al., 2004; Shinohara et al., 2002; Jyung et al., 1989). It follows that treatment to preserve auditory sensory neurons following deafferentation is of therapeutic significance and may lead to an increased benefit of the cochlear implant. Degeneration of the spiral ganglion neurons occurs as a result of the loss of sensory hair cells. This is in line with the neurotrophic factor hypothesis (Mattson, 1998), which suggests that deafferentation induces neurotrophic factor deprivation and upregulation of cell death pathways. This hypothesis and the specific role of neurotrophic factors have been tested in several experimental studies (Gillespie and Shepherd, 2005). Ylikoski and associates (1998) showed that intracochlear infusion of glial cell-derived neurotrophic factor (GDNF) enhanced the survival of auditory neurons after noise-induced inner hair cell damage. Shinohara and colleagues (2002) demonstrated that a combination of the neurotrophic factors brain-derived neurotrophic factor (BDNF) and ciliary neurotrophic factor (CNTF), applied directly to the inner ear fluids, significantly increased both the population of surviving auditory neurons following deafening, and the efficacy of electrical stimulation as measured by electrically-evoked auditory brainstem responses (eABR). Similar findings on spiral ganglion neuron survival have been found in other studies (Gillespie et al., 2003, 2004; Shepherd et al., 2005).
In humans, the secondary degeneration of spiral ganglion neurons occurs over several years following hair cell damage, thus allowing quite a long period between the first signs of severe hearing impairment and final selection for cochlear implantation. In the guinea pig, on the other hand, the neuronal degeneration is a much more rapid process, and significantly reduced electrical responsiveness can be detected within weeks of sensory hair cell loss. This makes the guinea pig model useful for studying procedures that may enhance the survival and function of spiral ganglion neurons and thus improve the efficacy of cochlear implants. However, to be of clinical relevance, the beneficial effects must also be maintained beyond the treatment period. The present study was performed to investigate whether the positive effects of local (intracochlear) GDNF infusion on survival and electrical responsiveness of auditory neurons are maintained up to 4 weeks after terminating active treatment.
Methods
Experimental design
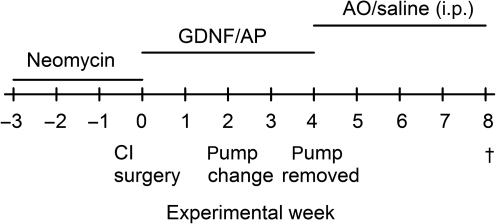
Guinea pigs with normal hearing were deafened by transtympanic injections of the ototoxic drug neomycin. After 3 weeks, when significant hearing loss was demonstrated by recording acoustically-evoked auditory brainstem responses (ABRs), the animals were implanted with an electrode device mimicking a cochlear implant. The electrode device was only used for eliciting eABR and not for electrical stimulation. At the same time an osmotic pump was implanted in order to slowly infuse GDNF into the inner ear. Treatment with GDNF or a control fluid (as control fluid, Ringer's acetate was used as a substitute for artificial perilymph) was continued for a total of 4 weeks, after which the animals were followed for another 4 weeks. Some of the post-treatment animals were given intraperitoneal injections with antioxidants to test whether reducing oxidative stress could provide further protection of the inner ear tissue. An outline of the experimental steps is shown in Figure 1.
FIG. 1.
Experimental design and time course for surgery, measurements, and drug application (CI, cochlear implant; GDNF, glial cell-derived neurotrophic factor; AP, artificial perilymph; i.p., intraperitoneal).
A total of 34 pigmented guinea pigs of both sexes (200–370 g; BioJet, Uppsala, Sweden) were used. All animals were tested for normal hearing using an ABR test prior to the experiments. All animal procedures were performed in accordance with the ethical guidelines of Karolinska Institutet, and were consistent with national regulations for the care and use of animals (approval nos. N 113/01 and N 468/03).
