Abstract
Traumatic brain injury (TBI) elicits a strong inflammatory response that contributes to the acute pathological processes seen following TBI, including cerebral edema and disruption of the blood–brain barrier (BBB), in addition to longer-term neurological damage and cognitive impairment. Proteasome inhibitors reduce vascular thrombotic and inflammatory events and consequently protect vascular function. In the present study we evaluated the neuroprotective effect of Velcade® (bortezomib), a potent and selective inhibitor of proteasomes, which is in clinical use for the treatment of multiple myeloma. When administered within 2 h after TBI onset, Velcade reduced inflammatory responses, lesion volume, and neurological functional deficits, and enhanced neuronal survival. Western blot and ELISA showed that Velcade decreased the expression of NF-κB. These results suggest that in the experimental setting, Velcade is an effective neuroprotective agent for the treatment of TBI.
Key words: neuroprotection, rats, traumatic brain injury, Velcade
Introduction
Traumatic brain injury (TBI) remains a major public health problem globally. An estimated 1.4 million people sustain TBI each year in the United States, and more than 5 million people are coping with disabilities due to TBI (Langlois et al., 2006). TBI induces a strong inflammatory response that contributes to the acute pathological processes seen following TBI; however, inflammatory regulators may play a beneficial role during periods of recovery and rehabilitation (Pan et al., 2007).
Proteasome inhibitors are a class of compounds that selectively block the activation of nuclear factor-κB (NF-κB) by inhibiting its release from IκB (Elliott et al., 2003; Magnani et al., 2000). Proteasome inhibitors have proven to be beneficial in the preclinical treatment of various types of cancer, stroke, myocardial infarction, and other inflammatory-related diseases (Di Napoli and Papa, 2003). By targeting the upstream regulator of inflammation, NF-κB, a proteasome inhibitor, thereby downregulates multiple levels of the inflammatory cascade, which results in a broad inhibition of the inflammatory response.
Velcade® (bortezomib), a proteasome inhibitor, was approved for use in the United States by the Food and Drug Administration to treat multiple myeloma, based on the results from the SUMMIT Phase II trial (Adams and Kauffman, 2004; Fisher et al., 2006). Notably, multiple myeloma increases proteasome levels in serum that decrease to normal levels in response to successful chemotherapy (Jakob et al., 2007). Studies in animals have indicated that Velcade may also have clinically significant effects in pancreatic cancer (Nawrocki et al., 2004; Shah et al., 2001). Preclinical and early clinical studies have been initiated to examine Velcade's effectiveness in treating other B-cell-related cancers (Schenkein, 2002), particularly some types of non-Hodgkin's lymphoma (O'Connor et al., 2005). Velcade attenuates murine collagen-induced arthritis, has important neuroprotective and anti-inflammatory effects on intracerebral hemorrhage (ICH; Lee et al., 2009; Sinn et al., 2007), and reduces infarction in rat models of focal cerebral ischemia (Henninger et al., 2006). Treatment with Velcade in an animal stroke model reduces adverse cerebrovascular events, including secondary thrombosis, inflammatory responses, and blood–brain barrier (BBB) disruption, and hence reduces infarct volume and neurological functional deficits when administered within 4 h after stroke onset. Velcade upregulates endothelial nitric oxide synthase (eNOS) expression, and blocks NF-κB activation (Zhang et al., 2006); however, there are no studies to date of the effect of Velcade on TBI. In the current study we therefore test the neuroprotective efficacy of Velcade in a model of TBI in rats.
Methods
All experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Henry Ford Hospital.
Animal model
A controlled cortical impact (CCI) model of TBI in rats was used in the present study (Lu et al., 2003a). Male Wistar rats weighing 300–350 g each were anesthetized intraperitoneally with chloral hydrate (350 mg/kg body weight). Rectal temperature was maintained at 37°C by using a feedback-regulated water heating pad. A CCI device was used to induce injury. The rats were placed in a stereotactic frame. Two 10-mm-diameter craniotomies were performed adjacent to the central suture, midway between the lambda and the bregma. The second craniotomy allowed for lateral movement of cortical tissue. The dura mater was kept intact over the cortex. Injury was induced by impacting the left cortex (ipsilateral cortex) with a pneumatic piston containing a 6-mm-diameter tip at a rate of 4 m/sec and 2.5 mm of compression. Velocity was measured with a linear velocity displacement transducer. Brain injury in this model is characterized by cystic cavity formation in the cortex, which causes asymmetric neurological deficits (Lu et al., 2003a), and selective cell damage in the hippocampal formation, causing spatial memory dysfunction (Chen et al., 2007; Lu et al., 2004). Therefore, sensorimotor and spatial memory tests were used to evaluate the functional response to injury and treatment after TBI.
Experimental groups
Adult male Wistar rats (n = 32) were injured with CCI and divided into two equal groups (n = 16). The first group was treated with 0.2 mg/kg Velcade (n = 8) or saline (n = 8) at 2 h after TBI. This dose of Velcade was selected based on data from stroke studies (Zhang et al., 2006). Modified Morris water maze testing (MWM) and modified Neurological Severity Scores (mNSS) were used to evaluate the spatial learning and motor-sensory functions, respectively. The rats were sacrificed 35 days after TBI. Brain samples from animals both in the Velcade- and saline-treated groups (n = 8 each) were processed for immunohistochemical studies. The second group (n = 16) was administered saline (n = 8) or Velcade (n = 8) at the same dose as in the first group. These rats were sacrificed 24 h after treatment. Five brain samples from each treatment group were processed for immunohistochemical study. Three brain samples from the boundary zone of injured rats from each treatment group were frozen and used for enzyme-linked immunosorbent assay (ELISA) and Western blot studies to detect the expression of NF-κB.
