Abstract
Objective
This article provides estimates of education differentials in life expectancy with and without cognitive impairment for the noninstitutionalized population aged 70 years and older in the United States.
Method
Life expectancy with cognitive impairment was calculated using multistate models, allowing transitions between cognitively intact and cognitively impaired states and from each of these states to death and allowing transition rates to vary across age and education. Four waves of the Assets and Health Dynamics of the Oldest Old survey were used.
Results
Those with low levels of education are more likely to become cognitively impaired and do so at an earlier age. After age 70, persons with low educational levels can expect to live 11.6 years, and persons with high education 14.1 years, without cognitive impairment. Length of life with cognitive impairment differs by education (1.6 years and 1.0 years at age 70, respectively) but differs little by age.
Discussion
Although those with higher education have lower rates of both cognitive impairment and mortality, those who do become cognitively impaired appear to be in poorer health, leading to a reduced probability of improved cognition and increased probability of mortality relative to those with lower educational levels.
Keywords: health expectancy, cognitive impairment, education, elderly, AHEAD, longitudinal studies
Cognitive impairment is a major health problem in old age and an area of growing concern for population health. Cognitive impairment contributes to diminished quality of life, decreases in active life expectancy (Gallo, Schoen, & Jones, 2000; Portrait, Lindeboom, & Deeg, 2001), and elevated mortality in old age (Aguero-Torres, Fratiglioni, Guo, Viitanen, & Winblad, 1999; Helmer, Joly, Letenneur, Commenges, & Dartigues, 2001). It also represents a major cause of institutionalization and increases the need for services among both those with impairment and their caregivers, such that a significant portion of health care resources is spent caring for older people with this condition (Langa et al., 2001; Manton, Corder, & Clark, 1993; Toseland, McCallion, Gerber, & Banks, 2002).
Because the population is aging and age is the strongest risk factor for cognitive impairment and dementia (Aronson et al., 1991), the prevalence of cognitive impairment is projected to increase (Brookmeyer, Gray, & Kawas, 1998). The length of life with cognitive impairment will increase as total life expectancy increases, unless the age at onset of cognitive impairment is delayed, perhaps by addressing modifiable risk factors (Haan & Wallace, 2004; Suthers, Kim, & Crimmins, 2003).
Like most health outcomes, the burden of cognitive impairment is not distributed equally in the population. Those with less education are more likely to be cognitively impaired (Albert, 1995; Lee, Kawachi, Berkman, & Grodstein, 2003). Low education is thought to be related to cognitive impairment because persons with lower education may reach old age with less cognitive reserve (Stern, 2002), poorer physical health (Kempen, Brilman, Ranchor, & Ormel, 1999; Seeman et al., 2004), and a greater risk for cognitive decline (Evans et al., 1993; Farmer, Kittner, Rae, Bartko, & Regier, 1995; Jacqmin-Gadda, Fabrigoule, Commenges, & Dartigues, 1997; Lee et al., 2003) and generally appear to be on an earlier aging trajectory relative to those with higher levels of education.
Low education and cognitive impairment are both related to higher mortality (Aguero-Torres et al., 1999; Christenson & Johnson, 1995; Helmer et al., 2001; Hummer, Rogers, & Eberstein, 1998; Steenland, Hu, & Walker, 2004). Although much of the literature emphasizes the long lives of people with dementia, mortality is estimated to be two to three times higher among cognitively impaired older persons relative to those without cognitive impairment (Aguero-Torres et al., 1999; Helmer et al., 2001).
The burden of cognitive impairment is related to the length of life spent with cognitive impairment, which depends on the incidence of impairment, recovery from impairment, and mortality levels. Health and mortality differences can interact to result in longer, healthier lives for some population groups and shorter or more disabled lives for other groups (Hayward & Heron, 1999). Healthy life expectancy is an approach that allows us to combine age-specific information on mortality and morbidity to estimate the average time that an individual will live in varying heath states given a set of health and mortality transitions. It provides a summary measure of both health quality and quantity of life in populations (Robine, Jagger, Mathers, Crimmins, & Suzman, 2003). Although health expectancy is a generic term for expectancy in a specific health state, most research has focused on disability-free life expectancy (Crimmins, Hayward, & Saito, 1994). Higher levels of education are related to longer disability-free life expectancy (Crimmins & Cambois, 2003; Crimmins, Hayward, & Saito, 1996; Crimmins & Saito, 2001; Land, Guralnik, & Blazer, 1994), but research has not addressed the relationship between education and life expectancy with and without cognitive impairment.