Treatment procedures
At 3 weeks post-deafening one group was sacrificed (Deaf 3 weeks; n = 6) and the cochleae were prepared for morphological analysis in order to determine the degree of structural changes present at the time of treatment initiation. The remaining animals were unilaterally implanted with a custom-made electrode device (Med-El GmbH, Innsbruck, Austria), consisting of an active electrode, a grounding electrode, a percutaneous connector, and a silicone tube connected to a mini-osmotic pump (model 2002 Alzet; DURECT Corp., Cupertino, CA). The mini-osmotic pump had an infusion rate of 0.5 μL/h, and delivered either 1 μg/mL GDNF or artificial perilymph. The animals were divided into two groups: one group (n = 20) received intracochlear infusion of GDNF for 4 weeks, and one group (n = 8) received intracochlear infusion of artificial perilymph for 4 weeks. After 4 weeks of GDNF treatment one group of animals was sacrificed (GDNF 4 weeks; n = 5) and their cochleae were prepared for structural analysis. The remaining animals (infused with GDNF or artificial perilymph) had the pump removed. The GDNF-treated animals were divided into two groups. One group received daily i.p. injections of the antioxidants Trolox® (a water-soluble vitamin E analogue; 1 mg/kg) and ascorbic acid (20 mg/kg) for 4 weeks (GDNF AO; n = 7). The other GDNF-treated group received i.p. injections of saline (GDNF saline; n = 8). The control group treated with artificial perilymph (Deaf 11 weeks; n = 8) received daily injections with saline i.p. These three post-treatment groups were followed for another 4 weeks. An overview of the different experimental groups is shown in Table 1. During the 8 weeks of treatment and post-treatment the auditory function was measured weekly by recording eABRs. After the final eABR measurement, the animals were sacrificed and the cochleae were processed for structural analysis. In addition to these groups, a normal and untreated group was included for structural comparison (normal; n = 8). In order to reduce the number of animals used, this group consisted of the contralateral cochleae from animals in the treated groups. The cochleae for the normal group were randomly chosen from all treated groups. Since treatments were applied using local intracochlear infusion, the contralateral ear was not expected to be significantly affected.
Table 1.
Overview of the Experimental Groups Used in the Study
| Experimental group | No of cochleae | Range of neomycin injections required to obtain sufficient deafening (average) | Average ABR threshold (±SD) at onset of treatment | Treatment | Post-treatment |
|---|---|---|---|---|---|
| Normal | 8 | – | – | – | – |
| Deaf 3 weeks | 6 | 2–6 (3.6) | 45.3 ± 2.7 | None | None |
| GDNF 4 weeks | 5 | 1–7 (3.4) | 51.4 ± 3.8 | 4 weeks of GDNF | None |
| GDNF AP | 7 | 1–5 (3.5) | 46.4 ± 3.8 | 4 weeks of GDNF | 4 weeks of antioxidants |
| GDNF saline | 8 | 1–6 (3.0) | 46.3 ± 2.3 | 4 weeks of GDNF | 4 weeks of saline |
| Deaf 11 weeks | 8 | 2–7 (4.2) | 50.5 ± 2.7 | 4 weeks of AP | 4 weeks of saline |
The normal group was used only for structural analysis and was consisted of samples obtained from the unexposed (not deafened, not treated) contralateral cochlea.
GDNF, glial cell-derived neurotrophic factor; AP, artificial perilymph; ABR, acoustically-evoked auditory brainstem response.
Implantation surgery
The guinea pigs were deeply anesthetized using xylazine (10 mg/kg i.m.) and ketamine (40 mg/kg i.m.). Ophthalmic ointment was applied to the eyes to prevent corneal ulcers due to a lack of blink reflex from the ketamine. The local anesthetic lidocaine was injected subcutaneously in the areas to be incised. A midline skin incision on the dorsal surface of the head was performed, starting posterior to the bregma, continuing down behind the ear, and ending at the base of the pinna. A hole was drilled through the skull at the midline, 1 cm posterior to the bregma. A stainless steel screw was placed through the hole to contact the dura of the brain. A small hole was made in the bulla ossea to expose the middle ear and visualize the cochlea. The combined electrode and microcannula device was then inserted approximately 2 mm through the round window. The mini-osmotic pump was placed under the skin on the back of the animal. The active electrode was inserted through the round window. The grounding electrode was placed in the middle ear cavity in contact with the middle ear wall. The percutaneous connector for electrical stimulation of the auditory nerve was cemented to the dorsal part of the skull with dental cement. For pump changes, the animal was anesthetized and subcutaneous lidocaine was injected at the surgical site.