Sensorimotor functional test
The measurement of sensorimotor function was performed using the mNSS (Adams and Kauffman, 2004; Clausen, 2004; Dechend et al., 1999). This measurement was conducted on all rats before injury, and on days 1, 4, 7, 14, 21, 28, and 35 after TBI. The mNSS is a composite of motor (muscle status and abnormal movement), sensory (visual, tactile, and proprioceptive), beam balance, and reflex tests. The motor tests of the mNSS include seven items, each with a maximum score of 3 points, which mainly assess the function of the motor representation area in the contralateral cortex. Damage to this area causes contralateral limb paralysis, leading to high scores on the mNSS motor tests. Sensory tests include two items, each with a maximum score of 2, that reflect a combination of visual, tactile, and deep sensations. A unilateral lesion in the sensory and motor representations of the forelimb in the somatosensory cortex can produce contralateral asymmetry (Day and Schallert, 1996; Day et al., 1999; Yamada et al., 1999). The placing test, included as a sensory test of the mNSS, also reflects an aspect of motor function, because the corticospinal pathway mediates the execution of the placing reaction, and lesions in this region produce an enduring forelimb-placing deficit (Reh and Kalil, 1982). Beam balance tests (part of the asymmetry test) contain seven items, each with a maximum score of 6, mainly reflecting hindlimb-placing performance, which is controlled by the contralateral cortical representation of motor function. Damage to this area causes dragging of the contralateral hindlimb (the hindlimb is not placed on the beam), or the hindlimb is placed on the vertical surface of the beam to help support the animal's weight and to aid in maintaining balance, which reflects a high score on the beam balance tests. The last part of the mNSS includes the pinna, corneal, and startle reflexes, and abnormal movements. In this model, injury to the left hemisphere of the cortex in rats causes sensory and motor functional deficits, with elevated scores on motor, sensory, and beam-balance tests in the early phase after injury (day 1 post-injury) (Lu et al., 2003a). Absent reflexes and abnormal movements are seen in rats with severe injury.
Spatial learning memory test
Our spatial memory testing procedure is a modification of the MWM, as described previously (Day and Schallert, 1996; Day et al., 1999; Lu et al., 2004; Yamada et al., 1999). Data collection was automated using the HVS Image 2020 Plus Tracking System (US HVS Image, San Diego, CA). The rats were tested on days 31–35 after TBI. At the start of a trial, the rat was randomly placed at one of four fixed starting points, randomly facing toward the wall (designated north, south, east, and west), and was allowed to swim for 90 sec or until it found the platform. The platform was located in a randomly changing position within the northeast quadrant throughout the test period (for example, sometimes equidistant from the center and the edge of the pool, against the wall, near the center of the pool, or at the edges of the northeast quadrant). If the animal was unable to find the platform within 90 sec, the experiment was terminated and a maximal score of 90 sec was assigned. The percentage of time traveled within the northeast (correct) quadrant was calculated relative to the total amount of time spent swimming before reaching the platform.
Tissue preparation
Rats from the first group (n = 16), both Velcade-treated (n = 8) and saline-treated (n = 8), were anesthetized intraperitoneally with ketamine and xylazine, and perfused transcardially with saline solution containing heparin 35 days after TBI. For histological studies, after saline perfusion the animals (n = 16) were perfused with 4% paraformaldehyde in 0.1 M PBS (pH 7.4). The brains were removed, post-fixed in 10% formalin for 1–2 days at room temperature, and then processed for paraffin sectioning. A series of 6-μm-thick tissue sections were cut using a microtome through each of seven standard blocks. A section from every block was stained with hematoxylin and eosin (H&E) for lesion volume calculation. The indirect lesion area was calculated (that is, the intact area of the ipsilateral hemisphere was subtracted from the area of the contralateral hemisphere; Swanson et al., 1990), and the lesion volume is presented as a volume percentage of the lesion compared with the contralateral hemisphere. The second group of animals (n = 16) were sacrificed 24 h after TBI. Five brain samples from both treatment groups were used for immunohistochemistry studies. After saline perfusion, three brain samples from differently-treated rats were frozen and were stored in a freezer at −80°C for Western blot and ELISA studies.
Immunohistochemistry
To examine the cerebral inflammatory responses after TBI, a monoclonal anti-rat ICAM-1 antibody (BD Biosciences, San Jose, CA) at a titer of 1:500 was used to detect adhesive molecule expression in the cerebral microvessels, and a polyclonal rabbit anti-human myeloperoxidase (MPO) antibody at a titer of 1:200 (Dako North America, Carpinteria, CA) was used to detect inflammatory cells. Matrix metalloproteinase-9 (MMP-9), a member of the MMP family of zinc-binding proteolytic enzymes, specifically degrades type IV collagen, a key structural component of the basement membrane that surrounds blood vessels, and promotes the breakdown of the BBB (Mun-Bryce and Rosenberg, 1998). To examine the integrity of cerebral microvessels, a mouse anti-rat type IV collagen mAb (M3F7; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA) at a titer of 1:200, and a mouse anti-rat MMP-9 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) at a titer of 1:200, were used and were counterstained with vWF 1:200 (Dako). These sections were obtained from the second group, sacrificed at 24 h after TBI. To examine the survival of neurons after different treatments following TBI, fluorescent immunohistochemical staining for microtubule-associated protein-2 at a titer of 1:400 (Chemicon, Temecula, CA) was used to identify neuronal cells in the dentate gyrus and the hippocampal CA3 region, and were counterstained with propidium iodide (PI) in the first group of animal samples.
Western blot analysis
Brain tissue from the lesion boundary zone in the second group of animals was washed once in 1× PBS and lysed in lysis buffer (20 mM Tris [pH 7.6], 100 mM NaCl, 1% Nonidet P-40, 0.1% SDS, 1% deoxycholic acid, 10% glycerol, 1 mM EDTA, 1 mM NaVO3, 50 mM NaF, cocktail I of protease inhibitors from CalBiochem, San Diego, CA). After sonication, soluble protein was obtained by centrifugation at 13,000g for 15 min at 4°C. The protein concentration of each sample was determined by bicinchoninic acid (BCA) protein assay (Pierce, Rockford, IL). For immunoblotting, equal amounts of cell lysate were subjected to SDS polyacrylamide electrophoresis on Novex Tris-Glycine pre-cast gels (Invitrogen, Carlsbad, CA), and the separated proteins were then electrotransferred to polyvinylidene fluoride (PVDF) membranes. The membranes were blocked with 2% I-Block (Applied Biosystems, Foster, CA) in PBS plus 0.1% Tween 20 for 1 h at room temperature, and then incubated with different primary antibodies overnight at 4°C. RelA is a member of the Rel/NF-κB family of transcription factors. To detect RelA expression, Western blot analysis was performed using an antibody against RelA (1: 1000; Santa Cruz Biotechnology). For control of protein loads, a β-actin antibody (1:5000; Sigma-Aldrich, St. Louis, MO) was used. After washing, the membranes were incubated with HRP-conjugated secondary antibodies (1:2500; Jackson ImmunoResearch Laboratories, West Grove, PA) in blocking buffer for 2 h at room temperature. Specific proteins were visualized using the SuperSignal West Pico chemiluminescence substrate system (Pierce). The intensity of the bands was measured using Scion image analysis software (Scion Cooperation, Frederick, MD).