Previously, estimates of the length of life expectancy with cognitive impairment based on the preferred multistate approach have not been available for U.S. national samples. Estimates of dementia-free life expectancy for several countries and a previous analysis of life expectancy with and without cognitive impairment using the Assets and Health Dynamics of the Oldest Old (AHEAD) sample all relied on prevalence data and information on mortality from vital statistics and the cross-sectional Sullivan method (Dubois & Hebert, 2006; Perenboom, Boshuizen, Breteler, Ott, & Van de Water, 1996; Ritchie & Polge, 2003; Suthers et al., 2003). However, the use of incident transitions from longitudinal data, both to better and worse health, is the theoretically preferred approach to estimating healthy life expectancy, because the transitions occur in a known time period and at a specified age, requiring fewer assumptions about the nature and timing of transitions (Laditka & Hayward, 2003). In addition, mortality by health state, which is not available from vital statistics data, is incorporated into multistate models. Mortality by health state is particularly important in this case, in which there are large differences in mortality by cognitive state. Using clinic-based data from the United Kingdom, Sauvaget, Jagger, and Arthur (2001) estimated cognitive impairment–free life expectancy using a modified multistate method but allowed only transitions into impairment, assuming no recovery. Movement of individuals in and out of disability states is well documented and is an important parameter in estimates of disability-free life expectancy (Crimmins et al., 1994; Manton, Stallard, & Corder, 1998), but improvement of cognitive functioning in large population surveys has rarely been examined (Larrieu et al., 2002; Liang, Borawski-Clark, Liu, & Sugisawa, 1996). It is important to be able to model such transitions as they occur in populations, even if transitions out of cognitive impairment are relatively rare. Research suggests that although recovery from cognitive impairment occurs infrequently, a significant portion of the causes of cognitive impairment may be reversible (Clarfield, 2003; Insel & Badger, 2001) and as many as 40% of mild cognitive impairment cases improve (Larrieu et al., 2002). Some of the most common reversible causes of dementia and cognitive impairment include treatable conditions such as depression and metabolic and nutrition-related risk factors (Bogner et al., 2007; Clarfield, 2003) or normal pressure hydrocephalus (Vacca, 2007). Treatment of these conditions may result in reversal of cognitive loss. In addition, some cognitive loss may be related to more acute health events such as stroke (Saxena, Ng, Koh, Yong, & Fong, 2007; Zhu et al., 1998) or cancer treatment (Hurria & Lachs, 2007) and may improve over time.
Liang et al. (1996) did model life expectancy with and without cognitive impairment in Japan using multistate methods that allowed for transitions in and out of cognitive impairment. Results suggested that education was related both to the probability of becoming impaired and to the probabilities of recovery and mortality among the impaired, demonstrating the importance of modeling all possible transitions, especially in a sample with different levels of education.
The purpose of this article is to examine educational differences in life expectancy with and without cognitive impairment among Americans aged 70 and over. This article expands on existing research by estimating life expectancy in a nationally representative sample of older persons in the United States using a multistate method and by incorporating analysis of education, a major risk factor for cognitive impairment and mortality. These models incorporate variability in mortality by cognitive state and education to determine cognitively intact and impaired life expectancies, representing an improvement over estimates assuming similar mortality across the population. Furthermore, our approach allows us to determine not only how the risk of cognitive impairment differs across groups but also to estimate the potentially reversible character of this process and to determine whether transition rates vary across education groups. Improved estimates of the burden of cognitive impairment among older persons and in specific subgroups of the older population from a nationally representative sample will also provide important information for health planning.
Method
Study Population
We examined cognitive state and mortality at four waves of the AHEAD study. AHEAD was the first large nationally representative survey to collect information on cognitive functioning (Herzog & Wallace, 1997). The first wave was conducted in 1993 among 7,443 noninstitutionalized persons aged 70 and over. At the first interview the response rate to the AHEAD survey was 80.4% (HRSonline, 2007). Follow-up interviews were conducted in 1995, 1998, and 2000. Changes in cognitive status and mortality between these interviews are the basis of health transitions. Because our estimates are based on transitions in cognitive state, we excluded 215 persons with only one interview from the 7,443 initial participants of AHEAD. Missing cases were older, had lower education, and were more likely to be males. Analyses are based on 7,228 respondents who completed at least two interviews either by self-report or proxy. A proxy provided data when the respondent was unable to participate directly. A proxy is most likely to be used when the person is ill, cognitively impaired, or very busy and active. Among eligible respondents at the first wave, 7% were proxies. Although the baseline population does not include institutionalized persons, individuals who moved to an institution after the first wave were included. We estimate that most of the institutionalized population is represented by the third wave, as 82% of the institutionalized population has been so for 5 years or less (Gabrel, 2000).
Interviews were conducted over the phone or in person; however, no significant difference in cognitive performance was found between participants with telephone and face-to-face assessments (Herzog & Rodgers, 1999). At the first wave, 74% of the respondents aged 70 to 79 were interviewed by phone and 67% of respondents aged 80 and over were interviewed in person. For most respondents, the same interview mode occurred in subsequent waves. Death of sample members including date and cause is reported in later waves by informants; the sample is also linked to the National Death Index through 2002. Life expectancy at age 70 computed from this sample is 14.4 years; life expectancy at age 70 for the United States in 1997, which is the midpoint of the period covered, was 14.3 years (National Center for Health Statistics, 1997).