Electrically-evoked auditory brainstem responses
The animals were anaesthetized and placed in a soundproof box. The eABRs were recorded using a SigGen System 2 signal analyzer (Tucker-Davies Technologies, Alachua, FL), using a previously described method (Hall, 1990). Responses were summed to alternate polarity monophasic current pulses, where each pair provides charge balancing. In all, 2048 responses to 50-μsec computer-generated monophasic current pulses, presented at 50 pps with alternating polarity on each presentation, were collected from a screw placed at the vertex (active), one subdermal needle electrode placed subcutaneously above the bulla ossea on the deafened ear (reference), and one in the hind leg (ground). The thresholds were monitored and defined as the lowest stimulus level in 10-μA steps that evoked at least a 0.3-μV reproducible waveform, as previously described (Maruyama et al., 2007; Yamagata et al., 2004).
Histology
After the final eABR measurements, the animals were deeply anesthetized with pentobarbital sodium (25 mg/kg i.p.), and transcardially perfused with saline (37°C), followed by cold glutaraldehyde (2.5% in 0.1 M phosphate buffer). The temporal bones were removed and the bulla ossea was opened up to expose the cochlea. Holes were made through the round window and the apex, and the cochlea was then gently flushed with glutaraldehyde and left in fixative overnight before decalcification in 0.1 M EDTA in phosphate buffer. After dehydration and embedding in JB-4 plastic (Polyscience Inc., Warrington, PA), 4-μm-thick sections were prepared for light microscopy. When reaching the mid-modiolar plane, defined as a section in which there were distinct profiles through all six cross-sections of Rosenthal's canal, every third section was collected. The mounted sections were stained with Paragon (toluidine blue and basic fuchsine). Six mid-modiolar plane sections from each animal were chosen, the areas of each Rosenthal's canal were measured (Sigma ScanPro; Aspire Software International, Ashburn, VA), and the number of spiral ganglion neuron profiles was counted. The criteria for a guinea pig spiral ganglion neuron were a cell with a diameter of 14–20 microns, and the presence of a nucleus with a diameter in the range of 7–10 microns. Mounting every third section was done to assure that a neuron was only counted once. The average density of the spiral ganglion neurons was then calculated. A total of 36 Rosenthal's canal sections were examined in each animal. In order to investigate if there were any differences in soma size between the groups, the diameter of the spiral ganglion neurons from the second turn (mid-region of the cochlea) was measured. This measurement was performed in the sections used for the cell counting.
Statistical analysis
For the statistical analysis in the eABR study, and for the calculation of the spiral ganglion neuron density, one-way ANOVA was used. As a post-hoc test Tukey's test was used. Data are presented as mean ± SEM.
Results
Effects on electrical responsiveness
In order to mimic the conditions in hearing impaired patients, the animals were deafened 3 weeks prior to GDNF treatment. In some subjects, neomycin was administered repeatedly in order to produce significant damage to the auditory sensory cells and indirectly to the spiral ganglion neurons. Representative images of the spiral ganglion in Rosenthal's canal from normal (not deafened) animals, and animals 3 weeks after application of neomycin are shown in Figure 2, illustrating significant loss of both sensory hair cells and spiral ganglion neurons following neomycin administration. The mean spiral ganglion cell density in the deafened animals was approximately 6 cells/10,000 mm2, corresponding to about 60% of the normal cell density (10 cells/10,000 mm2). There was no significant difference (p > 0.05) between hearing thresholds (recorded using click-evoked ABR) in the different groups when GDNF treatment was initiated (experimental week 0; Fig. 1).