Enzyme-linked immunosorbent assay
The PathScan Phospha-NF-κB P65 Sandwich ELISA kit (#7173; Cell Signaling Technology, Inc., Beverly, MA) was used for protein quantitative analysis on the same brain tissue used for Western blot. First, 100 μL of each diluted sample solution was added to the coated microwells and the tops of the wells were sealed firmly. The samples were incubated for 2 h at 37°C, followed by the addition of 100 μL of detection antibodies for 1 h at 37°C. HRP-linked secondary antibody was added to each well for 30 min at 37°C. The reaction was stopped by adding 100 μL of stop solution to each well. The plates were washed four times with washing buffer (PBS [pH 7.4] containing 0.1% [v/v] Tween 20) after each step. The absorbance at 450 nm was measured within 30 min after adding stop solution.
Statistical analysis
All data are presented as the means ± standard deviation. Data were analyzed using an analysis of variance for repeated measures of functional test data (Lu et al., 2003b). A paired t-test was used to examine the difference in cell counts in the ipsilateral hemisphere, as well as the functional changes that occurred in the Velcade-treated and saline groups. All measures were analyzed by observers blinded to individual treatment.
Results
Effects of Velcade on TBI outcome
Modified Neurological Severity Scores
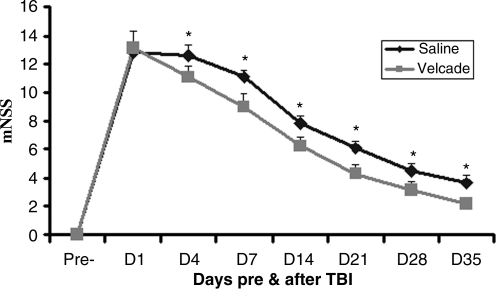
Injury in the left hemisphere of the cortex in rats caused neurological functional deficits as measured by mNSS. The higher the modified mNSS, the worse the sensorimotor function. Figure 1 shows the changes in sensorimotor function in injured rats after different treatments. Compared to the scores of the saline group, treatment with Velcade significantly improved sensorimotor function on days 4 (p = 0.024), 7 (p = 0.027), 14 (p = 0.012), 21 (p = 0.042), 28 (p = 0.040), and 35 (p = 0.027) after TBI.
FIG. 1.
This graph shows changes in sensorimotor scores in saline- and Velcade-treated rats after traumatic brain injury (TBI). The scores of the Velcade-treated rats were significantly lower than those for the saline-treated rats from days 4–35 after TBI. Data are presented as the mean ± standard deviation (n = 8/group; *p < 0.05 versus saline; mNSS, modified Neurological Severity Score).
Spatial learning function
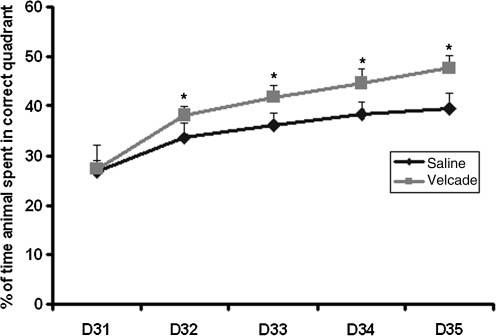
Spatial learning was tested during the last 5 days (days 31–35 post-injury) using the MWM test without prior training before injury. Spatial learning function was significantly improved in rats treated with Velcade compared with that of the saline-treated group on days 32 (p = 0.022), 33 (p = 0.042), 34 (p = 0.040), and 35 (p = 0.00015) (Fig. 2). These data demonstrate that Velcade improves spatial learning function after TBI.
FIG. 2.
The graph demonstrates the changes in spatial learning ability in saline- and Velcade-treated rats after traumatic brain injury (TBI). The Velcade-treated rats exhibited significant functional improvement compared with the saline-treated rats 32–35 days after TBI. Data are presented as the mean ± standard deviation (n = 8/group; *p < 0.05 versus saline).
Lesion volume
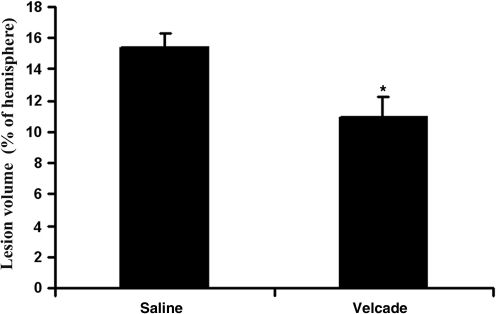
Figure 3 shows that treatment with Velcade (0.2 mg/kg) administered 2 h after TBI significantly reduced the lesion volume in the injured cortex compared to that of the saline group (n = 8 per group) at 35 days after TBI (p1 < 0.0001).
FIG. 3.
This bar graph shows that the lesion volume of the Velcade-treated group was significantly reduced compared to that of the saline-treated group when examined at day 35 after traumatic brain injury. Data are presented as the mean ± standard deviation (n = 8/group; *p < 0.0001).