Variables
Cognition measures
Cognitive function for self-respondents in AHEAD is based on a summary performance-based measure that reflects a range of cognitive abilities, including memory, language, orientation, and attention. The summary measure comprises four cognitive tests: the Telephone Interview for Cognitive Status, which includes 10 items relating to knowledge, language, and orientation (e.g., naming objects and the president, the date); a serial 7s subtraction test (respondents were asked to subtract 7 from 100 and then to continue subtracting 7 from the calculated number for a total of five times); an immediate word recall task (participants were asked to recall a list of 10 words); and a delayed word recall task (participants were asked to recall the same list of 10 words after a 5-minute delay; Herzog & Wallace, 1997). The summary score is calculated by pooling the results of these tests and ranges from 0 (severely impaired) to 35 (high cognitive functioning). At baseline, participants with a score less than or equal to 8 were considered cognitively impaired, with others classified as cognitively intact. Estimates of the age-specific prevalence of cognitive impairment in the United States using this definition based on the first wave of the AHEAD sample supplemented with information on the institutionalized population from the third wave are similar to those from the Framingham study (Suthers et al., 2003).
A common problem in repeated cognitive testing is the practice effect, in which participants learn how to answer tests, resulting in artificial improvement of cognitive function score with repeated testing (Rabbitt, Diggle, Holland, & McInness, 2004). Practice effects appear to be particularly important from the first to second testing. Because previous research indicated an average practice effect of approximately 1 point in the summary score, we accounted for the practice effect by changing the definition of cognitive impairment to a score of less than or equal to 9 for the last three waves (Alley, Suthers, & Crimmins, 2007).
A second issue in constructing the cognitive summary score is that the number of respondents who declined to participate in the Serial 7s Test was significantly higher than refusals on other cognitive tests. At baseline, 12% (n = 769) of the 6,651 self-respondents refused to answer Serial 7s questions; less than 1% of participants refused the word recall lists or individual items on the Telephone Instrument for Cognitive Status. Although the number of refusals changed across subsequent data collection points, the pattern of disproportionately greater refusals on the Serial 7s Test is consistent at every time point. Because these data are not missing at random (Herzog & Wallace, 1997), it is important to take account of missing data rather than to delete missing cases or assign them a score of 0 (Rodgers, Ofstedal, & Herzog, 2003). Serial 7s values for participants who refused were imputed cross-sectionally based on values on the other three cognitive tests of the same wave. This imputation procedure was applied on 603 persons at the first wave, 283 at the second wave, 158 at the third wave, and 103 at the last wave. These imputations led to few changes in participant cognitive status. Across Waves 1, 2, and 3, imputation resulted in a change of cognitive status for only 16 persons. Imputation had a greater effect on the last interview, changing the status of 89 persons. The impact of imputation on transition rates is also minor. Imputation increased the number of transitions by 82, from 16,786 transitions across the four waves to 16,868 transitions. In general, imputations increased cognitively impaired life expectancy relative to coding refusals as missing; however, these differences were not significant. Results shown below are based on analysis of imputed values.
For persons whose information is provided by proxies, the cognitive measure is based on seven questions about the sample individual’s behavior that reflect cognitive status. Proxies are asked whether the respondent got lost in familiar environments, frequently wandered, had hallucinations, was unable to be left alone, was poor in making judgments, was poor in organizing daily activities, or was poor in using memory skills. If the respondent was reported to display two or more of these behaviors, he or she was defined as cognitively impaired. Although it is possible to have different proxies at different interviews, 86% of those with proxies at the first two waves had the same proxy both times.
Education
Analyses were stratified based on self-reported years of education. We defined two levels of education: low education if the respondents completed less than 12 years of education and high education if the participants completed at least 12 years, which is equivalent to the completion of secondary school in the United States.
Life table models
Life expectancies with and without cognitive impairment are calculated using IMaCh (Interpolated Markov Chain) software version 0.97, which models transitions among states as a discrete-time Markov chain (Lièvre, Brouard, & Heathcote, 2003). The model allows transitions between cognitively intact and cognitively impaired states and from each of these cognitive states to death. Hence, we estimate age-specific probabilities of the onset of cognitive impairment, recovery to a cognitively intact state, and mortality among people with and without cognitive impairment, that is, the probability an individual in state i (i = cognitively intact or impaired) at age x (measured in single year) will be in state j (j = cognitively intact, impaired, or dead) at age x + 1. The IMaCh program first estimates the age-specific transition probabilities with a multinomial logistic regression and then uses these probabilities to estimate life expectancies with and without cognitive impairment. This approach does not require a fixed interval length and incorporates information from cases with missed interviews using an interpolation method. Standard deviations are used to calculate 95% confidence intervals for the transition probabilities and the health expectancies.