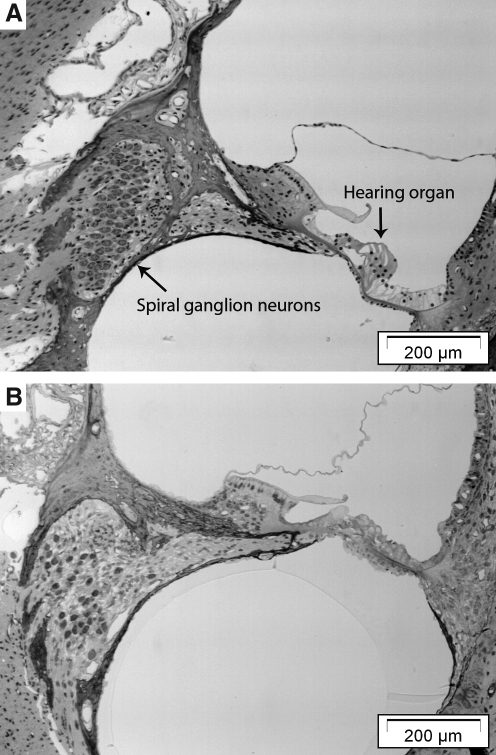
FIG. 2.
Light micrographs showing representative sections through (A) a normal (not defeaned and not treated) cochlea, and (B) a cochlea from a guinea pig 3 weeks following deafening (Deaf 3 weeks). In the deafened animal the hearing organ has collapsed and no hair cells can be identified. There was also a significant decrease in the number of spiral ganglion neurons (the density in B was 6 cells/10,000 mm2, compared to 10 cells/10,000 mm2 in the normal animal shown in A).
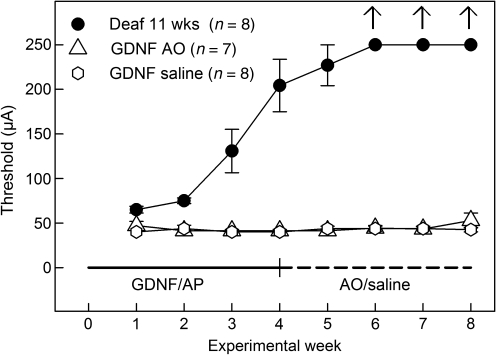
In Figure 3, the changes in electrical responsiveness (recorded as eABR thresholds) throughout the experiment are shown. Already at the time of the first eABR measurement (i.e., 1 week after starting GDNF or artificial perilymph infusion; experimental week 1), the control group (Deaf 11 weeks) that was given artificial perilymph had a significantly higher eABR threshold than the GDNF-treated animals (p < 0.05). The two GDNF-treated groups displayed approximately the same thresholds throughout the eight experimental weeks (no statistically significant differences between the GDNF groups). The thresholds continued to increase in the control group, and from experimental week 6 an electrical response could not be elicited using the present equipment.
FIG. 3.
Recordings of eABR thresholds throughout the experiment. No significant difference between the GDNF groups was seen throughout the experiment. There was a significant increase (p < 0.05) in eABR thresholds at experimental week 1 for the Deaf 11 weeks group compared to the GDNF-treated groups. At week 3 the difference had increased significantly (p < 0.001), and after week 6 no electrical responses could be elicited in the control group (eABR, electrically-evoked auditory brainstem responses; GDNF, glial cell-derived neurotrophic factor; AP, artificial perilymph; AO, antioxidant).
Preservation of the spiral ganglion cell population
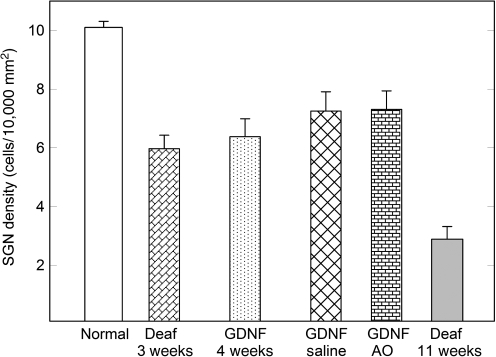
It has been hypothesized that the electrical responsiveness of the inner ear is closely related to the number of remaining spiral ganglion neurons in Rosenthal's canal. A total of six groups were thus analyzed for the density of spiral ganglion neurons (number of neurons per 10,000 μm2) at different time points with respect to the treatment schedule (Fig. 4). This included a normal group, consisting of cochleae that had not been subjected to any deafening or treatment procedures, and a group representing the starting point prior to any treatment (animals sacrificed after 3 weeks of deafening). After 4 weeks of GDNF treatment (experimental week 4 in Fig. 3), the density of spiral ganglion neurons was approximately the same as in the group of animals sacrificed immediately after deafening without any treatment (no significant difference). GDNF-treated animals were followed for an additional 4 weeks (without GDNF treatment). Half of these subjects were given i.p. injections with antioxidants (ascorbic acid and Trolox), and half received saline. As seen in the figure, the spiral ganglion cell population did not decrease during this 4-week post-treatment period. If anything, the cell density was somewhat higher; however, there was no substantial difference between the two GDNF-treated groups. In contrast, the control group consisting of animals receiving no treatment at all (Deaf 11 weeks) displayed a significantly lower mean spiral ganglion neuron density than all the other groups (p < 0.001). At this time point, 11 weeks following deafening without any active treatment, the auditory neuron density was about 40% of the GDNF-treated groups, and about 30% of the normal cell density.