Histological analysis
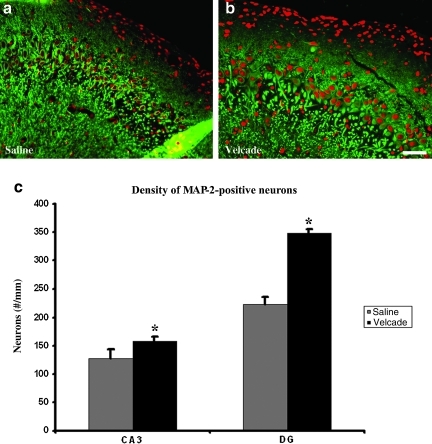
Brain tissue from five animals from each treatment in the first group used for functional testing (sacrificed at 35 days) was prepared for paraffin sectioning. Three sections at 100-μm intervals from block E (containing the dentate gyrus and the dorsal hippocampus) were selected for fluorescent immunohistochemical staining for microtubule-associated protein-2 (MAP-2), to identify neuronal cells in the dentate gyrus and the hippocampal CA3 region, and were counterstained with PI. The number of MAP-2-positive cells in these two regions of the ipsilateral hemisphere was counted under a 20× objective using the MCID system, and then divided by the corresponding length (mm) to calculate the density of the surviving neuronal cells (Lu et al., 2004). These data showed that treatment with Velcade significantly reduced the loss of neurons in the dentate gyrus (p < 0.0001), and the hippocampal CA3 region (p = 0.0196), compared with the saline group in rats 35 days after TBI (n = 5 per group; Fig. 4).
FIG. 4.
The bar graph (c) and micrographs (a and b) show the numbers of microtubule-associated protein-2 (MAP-2) positive cells in the CA3 and dentate gyrus (DG) areas of saline- and Velcade-treated rats examined at day 35 after traumatic brain injury (TBI). The Velcade-treated rats showed significantly increased numbers of surviving neurons than the saline-treated rats. Data are presented as the mean ± standard deviation (CA3 p < 0.05; DG p < 0.05; scale bar = 50 μm; n = 8).
Effects of treatment on inflammatory responses
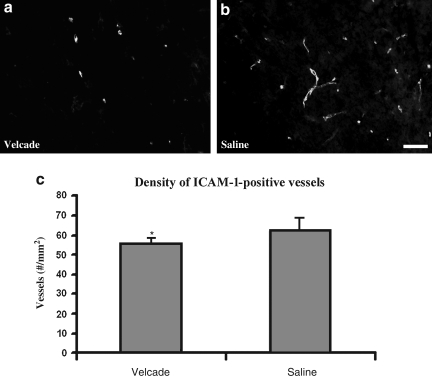
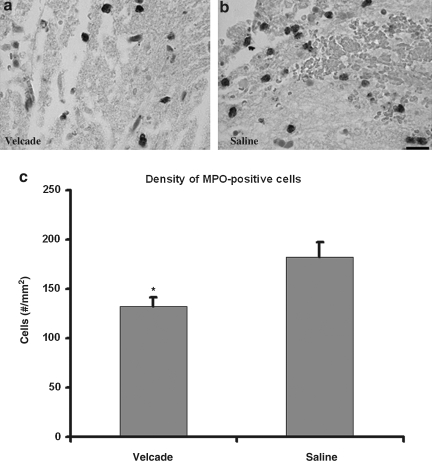
TBI exacerbates inflammatory responses. In the present study, treatment with Velcade 2 h after TBI significantly reduced the number of intracellular adhesion molecule-1 (ICAM-1)-immunoreactive vessels (n = 5 per group, p = 0.032; Fig. 5), and MPO-immunoreactive cells (n = 5 per group, p = 0.037; Fig. 6), detected 24 h after treatment compared with the saline-treated group. ICAM-positive vessels and MPO-positive cells were present primarily in the boundary zone, which includes injured cortical tissue and injured hippocampus. These data demonstrate that treatment with Velcade reduces the cerebral inflammatory response.
FIG. 5.
(a and b) Micrographs showing ICAM-1-positive vessels in the boundary zone of saline- and Velcade-treated rats (scale bar = 50 μm). (c) Bar graph showing that the density of ICAM-1-positive vessels in the Velcade-treated rats was significantly lower than that in the saline-treated rats examined 24 h after TBI (*p < 0.05, n = 5/group; ICAM-1, intracellular adhesion molecule-1; TBI, traumatic brain injury).
FIG. 6.
Bar graph (c) and micrographs (a and b) show the myeloperoxidase (MPO)-positive cells in brown in the boundary zone of saline- and Velcade-treated rats. The density of MPO-positive cells in the boundary zone of Velcade-treated rats was significantly lower than that of the saline-treated group examined at 24 h after traumatic brain injury (*p < 0.05, n = 5/group; scale bar = 25 μm).
Effects of treatment on microvascular integrity
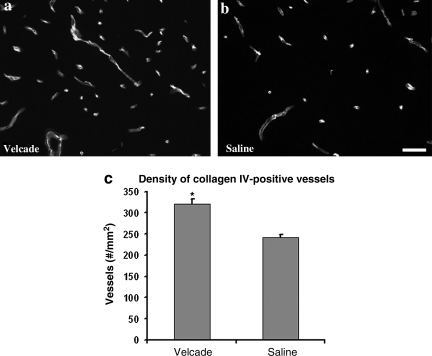
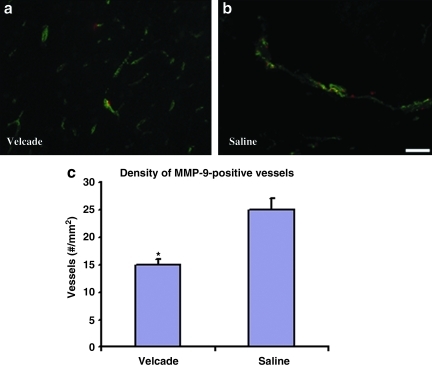
Treatment with Velcade at 2 h significantly increased collagen IV-immunoreactive vessels (n = 5 per group, p < 0.0001; Fig. 7), and reduced MMP-9 immunoreactive vessels (n = 5 per group, p = 0.007; Fig. 8) in the ipsilateral cortex compared with the saline-treated group examined at 24 h after TBI. Thus our data suggest that treatment with Velcade promotes microvascular integrity.
FIG. 7.
Bar graph (c) and micrographs (a and b) show differences in collagen IV-positive vessels of the boundary zone in rats with different treatments after traumatic brain injury (TBI). The density of collagen IV-positive vessels in the Velcade-treated group was significantly higher than that of the saline-treated group when examined at 24 h after TBI (*p < 0.05, n = 5/group; scale bar = 50 μm).
FIG. 8.