Results
Sample Description
The characteristics of the sample are shown in Table 1. Multistate analyses are based on 7,228 persons who answered at least two interviews during the period 1994 to 2000. The sample at the first wave includes 7,138 persons who completed the first interview; 90 additional participants had missing data at the first wave but were interviewed at least twice in later waves. After 6 years of follow-up, one third of the sample (n = 2,702) had died. At baseline, the mean age was 77, almost two thirds of the sample were females (62%), and 42% had a low level of education. Because the sample ages with time and the risk of dying differs by gender, health status, and education, at the fourth wave 65% of the survivors were females and only 37% had a low education level. Only 6% were cognitively impaired at the first wave, but this proportion increased to 11% by the last wave.
Table 1.
Sample Description Based on 7,228 Individuals With Two or More Interviews
| Wave 1 | Wave 2 | Wave 3 | Wave 4 | |
|---|---|---|---|---|
| Average date of interview | January 1994 | January 1996 | April 1998 | May 2000 |
| Interview completed (n) | 7,138 | 6,028 | 5,020 | 4,109 |
| Dead (n) | — | 794 | 1,810 | 2,702 |
| Missinga (n) | 90 | 406 | 398 | 417 |
| Proxy (%) | 10.8 | 13.0 | 15.4 | 17.1 |
| Mean age (years) | 77.4 | 79.5 | 81.2 | 82.8 |
| Female (%) | 62.3 | 62.8 | 63.7 | 64.6 |
| Cognitively impaired (%) | 6.4 | 8.0 | 10.0 | 10.7 |
| Less than 12 years education (%) | 42.0 | 40.3 | 38.4 | 36.7 |
Missing includes nonparticipants to the survey and persons who did not provide answers to the cognition items.
Differentials in Prevalence of Cognitive Impairment by Education at Baseline
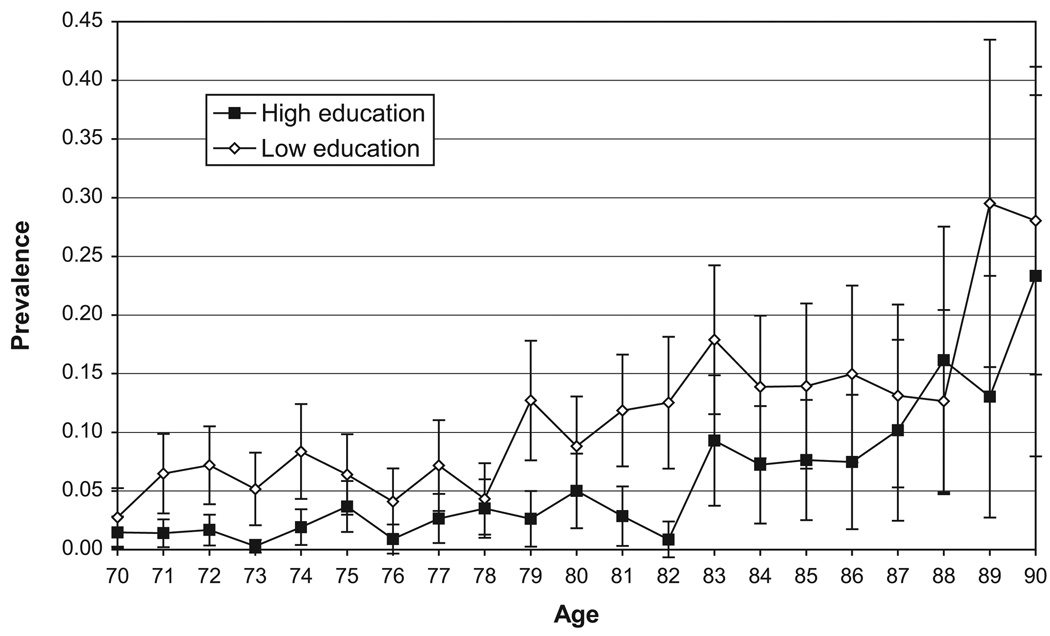
The age-specific prevalence of cognitive impairment at the first wave by gender and educational level is presented in Figure 1. The proportion with cognitive impairment among those aged 70 and older with low levels of education is 11.5%, versus 3.5% among the higher educated. At almost every age the prevalence of cognitive impairment is higher for the lower educated than for the higher educated, but these differences are not uniformly significant.
Figure 1.