FIG. 4.
Spiral ganglion neuron (SGN) density in all groups. After 3 weeks of deafening (Deaf 3 weeks), the density of spiral ganglion neurons was about 60% of that in normal untreated animals (p < 0.001). No significant difference was found between the GDNF-treated groups. There was a significant difference (p < 0.001) between the deafened untreated group (Deaf 11 weeks) and the GDNF-treated groups. After 11 weeks of deafness only 30% of the spiral ganglion cell density remained compared to that of a normal untreated animal (GDNF, glial cell-derived neurotrophic factor; AO, antioxidant).
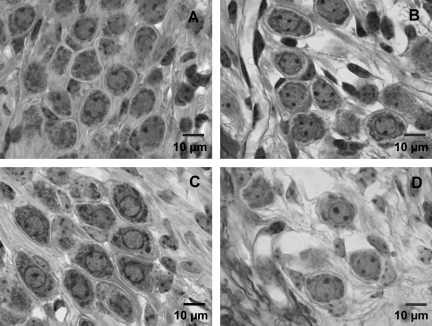
Given reports that local neurotrophin treatment may lead to increased size of the cell soma that could bias the density measurements, the diameter of the cell somas in the second turn of the cochlea were measured in normal animals and in GDNF-saline and Deaf 11 weeks animals (Fig. 5). The choice of monitoring changes in the second turn and not in the first turn was made to see the overall effect along the cochlea rather than one of the extremes. The protective effect of GDNF treatment seen as higher spiral ganglion neuron density compared to control animals was clearly also seen in the second turn in the cell somas, and there were significant differences between the groups (Table 2). However, although the GDNF-treated neurons were slightly larger (p < 0.01) than normal (untreated neurons), they were significantly smaller than spiral ganglion cells from the artificial perilymph-treated group (Deaf 11 weeks; p < 0.05).
FIG. 5.
High-magnification (100 × ) light micrographs from Rosenthal's canal showing representative spiral ganglion neurons obtained from (A) a normal (not deafened and not treated) animal, (B) an animal 3 weeks following deafening (Deaf 3 weeks), (C) from the GDNF-saline group (animals deafened followed by 4 weeks of treatment and 4 weeks saline post-treatment), and (D) an animal 11 weeks after deafening and without any treatment (Deaf 11 weeks; scale bar = 10 μm; GDNF, glial cell-derived neurotrophic factor).
Table 2.
Measurements of the Soma Diameter in Spiral Ganglion Neurons from Normal (Untreated) Animals, Deafened GDNF-Treated Animals (GDNF-Saline), and Deafened Control Animals (deaf 11 weeks)
| |
|
|
p Value |
|
|---|---|---|---|---|
| Group | Mean diameter ± SEM (μm) | Range (μm) | versus normal | versus deaf 11 weeks |
| Normal | 14.7 ± 0.13 | 9.9–20.8 | p < 0.001 | |
| GDNF-saline | 15.3 ± 0.16 | 9.0–22.2 | p < 0.01 | p < 0.05 |
| Deaf 11 weeks | 16.1 ± 0.29 | 10.4–23.5 | p < 0.001 | |
GDNF, glial cell-derived neurotrophic factor.