Bar graph (c) and micrographs (a and b) showing changes in matrix metalloproteinase-9 (MMP-9) in the boundary zone of saline- and Velcade-treated rats. Compared to the saline-treated group examined at 24 h after traumatic brain injury (TBI), the Velcade-treated group shows significantly lower MMP-9 density after double-staining (*p < 0.05, n = 5/group; scale bar = 50 μm).
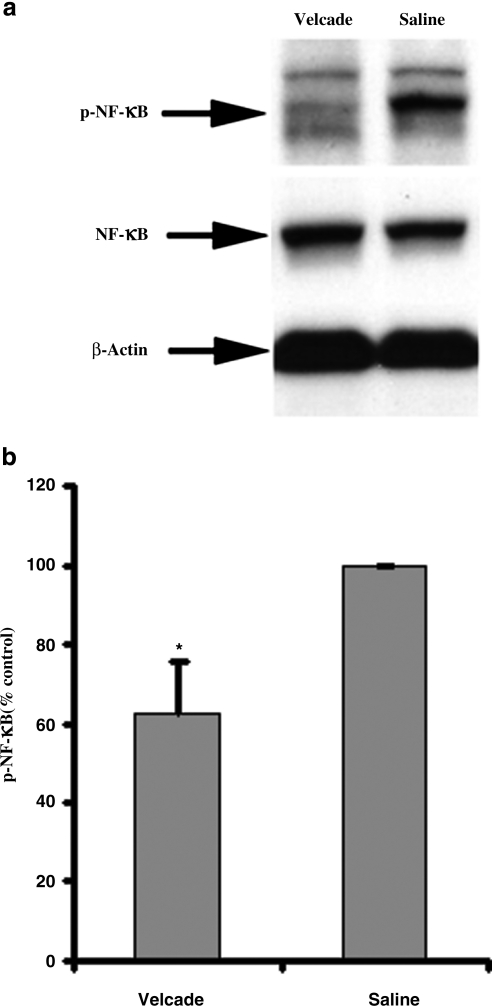
NF-κB level quantified with ELISA array and Western blot
Western blot analysis showed that expression of NF-κB in brain tissue at the lesion boundary zone at 24 h after TBI was no different in Velcade-treated rats than in the saline-treated group. However, the expression of phospho-NF-κB in Velcade-treated rats was significantly decreased compared to that in the saline group, using both ELISA (p = 0.0043) and Western blot (n = 3; p < 0.0001; Fig. 9).
FIG. 9.
The effect of Velcade on nuclear factor-κB (NF-κB) phosphorylation in the boundary zone in rats examined at 24 h after TBI. (a) Western blot detection of NF-κB and phospho-NF-κB. (b) Phospho-NF-κB (p-NF-κB) expression as detected by ELISA (n = 3, p = 0.0043; TBI, traumatic brain injury; ELISA, enzyme-linked immunosorbent assay).
Discussion
Our major findings in the present study were: (1) Velcade (0.2 mg/kg) injected IV 2 h after TBI reduced functional deficits and improved spatial learning ability; (2) Velcade reduced inflammatory responses and lesion volume; (3) treatment with Velcade significantly reduced the loss of neurons in the dentate gyrus and the hippocampal CA3 compared with the saline-treated group 35 days after TBI; (4) the activation of NF-κB in the boundary zone of injured brain tissue in rats was decreased with the administration of Velcade IV; and (5) treatment with Velcade protects microvascular integrity. These results suggest that Velcade may be useful in the treatment of TBI.
TBI elicits a strong inflammatory response that is a complex process and may involve differential regulation and response at different post-TBI time points (Lloyd et al., 2008). While cytokines and inflammatory regulators may play a beneficial role in the later periods of recovery and rehabilitation, such factors appear detrimental to patient outcome in the acute stages of head injury (Pan et al., 2007; Shuaib et al., 2002; Williams et al., 2006). As the inflammatory response typically develops hours or days after TBI, inflammatory mediators such as adhesion molecules and proinflammatory cytokines participating in this secondary inflammatory cascade are potential therapeutic targets for brain injury (Del Zoppo, 1997). TBI triggers endothelial dysfunction, which promotes inflammatory responses, procoagulant events, and BBB disruption (Clausen, 2004). Activated endothelial cells upregulate adhesion molecules and cytokines, which facilitates microvascular endothelial-leukocyte adhesion, evokes microvascular perfusion deficits, and contributes to further breakdown of the BBB (Clausen, 2004). The secretion of MMP-9 increases BBB permeability (Mun-Bryce and Rosenberg, 1998; Rosenberg et al., 2001).
NF-κB is an important transcription factor, regulating many genes that play key roles in embryonic development, immune and inflammatory responses, lymphoid differentiation, and apoptosis (Baeuerle and Baltimore, 1996; Baldwin, 1996; Verma et al., 1995). NF-κB is activated in the brain after focal ischemia, global ischemia, and TBI (Carroll et al., 2000; Chen et al., 2007; Clemens et al., 1997; Plesnila et al., 2007; Schneider et al., 1999). The ubiquitin-proteasome pathway is a principal pathway for regulating NF-κB activation (Karin and Delhase, 2000). In the quiescent state, NF-κB is present in the cytoplasm in an inactive complex with the inhibitory protein IκB. In response to various stimuli, IκB undergoes phosphorylation and ubiquitination, and is subsequently degraded by the proteasome (Palombella et al., 1994). Activated NF-κB is then translocated into the nucleus, where it binds to the promoter of a large number of genes, including IL-1β, IL-6, TNF-α, selectins, ICAM-1, tissue factor, MMPs, and PAI-1 (Barnes and Karin, 1997; Dechend et al., 1999; Monaco et al., 2004). Although a controversial role of NF-κB in the regulation of cell survival and death has also been suggested, there is substantial evidence that NF-κB activation in brain contributes to neuronal death (Mattson and Camandola, 2001). Occlusion of the middle cerebral artery results in less brain damage in mice lacking the p50 subunit of NF-κB compared with wild-type mice (Nurmi et al., 2004; Schneider et al., 1999). Thus treatment strategies that inhibit NF-κB activation may reduce adverse vascular events and may reduce the loss of neuronal cells after TBI. Our data show that treatment with Velcade decreased the expression of phospho-NF-κB, and hence significantly reduced the number of ICAM-1-immunoreactive vessels and MPO-immunoreactive cells at 24 h after treatment, demonstrating that Velcade plays a role in anti-inflammatory effects after TBI.