Prevalence of Cognitive Impairment by Education at Baseline
Educational Differences in Onset of Cognitive Impairment and Recovery From Cognitive Impairment
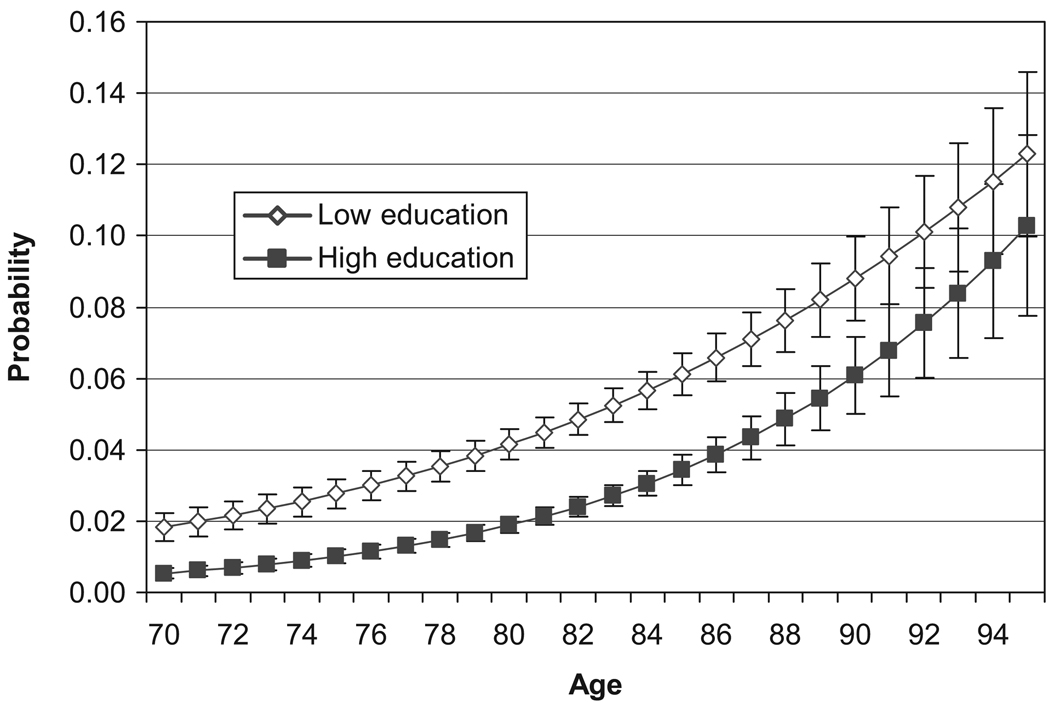
Annual probabilities of becoming cognitively impaired by education are presented in Figure 2. Not surprisingly, the onset of cognitive impairment increases with age. The probability of becoming cognitively impaired is lower for the higher education group and the difference is statistically significant for all ages until 91. At age 90, the likelihood of becoming cognitively impaired is about 9% for those with low education and about 6% for those with high education.
Figure 2.
Transition Rates From Noncognitively Impaired to Cognitively Impaired by Age and Education
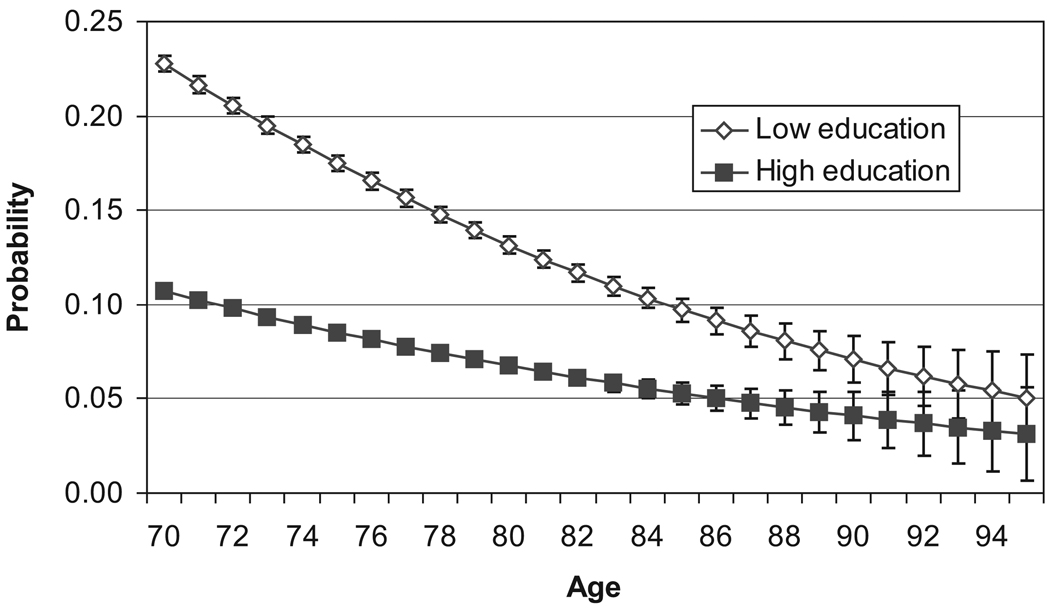
We also find differences in the likelihood of return to intact cognitive functioning by age and education (Figure 3). The probability of recovery from impairment declines with age and is higher among the low education group. A 70-year-old classified as cognitively impaired has a .23 probability of being scored cognitively intact at age 71 if he or she has less than a high school education and .10 probability with a higher educational level. With increasing age, the recovery rate remains higher among the lower educated, but the difference by education becomes smaller and is not significant after age 90. We discuss the characteristics of those who recover and the implications of these relatively high levels of return to recovery later in the article.