Discussion
Cochlear function depends on an intricate interplay between many tissues and different cell types. For detecting and then conveying auditory information to the nervous system, there are, however, two main players: the sensory inner and outer hair cells in the hearing organ, and the spiral ganglion neurons connecting these sensory elements to the cochlear nucleus in the brainstem. Both are essential for normal hearing, but the development and clinical success of the cochlear implant, which functionally bypasses the malfunctioning sensory cells, have demonstrated that the spiral ganglion neurons alone may assume a significant role when combined with electrical stimulation. Thus maintaining a population of appropriately functioning spiral ganglion neurons is of great importance for hearing-impaired patients fitted with cochlear implants. With the introduction of cochlear implants based on electro-acoustic stimulation (EAS) technology, that are used in patients with less severe cochlear degeneration and partially preserved hearing, there has been increased interest in compounds that may preserve spiral ganglion function.
We have previously demonstrated using the present guinea pig model for cochlear implants, that neurotrophic factors such as BDNF and GDNF can significantly reduce secondary degeneration of spiral ganglion neurons following experimental deafening up to 6 weeks after trauma (Maruyama et al., 2007, 2008; Yamagata et al., 2004; Shinohara et al., 2002) The neurotrophic intervention leads to maintained electrical responsiveness of the spiral ganglion neurons, manifest as significantly lower stimulus thresholds for electrically-evoked auditory brainstem responses (Maruyama et al., 2007, 2008; Yamagata et al., 2004; Shinohara et al., 2002). These results are confirmed in the present study, that showed significant benefits of GDNF treatment in animals 3 weeks after deafening. In a clinical context, it could be argued that this time period is still much too short to be comparable to the situation in the human inner ear following trauma. However, it should be noted that spiral ganglion cell loss was already 40% at this time point, which would correspond to a significant hearing deficit. The degeneration of human spiral ganglion neurons is considerably slower than that seen in the guinea pig, and is dependent on the etiology of deafness, and comparisons of the different time periods should thus be made with care. The guinea pig inner ear responds much more rapidly to ototoxic stress, which makes it a convenient model for studying interventional therapy approaches. The results show that without GDNF intervention, spiral ganglion cell loss continues, thus supporting the neurotrophic factor hypothesis (Mattson, 1998). The loss of spiral ganglion neurons is accompanied by reduced electrical responsiveness (as measured with eABRs), consistent with previous findings (Jyung et al., 1989), suggesting a strong correlation between spiral ganglion cell density and the electrical response threshold. However, as suggested in a recent study showing positive functional effects of acute uridine triphosphate treatment with no clear relationship to neuronal density, these substances may act not only by rescuing cells, but also by influencing cellular functions (Fransson et al., 2009).
The present estimation of cell densities in different experimental groups is based on the assumption that the size of the cell body is comparable, irrespectively of treatment. However, a few reports have demonstrated that administration of exogenous neurotrophins caused an increased soma size in a deafened guinea pig model (Shepherd et al., 2005; Zhou and Wu, 2006; Glueckert et al., 2008) compared to guinea pigs treated with artificial perilymph. If the size of the soma is significantly larger, there is a risk that cells may be counted twice and thus cause a false increase in cell density. Our study showed that, compared to normal untreated animals, both GDNF treatment and untreated deafening (cf. Table II) caused a small but significant increase in soma size. However, the size of the spiral ganglion neurons in the GDNF treated group was significantly smaller than in deafened guinea pigs receiving only artificial perilymph. Thus, if anything, the small differences in cell size could have reduced the difference in spiral ganglion cell density between these two groups.
The present study extends previous results in one important aspect. Gillespie and co-workers (2003) previously reported that the protective effects of BDNF on the spiral ganglion neuron population disappeared within 2 weeks after cessation of treatment, and suggested on this basis that such treatment would have minimal clinical benefit unless it were continued indefinitely. On the other hand, Maruyama and associates (2008) reported that the positive effects on eABR thresholds were maintained 2 weeks after the end of intracochlear GDNF treatment. It is possible that the discrepancy is due to differences in the action of BDNF and GDNF. Here we demonstrate that even up to 4 weeks after the end of GDNF treatment, there is a significant beneficial effect on cell survival and electrical responsiveness. In light of the neurotrophic factor hypothesis (Mattson, 1998), this is somewhat unexpected; if the loss of trophic support from the hair cells causes rapid degeneration of spiral ganglion neurons, which can be halted by exogenous infusion of GDNF, why doesn't the removal of GDNF re-initiate the degenerative processes? It is possible, as suggested by Maruyama and colleagues (2008), that after a critical period of time following inner ear injury, endogenous survival factors are activated, and are able to maintain the surviving spiral ganglion cell population. To address this issue, experiments are required in which the post-treatment period is significantly longer.