By targeting the upstream regulator of inflammation NF-κB, Velcade downregulates multiple levels of the inflammatory cascade, which results in broad inhibition of the inflammatory response. The nuclear factor-κB family of transcription factors is intimately involved in the regulation of the inflammatory responses that play a fundamental role in damage to articular tissues (Roman-Blas and Jimenez, 2008). Early treatment with Velcade induced a reduction in early hematoma growth and mitigated the development of brain edema, coupled with a marked inhibitory effect on inflammation in ICH (Sinn et al., 2007). Treatment with Velcade reduces adverse cerebrovascular events, including secondary thrombosis, inflammatory responses, and BBB disruption, and hence reduces infarct volume and neurological functional deficits when administered within 4 h after stroke onset (Zhang et al., 2006). Velcade inhibits the expression of adhesion molecules and proinflammatory cytokines in endothelial cells. Our data indicate that treatment with Velcade (0.2 mg/kg) initiated 2 h after TBI reduces inflammatory responses, such that it decreases the loss of neurons in the DG and CA3 areas, reduces lesion volume and neurological functional deficits, and enhances the spatial learning function and increases vascular integrity. However, the effects of Velcade on endothelial cell gene expression, which provokes vascular inflammatory responses, thrombosis, and BBB damage, have not yet been studied in TBI. Therefore the mechanisms by which treatment with Velcade reduces endothelial inflammation and permeability require further investigation at the level of genes and molecular expression after TBI. Further studies investigating the therapeutic window and dose responses for Velcade treatment of TBI are warranted. The present study is proof of principle that Velcade (as in stroke) has a potent therapeutic benefit for TBI. Velcade is a proteasome inhibitor with strong antitumor activity against many tumors, primarily multiple myeloma (Koreth et al., 2009). Some concern regarding the safety of Velcade has been raised, with studies showing neuropathic pain as the main side effect and dose-limiting factor in clinical practice after multiple-dose long-term administration (Gilardini et al., 2008). The chronic administration (4 weeks) of Velcade (0.08–0.2 mg/kg twice or three times daily) reduced sensory nerve conduction velocity in the tail in rats (Cavaletti et al., 2007). However, recovery was complete after the 4-week follow-up period.
Conclusion
In conclusion, here we demonstrated that treatment with Velcade effectively reduces lesion volume, inflammatory responses, and loss of neurons in the DG and CA3 areas. Velcade also enhances microvascular integrity and spatial learning. In addition, treatment with Velcade decreases the activation of phosphorylated NF-κB in the lesion boundary zone brain tissue in rats after TBI. Thus Velcade appears to be neuroprotective and may be useful in the treatment of TBI.
Acknowledgments
This work was supported by National Institutes of Health grants PO1 NS42345 and RO1 NS42259. Special thanks to Susan MacPhee-Gray for editorial assistance.
Author Disclosure Statement
No conflicting financial interests exist.
References
- Adams J. Kauffman M. Development of the proteasome inhibitor Velcade (Bortezomib) Cancer Invest. 2004;22:304–311. doi: 10.1081/cnv-120030218. [DOI] [PubMed] [Google Scholar]
- Baeuerle P.A. Baltimore D. NF-kappa B: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- Baldwin A.S., Jr The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu. Rev. Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- Barnes P.J. Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N. Engl. J. Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- Carroll J.E. Hess D.C. Howard E.F. Hill W.D. Is nuclear factor-kappaB a good treatment target in brain ischemia/reperfusion injury? Neuroreport. 2000;11:R1–R4. [PubMed] [Google Scholar]
- Cavaletti G. Gilardini A. Canta A. Rigamonti L. Rodriguez-Menendez V. Ceresa C. Marmiroli P. Bossi M. Oggioni N. D'Incalci M. DeCoster R. Bortezomib-induced peripheral neurotoxicity: a neurophysiological and pathological study in the rat. Exp. Neurol. 2007;204:317–325. doi: 10.1016/j.expneurol.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Chen G. Shi J. Ding Y. Yin H. Hang C. Progesterone prevents traumatic brain injury-induced intestinal nuclear factor kappa B activation and proinflammatory cytokines expression in male rats. Mediators Inflamm. 20072007:93431. doi: 10.1155/2007/93431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen F. Delayed cell death after traumatic brain injury—Role of reactive oxygen species. Comprehensive summaries of Uppsala Dissertations from the Faculty of Medicine. 2004;1359:1–75. [Google Scholar]
- Clemens J.A. Stephenson D.T. Smalstig E.B. Dixon E.P. Little S.P. Global ischemia activates nuclear factor-kappa B in forebrain neurons of rats. Stroke. 1997;28:1073–1080. doi: 10.1161/01.str.28.5.1073. ; discussion 1080–1071. [DOI] [PubMed] [Google Scholar]
- Day L.B. Schallert T. Anticholinergic effects on acquisition of place learning in the Morris water task: spatial mapping deficit or inability to inhibit nonplace strategies? Behav. Neurosci. 1996;110:998–1005. doi: 10.1037//0735-7044.110.5.998. [DOI] [PubMed] [Google Scholar]