Figure 3.
Transition Rates From Cognitively Impaired to Cognitively Intact by Age and Education
Differentials in Mortality
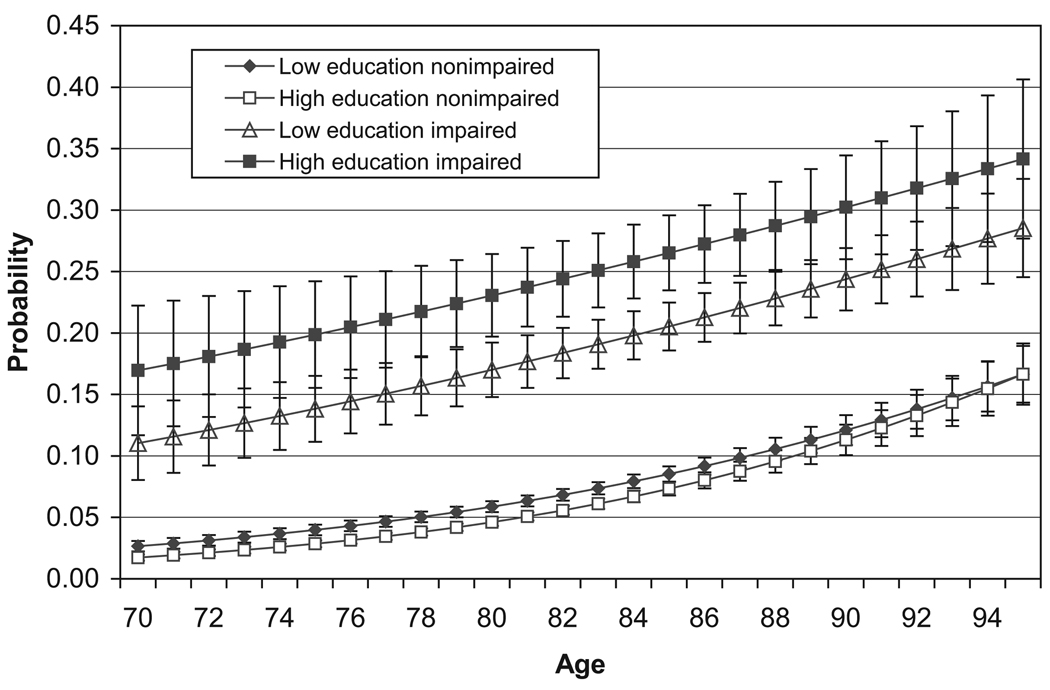
Annual mortality rates for people with and without cognitive impairment are presented in Figure 4. Persons with cognitive impairment have higher mortality rates than those without cognitive impairment, but how much higher depends on both education and age. Surprisingly, the risk of dying among those with cognitive impairment is generally higher for the more educated than for the low education group, although this difference is not always significant. For those without cognitive impairment, there is little difference by education in the risk of dying, meaning that the effects of mortality differences by education in the length of cognitively impaired life will be due to differences among the impaired. At age 80 those without cognitive impairment have about a 5% risk of dying; those with cognitive impairment and low education have a risk three times higher (17%), whereas the risk for those with cognitive impairment and high education is more than four times as high (23%). This result suggests that the highly educated cognitively impaired group consists of more individuals with more serious health problems, who are more likely to die.
Figure 4.
Death Rates by Age, Education, and Cognitive Impairment
Differentials in Life Expectancy With and Without Cognitive Impairment
Education differences in both total life expectancy and expectancies with and without cognitive impairment based on these transition probabilities are presented in Table 2. Life expectancy and cognitively intact life expectancy decrease dramatically with age, whereas life expectancy with cognitive impairment remains relatively stable in the population as age increases. This is true for the total population and for both educational groups. On average, the higher educated group can expect to live more years without cognitive impairment than the lower educated group and can expect a shorter time in the cognitively impaired state.
Table 2.
Life Expectancy (LE) With and Without Cognitive Impairment
| Age | Total LE | Cognitively Intact LE |
LE With Impairment |
Percentage Total LE With Impairment |
|---|---|---|---|---|
| Total sample | ||||
| 70 | 14.41 (14.10–14.72) | 13.12 (12.83–13.41) | 1.29 (1.19–1.39) | 9.0% |
| 80 | 8.50 (8.27–8.73) | 7.13 (6.92–7.34) | 1.37 (1.25–1.49) | 16.1% |
| 90 | 4.63 (4.39–4.88) | 3.12 (2.90–3.34) | 1.51 (1.32–1.70) | 32.6% |
| Low education | ||||
| 70 | 13.24 (12.77–13.72) | 11.61 (11.16–12.07) | 1.63 (1.45–1.80) | 12.3% |
| 80 | 8.01 (7.71–8.31) | 6.33 (6.05–6.60) | 1.68 (1.51–1.85) | 21.0% |
| 90 | 4.6 (4.28–4.92) | 2.87 (2.58–3.16) | 1.73 (1.47–2.00) | 37.6% |
| High education | ||||
| 70 | 15.08 (14.67–15.49) | 14.06 (13.67–14.45) | 1.02 (0.90–1.15) | 6.8% |
| 80 | 8.82 (8.47–9.17) | 7.72 (7.40–8.04) | 1.1 (0.94–1.26) | 12.5% |
| 90 | 4.65 (4.28–5.02) | 3.41 (3.06–3.75) | 1.24 (0.98–1.51) | 26.7% |
Note: Confidence intervals given in parentheses.