As it has been reported that the protective effects of neurotrophic intervention disappear in a couple of weeks following cessation of treatment, we included a post-treatment group receiving i.p. injections of the antioxidants ascorbic acid and Trolox. These antioxidants have previously been shown to maintain the spiral ganglion cell population and electrical responsiveness (Maruyama et al., 2007), and when administered together with GDNF, further increase the electrical responsiveness of the spiral ganglion neurons (Maruyama et al., 2008). In the present study, however, no beneficial effects of antioxidants could be detected. This can be interpreted in at least two possible ways. One is that the degenerative processes had progressed so far that formation of free radicals was no longer significantly involved (i.e., the therapeutic window for antioxidants had closed). Another interpretation is that there was no longer any degenerative process occurring. This would suggest that GDNF treatment, possibly in combination with the activation of endogenous survival factors, had permanently stopped the degeneration of spiral ganglion neurons. This in turn would suggest that the positive effects of GDNF treatment could be maintained well beyond the 4-week post-treatment period studied in this article.
The results detailed here indicate that local growth factor treatment may help prevent loss of electrical responsiveness and prevent auditory nerve cell death, and that this effect may outlast the period of treatment, and may thus enhance the benefits of cochlear implant therapy for the deaf.
Acknowledgments
This work was supported by the European Commission (FP6 Integrated Project EUROHEAR LSHG-CT-20054-512063), the Swedish Research Council, the Foundation Tysta Skolan, the Petrus and Augusta Hedlund Foundation, National Institutes of Health grant DC03820, the Ruth and Lynn Townsend Professorship, the Stockholm County Council (ALF projects 20050538 and 20060165), and in part by the Swedish Council for Working Life and Social Research through the FAS Center program Hearing Disabilities in Working Life and Society. Support was also provided by an institutional grant from the Swedish Foundation for International Cooperation in Research and Higher Education
Author Disclosure Statement
No competing financial interests exist.
References
- Agterberg M.J. Versnel H. van Dijk L.M. de Groot J.C. Klis S.F. Enhanced survival of spiral ganglion cells after cessation of treatment with brain-derived neurotrophic factor in deafened guinea pigs. J. Assoc. Res. Otolaryngol. 2009;10:355–367. doi: 10.1007/s10162-009-0170-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson A. Järlebark L.E. Ulfendahl M. In vivo infusion of UTP and uridine to the deafened guinea pig inner ear: effects on response thresholds and neural survival. J. Neurosci. Res. 2009;87:1712–1717. doi: 10.1002/jnr.21969. [DOI] [PubMed] [Google Scholar]
- Gillespie L.N. Clark G.M. Bartlett P.F. Marzella P.L. BDNF-induced survival of auditory neurons in vivo: Cessation of treatment leads to accelerated loss of survival effects. J. Neurosci. Res. 2003;71:785–790. doi: 10.1002/jnr.10542. [DOI] [PubMed] [Google Scholar]
- Gillespie L.N. Clark G.M. Marzella P.L. Delayed neurotrophin treatment supports auditory neuron survival in deaf guinea pigs. Neuroreport. 2004;15:1121–1125. doi: 10.1097/00001756-200405190-00008. [DOI] [PubMed] [Google Scholar]