- Day L.B. Weisand M. Sutherland R.J. Schallert T. The hippocampus is not necessary for a place response but may be necessary for pliancy. Behav. Neurosci. 1999;113:914–924. doi: 10.1037//0735-7044.113.5.914. [DOI] [PubMed] [Google Scholar]
- Dechend R. Maass M. Gieffers J. Dietz R. Scheidereit C. Leutz A. Gulba D.C. Chlamydia pneumoniae infection of vascular smooth muscle and endothelial cells activates NF-kappaB and induces tissue factor and PAI-1 expression: a potential link to accelerated arteriosclerosis. Circulation. 1999;100:1369–1373. doi: 10.1161/01.cir.100.13.1369. [DOI] [PubMed] [Google Scholar]
- Del Zoppo G.J. Microvascular responses to cerebral ischemia/inflammation. Ann. NY Acad. Sci. 1997;823:132–147. doi: 10.1111/j.1749-6632.1997.tb48386.x. [DOI] [PubMed] [Google Scholar]
- Di Napoli M. Papa F. Curr. Opin. Investig. Drugs. 2003;4:333–341. MLN-519. Millennium/PAION. [PubMed] [Google Scholar]
- Elliott P.J. Zollner T.M. Boehncke W.H. Proteasome inhibition: a new anti-inflammatory strategy. J. Mol. Med. 2003;81:235–245. doi: 10.1007/s00109-003-0422-2. [DOI] [PubMed] [Google Scholar]
- Fisher R.I. Bernstein S.H. Kahl B.S. Djulbegovic B. Robertson M.J. De Vos S. Epner E. Krishnan A. Leonard J.P. Lonial S. Stadtmauer E.A. O'Connor O.A. Shi H. Boral A.L. Goy A. Multicenter phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. J. Clin. Oncol. 2006;24:4867–4874. doi: 10.1200/JCO.2006.07.9665. [DOI] [PubMed] [Google Scholar]
- Gilardini A. Marmiroli P. Cavaletti G. Proteasome inhibition: A promising strategy for treating cancer, but what about neurotoxicity? Curr. Med. Chem. 2008;15:3025–3035. doi: 10.2174/092986708786848622. [DOI] [PubMed] [Google Scholar]
- Henninger N. Sicard K.M. Bouley J. Fisher M. Stagliano N.E. The proteasome inhibitor Velcade reduces infarction in rat models of focal cerebral ischemia. Neurosci. Lett. 2006;398:300–305. doi: 10.1016/j.neulet.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Jakob C. Egerer K. Liebisch P. Turkmen S. Zavrski I. Kuckelkorn U. Heider U. Kaiser M. Fleissner C. Sterz J. Kleeberg L. Feist E. Burmester G.R. Kloetzel P.M. Sezer O. Circulating proteasome levels are an independent prognostic factor for survival in multiple myeloma. Blood. 2007;109:2100–2105. doi: 10.1182/blood-2006-04-016360. [DOI] [PubMed] [Google Scholar]
- Karin M. Delhase M. The I kappa B kinase (IKK) and NF-kappa B: key elements of proinflammatory signalling. Semin. Immunol. 2000;12:85–98. doi: 10.1006/smim.2000.0210. [DOI] [PubMed] [Google Scholar]
- Koreth J. Alyea E.P. Murphy W.J. Welniak L.A. Proteasome inhibition and allogeneic hematopoietic stem cell transplantation: A review. Biol. Blood Marrow Transplant. 2009;15:1502–1512. doi: 10.1016/j.bbmt.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois J.A. Rutland-Brown W. Wald M.M. The epidemiology and impact of traumatic brain injury: a brief overview. J. Head Trauma Rehabil. 2006;21:375–378. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- Lee S.W. Kim J.H. Park Y.B. Lee S.K. Bortezomib attenuates murine collagen-induced arthritis. Ann. Rheum. Dis. 2009;68:1761–1767. doi: 10.1136/ard.2008.097709. [DOI] [PubMed] [Google Scholar]
- Lloyd E. Somera-Molina K. Van Eldik L.J. Watterson D.M. Wainwright M.S. Suppression of acute proinflammatory cytokine and chemokine upregulation by post-injury administration of a novel small molecule improves long-term neurologic outcome in a mouse model of traumatic brain injury. J. Neuroinflammation. 2008;5:28. doi: 10.1186/1742-2094-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D. Goussev A. Chen J. Pannu P. Li Y. Mahmood A. Chopp M. Atorvastatin reduces neurological deficit and increases synaptogenesis, angiogenesis, and neuronal survival in rats subjected to traumatic brain injury. J. Neurotrauma. 2004;21:21–32. doi: 10.1089/089771504772695913. [DOI] [PubMed] [Google Scholar]
- Lu D. Mahmood A. Zhang R. Copp M. Upregulation of neurogenesis and reduction in functional deficits following administration of DEtA/NONOate, a nitric oxide donor, after traumatic brain injury in rats. J. Neurosurg. 2003a;99:351–361. doi: 10.3171/jns.2003.99.2.0351. [DOI] [PubMed] [Google Scholar]
- Lu M. Chen J. Lu D. Yi L. Mahmood A. Chopp M. Global test statistics for treatment effect of stroke and traumatic brain injury in rats with administration of bone marrow stromal cells. J. Neurosci. Methods. 2003b;128:183–190. doi: 10.1016/s0165-0270(03)00188-2. [DOI] [PubMed] [Google Scholar]
- Magnani M. Crinelli R. Bianchi M. Antonelli A. The ubiquitin-dependent proteolytic system and other potential targets for the modulation of nuclear factor-kB (NF-kB) Curr. Drug Targets. 2000;1:387–399. doi: 10.2174/1389450003349056. [DOI] [PubMed] [Google Scholar]
- Mattson M.P. Camandola S. NF-kappaB in neuronal plasticity and neurodegenerative disorders. J. Clin. Invest. 2001;107:247–254. doi: 10.1172/JCI11916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco C. Andreakos E. Kiriakidis S. Mauri C. Bicknell C. Foxwell B. Cheshire N. Paleolog E. Feldmann M. Canonical pathway of nuclear factor kappa B activation selectively regulates proinflammatory and prothrombotic responses in human atherosclerosis. Proc. Natl. Acad. Sci. USA. 2004;101:5634–5639. doi: 10.1073/pnas.0401060101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mun-Bryce S. Rosenberg G.A. Matrix metalloproteinases in cerebrovascular disease. J. Cereb. Blood Flow Metab. 1998;18:1163–1172. doi: 10.1097/00004647-199811000-00001. [DOI] [PubMed] [Google Scholar]