At age 70, the remaining lifetime without impairment is 14.1 years for the highly educated and 11.6 years for the low education group. This can also be interpreted as an average age of onset of cognitive impairment of 84.1 years for those with high education and 81.6 years for those with lower education. At the same age, the highly educated will spend 1 year, or 7% of remaining life, with impairment, whereas the low education group will spend 1.6 years, or 12% of remaining life, cognitively impaired. These proportions increase rapidly with age. At age 90, those with low education will spend about one third (37.6%) of their time with impairment; this percentage is reduced to 26.7% if the person is highly educated. However, life expectancy with impairment remains quite stable with age, leading us to observe a potentially incompressible time spent in impairment.
Recovery From Cognitive Impairment
Our data suggest a somewhat higher rate of recovery from cognitive impairment than might be expected, and we find a significantly higher recovery rate for those with less education than for those with more education. We address two issues in this section: (a) How does this high recovery rate affect our conclusions about the length of cognitively impaired life expectancy and educational differentials? (b) Is there an explanation for the high rate of recovery and educational differences in recovery?
To test the robustness of our life expectancy differentials by education, we recalculated the health expectancy for the low education group assuming that their recovery rate was the same as that calculated for the highly educated. Results are presented in Table 3. Reducing the recovery rate among the low education group (approximately dividing it by 2) does not significantly change our estimates of life expectancy with cognitive impairment. Although the point estimate is 1.8 years under simulated conditions, rather than the 1.6 years reported before, these estimates have largely overlapping confidence intervals. This is because the prevalence of impairment is very small in younger ages where the incidence of recovery is higher.
Table 3.
Recalculated Life Expectancy (LE) for Low Educated Assuming Recovery Rate of High Educated Group
| Age | Total LE | Cognitively Intact LE | LE With Impairment |
|---|---|---|---|
| 70 | 12.81 (12.34–13.29) | 10.99 (10.50–11.47) | 1.83 (1.61–2.04) |
| 80 | 7.75 (7.46–8.04) | 5.93 (5.65–6.20) | 1.82 (1.64–2.01) |
| 90 | 4.47 (4.16–4.79) | 2.66 (2.38–2.94) | 1.82 (1.55–2.09) |
Note: Confidence intervals given in parentheses.
To further understand the factors that might be linked to recovery, we look at the characteristics of those who are cognitively impaired at Wave 1 who either recover from impairment by Wave 2 or remain impaired at Wave 2 (Table 4). Those who recovered cognitive functioning are less likely to be female relative to those who remain cognitively unimpaired (especially among the higher educated). People who recover cognitive functioning are less likely to have a proxy respondent at both interviews and more likely to change from proxy to performance testing that would be appropriate for people who recover functioning.
Table 4.