- Gillespie L.N. Shepherd R.K. Clinical application of neurotrophic factors: the potential for primary auditory neuron protection. Eur. J. Neurosci. 2005;22:2123–2133. doi: 10.1111/j.1460-9568.2005.04430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glueckert R. Bitsche M. Miller J.M. Zhu Y. Prieskorn D.M. Altschuler R.A. Schrott-Fischer A. Deafferentation-associated changes in afferent and efferent processes in the guinea pig cochlea and afferent regeneration with chronic intrascalar brain-derived neurotrophic factor and acidic fibroblast growth factor. J. Comp. Neurol. 2008;507:1602–1621. doi: 10.1002/cne.21619. [DOI] [PubMed] [Google Scholar]
- Hall R.D. Estimation of surviving spiral ganglion cells in the deaf rat using the electrically evoked auditory brainstem response. Hear. Res. 1990;49:155–168. doi: 10.1016/0378-5955(90)90102-u. [DOI] [PubMed] [Google Scholar]
- Incesulu A. Nadol J.B., Jr. Correlation of acoustic threshold measures and spiral ganglion cell survival in severe to profound sensorineural hearing loss: implications for cochlear implantation. Ann. Otol. Rhinol. Laryngol. 1998;107:906–911. doi: 10.1177/000348949810701102. [DOI] [PubMed] [Google Scholar]
- Jyung R.W. Miller J.M. Cannon S.C. Evaluation of eighth nerve integrity by the electrically evoked middle latency response. Otolaryngol. Head Neck Surg. 1989;101:670–682. doi: 10.1177/019459988910100610. [DOI] [PubMed] [Google Scholar]
- Maruyama J. Miller J.M. Ulfendahl M. Glial cell line-derived neurotrophic factor and antioxidants preserve the electrical responsiveness of the spiral ganglion neurons after experimentally induced deafness. Neurobiol. Dis. 2008;29:14–21. doi: 10.1016/j.nbd.2007.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama J. Yamagata T. Ulfendahl M. Bredberg G. Altschuler R.A. Miller J.M. Effects of antioxidants on auditory nerve function and survival in deafened guinea pigs. Neurobiol. Dis. 2007;25:309–318. doi: 10.1016/j.nbd.2006.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson M.P. Neuroprotective strategies based on targeting of postreceptor signaling events. In: Mattson M.P., editor. Neuroprotective Signaling Transduction. Humana Press; Totowa, NJ: 1998. pp. 301–335. 345. [Google Scholar]
- Nadol J.B., Jr. Young Y.S. Glynn R.J. Survival of spiral ganglion cells in profound sensorineural hearing loss: implications for cochlear implantation. Ann. Otol. Rhinol. Laryngol. 1989;98:411–416. doi: 10.1177/000348948909800602. [DOI] [PubMed] [Google Scholar]
- Shepherd R.K. Coco A. Epp S.B. Crook J.M. Chronic depolarization enhances the trophic effects of brain-derived neurotrophic factor in rescuing auditory neurons following a sensorineural hearing loss. J. Comp. Neurol. 2005;486:145–158. doi: 10.1002/cne.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara T. Bredberg G. Ulfendahl M. Pyykko I. Olivius N.P. Kaksonen R. Lindstrom B. Altschuler R. Miller J.M. Neurotrophic factor intervention restores auditory function in deafened animals. Proc. Natl. Acad. Sci. USA. 2002;99:1657–1660. doi: 10.1073/pnas.032677999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata T. Miller J.M. Ulfendahl M. Olivius N.P. Altschuler R.A. Pyykko I. Bredberg G. Delayed neurotrophic treatment preserves nerve survival and electrophysiological responsiveness in neomycin-deafened guinea pigs. J. Neurosci. Res. 2004;78:75–86. doi: 10.1002/jnr.20239. [DOI] [PubMed] [Google Scholar]
- Ylikoski J. Pirvola U. Virkkala J. Suvanto P. Liang X.Q. Magal E. Altschuler R. Miller J.M. Saarma M. Guinea pig auditory neurons are protected by glial cell line-derived growth factor from degeneration after noise trauma. Hear. Res. 1998;124:17–26. doi: 10.1016/s0378-5955(98)00095-1. [DOI] [PubMed] [Google Scholar]
- Zhou L.H. Wu W. Survival of injured spinal motoneurons in adult rat upon treatment with glial cell line-derived neurotrophic factor at 2 weeks but not at 4 weeks after root avulsion. J. Neurotrauma. 2006;23:920–927. doi: 10.1089/neu.2006.23.920. [DOI] [PubMed] [Google Scholar]