- Nawrocki S.T. Sweeney-Gotsch B. Takamori R. Mcconkey D.J. The proteasome inhibitor bortezomib enhances the activity of docetaxel in orthotopic human pancreatic tumor xenografts. Mol. Cancer Ther. 2004;3:59–70. [PubMed] [Google Scholar]
- Nurmi A. Lindsberg P.J. Koistinaho M. Zhang W. Juettler E. Karjalainen-Lindsberg M.L. Weih F. Frank N. Schwaninger M. Koistinaho J. Nuclear factor-kappaB contributes to infarction after permanent focal ischemia. Stroke. 2004;35:987–991. doi: 10.1161/01.STR.0000120732.45951.26. [DOI] [PubMed] [Google Scholar]
- O'Connor O.A. Wright J. Moskowitz C. Muzzy J. Macgregor-Cortelli B. Stubblefield M. Straus D. Portlock C. Hamlin P. Choi E. Dumetrescu O. Esseltine D. Trehu E. Adams J. Schenkein D. Zelenetz A.D. Phase II clinical experience with the novel proteasome inhibitor bortezomib in patients with indolent non-Hodgkin's lymphoma and mantle cell lymphoma. J. Clin. Oncol. 2005;23:676–684. doi: 10.1200/JCO.2005.02.050. [DOI] [PubMed] [Google Scholar]
- Palombella V.J. Rando O.J. Goldberg A.L. Maniatis T. The ubiquitin-proteasome pathway is required for processing the NF-kappa B1 precursor protein and the activation of NF-kappa B. Cell. 1994;78:773–785. doi: 10.1016/s0092-8674(94)90482-0. [DOI] [PubMed] [Google Scholar]
- Pan D.S. Liu W.G. Yang X.F. Cao F. Inhibitory effect of progesterone on inflammatory factors after experimental traumatic brain injury. Biomed. Environ. Sci. 2007;20:432–438. [PubMed] [Google Scholar]
- Plesnila N. Von Baumgarten L. Retiounskaia M. Engel D. Ardeshiri A. Zimmermann R. Hoffmann F. Landshamer S. Wagner E. Culmsee C. Delayed neuronal death after brain trauma involves p53-dependent inhibition of NF-kappaB transcriptional activity. Cell Death Differ. 2007;14:1529–1541. doi: 10.1038/sj.cdd.4402159. [DOI] [PubMed] [Google Scholar]
- Reh T. Kalil K. Functional role of regrowing pyramidal tract fibers. J. Comp. Neurol. 1982;211:276–283. doi: 10.1002/cne.902110306. [DOI] [PubMed] [Google Scholar]
- Roman-Blas J.A. Jimenez S.A. Targeting NF-kappaB: a promising molecular therapy in inflammatory arthritis. Int. Rev. Immunol. 2008;27:351–374. doi: 10.1080/08830180802295740. [DOI] [PubMed] [Google Scholar]
- Rosenberg G.A. Cunningham L.A. Wallace J. Alexander S. Estrada E.Y. Grossetete M. Razhagi A. Miller K. Gearing A. Immunohistochemistry of matrix metalloproteinases in reperfusion injury to rat brain: activation of MMP-9 linked to stromelysin-1 and microglia in cell cultures. Brain Res. 2001;893:104–112. doi: 10.1016/s0006-8993(00)03294-7. [DOI] [PubMed] [Google Scholar]
- Schenkein D. Proteasome inhibitors in the treatment of B-cell malignancies. Clin. Lymphoma. 2002;3:49–55. doi: 10.3816/clm.2002.n.011. [DOI] [PubMed] [Google Scholar]
- Schneider A. Martin-Villalba A. Weih F. Vogel J. Wirth T. Schwaninger M. NF-kappaB is activated and promotes cell death in focal cerebral ischemia. Nat. Med. 1999;5:554–559. doi: 10.1038/8432. [DOI] [PubMed] [Google Scholar]
- Shah S.A. Potter M.W. Mcdade T.P. Ricciardi R. Perugini R.A. Elliott P.J. Adams J. Callery M.P. 26S proteasome inhibition induces apoptosis and limits growth of human pancreatic cancer. J. Cell Biochem. 2001;82:110–122. doi: 10.1002/jcb.1150. [DOI] [PubMed] [Google Scholar]
- Shuaib A. Xu Wang C. Yang T. Noor R. Effects of nonpeptide V(1) vasopressin receptor antagonist SR-49059 on infarction volume and recovery of function in a focal embolic stroke model. Stroke. 2002;33:3033–3037. doi: 10.1161/01.str.0000039405.31526.06. [DOI] [PubMed] [Google Scholar]
- Sinn D.I. Lee S.T. Chu K. Jung K.H. Kim E.H. Kim J.M. Park D.K. Song E.C. Kim B.S. Yoon S.S. Kim M. Roh J.K. Proteasomal inhibition in intracerebral hemorrhage: neuroprotective and anti-inflammatory effects of bortezomib. Neurosci. Res. 2007;58:12–18. doi: 10.1016/j.neures.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Smith D.H. Soares H.D. Pierce J.S. Perlman K.G. Saatman K.E. Meaney D.F. Dixon C.E. Mcintosh T.K. A model of parasagittal controlled cortical impact in the mouse: cognitive and histopathologic effects. J. Neurotrauma. 1995;12:169–178. doi: 10.1089/neu.1995.12.169. [DOI] [PubMed] [Google Scholar]
- Swanson R.A. Morton M.T. Tsao-Wu G. Savalos R.A. Davidson C. Sharp F.R. A semiautomated method for measuring brain infarct volume. J. Cereb. Blood Flow Metab. 1990;10:290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- Verma I.M. Stevenson J.K. Schwarz E.M. Van Antwerp D. Miyamoto S. Rel/NF-kappa B/I kappa B family: intimate tales of association and dissociation. Genes Dev. 1995;9:2723–2735. doi: 10.1101/gad.9.22.2723. [DOI] [PubMed] [Google Scholar]
- Williams A.J. Dave J.R. Tortella F.C. Neuroprotection with the proteasome inhibitor MLN519 in focal ischemic brain injury: relation to nuclear factor kappaB (NF-kappaB), inflammatory gene expression, and leukocyte infiltration. Neurochem. Int. 2006;49:106–112. doi: 10.1016/j.neuint.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Yamada K. Tanaka T. Mamiya T. Shiotani T. Kameyama T. Nabeshima T. Improvement by nefiracetam of beta-amyloid-(1–42)-induced learning and memory impairments in rats. Br. J. Pharmacol. 1999;126:235–244. doi: 10.1038/sj.bjp.0702309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L. Zhang Z.G. Liu X. Hozeska A. Stagliano N. Riordan W. Lu M. Chopp M. Treatment of embolic stroke in rats with bortezomib and recombinant human tissue plasminogen activator. Thromb. Haemost. 2006;95:166–173. [PubMed] [Google Scholar]