Figure 2. Importin α2 and Ntf2 Levels Differ in X. Laevis and X. Tropicalis.
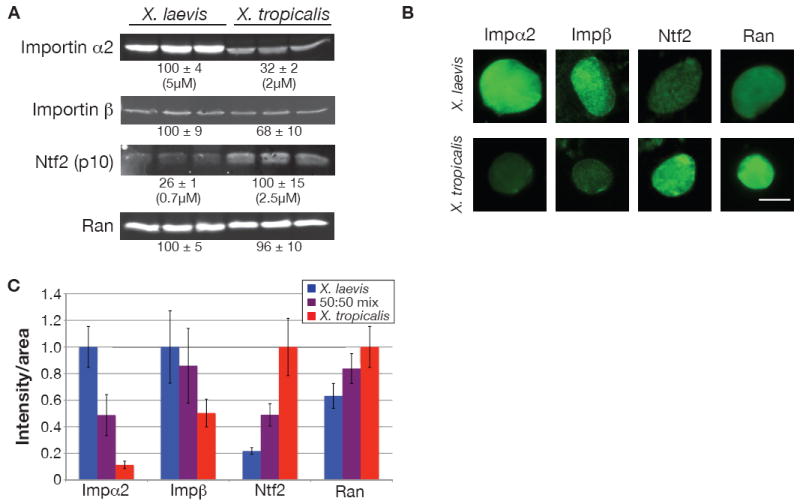
(A) 25 μg of protein from three different X. laevis and X. tropicalis egg extracts were separated by SDS-PAGE, transferred to nitrocellulose, and probed with antibodies against the indicated proteins. Values below each set of three lanes represent relative protein amounts (mean ± SD, n=3) quantified by infrared fluorescence. Absolute concentrations were determined by comparing band intensities to known concentrations of recombinant importin α2 or Ntf2 on the same blot. Two different antibodies against importin α2 and Ntf2 showed similar differences between the two species.
(B) Nuclei at 80 min were processed for immunofluorescence using the same antibodies as in (A) and representative images are shown. For a given antibody, images were acquired with the same exposure time and scaled identically. Scale bar, 20 μm.
(C) Quantification of nuclei displayed in (B). Nuclear fluorescence intensity per unit area was calculated for at least 50 nuclei per condition, averaged, and normalized to 1.0 (arbitrary units). Error bars represent SD. Two different antibodies against importin α2 and Ntf2 showed similar differences between the two species.
See also Figure S2.