Characteristics at Wave 1 of All Participants, Cognitively Unimpaired Participants, Impaired Participants, Those Who Recovered by Wave 2, and Those Who Remained Cognitively Impaired at Wave 2
| Total at Wave 1 | Unimpaired at Wave 1 |
Impaired at Wave 1 |
Recovery by Wave 2 |
Remain Cognitively Impaired at Wave 2 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Low Ed | High Ed | Low Ed | High Ed | Low Ed | High Ed | Low Ed | High Ed | Low Ed | High Ed | |
| Number of cases | 3,163 | 3,975 | 2,794 | 3,837 | 369 | 138 | 79 | 15 | 148 | 58 |
| Age (in years) | 78.8 | 76.6 | 78.1 | 76.4 | 82.1 | 81.6 | 80.0 | 77.4 | 82.6 | 81.6 |
| Female | 61.9 | 62.4 | 61.9 | 62.4 | 63.9 | 62.8 | 57.1*** | 36.2*** | 75.1 | 72.5 |
| Telephone interview | 53.1 | 66.3* | 54.4** | 66.6*, ** | 46.5 | 58.9* | 56.6 | 64.9 | 49.6 | 62.7 |
| Proxy Wave 1 | 15.6 | 7.3* | 11.9** | 5.0*, ** | 47.0 | 73.8* | 28.1 | 62.5* | 53.2 | 79.3* |
| Proxy Wave 1/2 | ||||||||||
| Perf/perf | 78.0 | 90.3* | 83.1** | 92.2*, ** | 28.5 | 9.5* | 43.6*** | 13.3 | 21.3 | 8.4* |
| Proxy/perf | 2.0 | 1.3* | 2.1 | 1.3* | 2.5 | 4.9 | 5.5*** | 21.5*** | 1.2 | 0.0 |
| Perf/proxy | 9.2 | 4.0* | 6.5** | 3.6*, ** | 26.3 | 15.0 | 28.3 | 24.1 | 25.4 | 12.3 |
| Proxy/proxy | 10.7 | 4.3* | 8.3** | 3.0*, ** | 42.6 | 70.5* | 22.6*** | 41.1*, *** | 52.1 | 79.3* |
| Can | 11.6 | 15.4* | 12 | 15.3* | 8.5 | 18.0* | 5.2 | 7.8 | 5.4 | 18.7* |
| Depressed | 32.0 | 19.7* | 30.5** | 19.6* | 53.0 | 25.6* | 55.2 | 17.6 | 51.6 | 18.0* |
| Diabetes | 15.5 | 10.7* | 15.6 | 10.7* | 15.3 | 14.8 | 14.4 | 24.7 | 13.3 | 14.6 |
| Hearing problem | 32.5 | 21.1* | 31.4** | 20.6*, ** | 41.5 | 35.2 | 40.3 | 41.0 | 39.1 | 42.5 |
| Heart disease | 35.3 | 28.9* | 35.2 | 29.0* | 37.1 | 26.4 | 29.5 | 16.6 | 34.8 | 27.6 |
| Hypertension | 53.2 | 47.0* | 53.6 | 47.4* | 53.2 | 45.2 | 55.3 | 26.6* | 49.9 | 54.1 |
| Stroke | 10.6 | 7.2* | 9.1** | 6.4*, ** | 22.9 | 29.2 | 17.1 | 41.5* | 15.7 | 22.6 |
p < .05, from chi-square test between education groups within impaired or recovery category.
p < .05, from chi-square test between unimpaired and impaired within education groups.
p < .05, from chi-square test between those who recovered and those who stayed impaired within education groups.
We hypothesized that those with treatable conditions such as depression and those recovering from strokes and cancer treatment might be more likely to recover. Within education groups, our data do not indicate a difference in depression or cancer between those who recover and those who stay impaired; however, the proportion with stroke among those with higher education recovering cognitive functioning is almost twice as high as among those who remain impaired (41.5 vs. 22.6). Although this is not a significant difference, it does suggest that there may be some link between stroke recovery and improving cognitive function among those with higher education. This could be linked to better rehabilitative treatment received or to the fact that those with better medical care and better understanding of medical conditions might report more mild strokes initially.
The characteristics in Table 4 can also add some insight into the lower recovery from impairment and higher mortality with impairment of those with higher education. Both of these findings led us to suggest that the highly educated impaired might be in worse overall health than those of lower education with impairment. A history of cancer is more common among those with higher education, which could be related to higher mortality. Impaired persons with higher education are more likely to have proxy respondents at both interviews than those with less education, which is an indicator of being in worse overall health. It is also possible that there is more individual variability in performance-based responses than those of proxies resulting in lower recovery for those of higher education.
Discussion
This article provides the first estimates of life expectancies by cognitive status and education calculated from longitudinal data using a multistate model. Several important findings emerge from this analysis. First, average life expectancy without cognitive impairment differs by education level: A person aged 70 years with less than a high school education can expect to spend almost 12 years or 88% of his or her remaining life without impairment, whereas an individual with 12 years of education or more can expect to spend 14 years or more than 90% of his or her remaining life cognitively intact. Second, life expectancy with cognitive impairment also differs by education. Those in the lower education group can expect to experience 1.6 years with cognitive impairment at age 70, whereas those in the higher education group can expect to average 1 year with cognitive impairment. Third, the expected years with cognitive impairment stays relatively constant as age increases within educational group. This suggests a relatively incompressible period of cognitive impairment for the average individual, similar to the terminal drop phenomenon (Wilson, Beckett, Bienias, Evans, & Bennett, 2003). Fourth, the characteristics of the cognitively impaired population differ by education in ways that meaningfully affect an individual’s probability of both recovery and death. Those with higher education are less likely to become cognitively impaired, but when they do, they appear to exhibit more severe cognitive impairment and to be in worse health. They are more likely to have cancer, less likely to recover cognitive function, and more likely to die than those with lower education. Finally, results show a small but significant amount of recovery from cognitive impairment. Those with cognitive impairment at age 80 (6% of participants) have approximately an 11% probability of recovery by the following year. However, because a small proportion of participants are cognitively impaired and an even smaller proportion are likely to recover, the effect of recovery on estimates of life expectancy with cognitive impairment is small.
Our analyses are based on a multistate model that employs a more realistic model of the processes that underlie differences in life expectancies in time period studied than prevalence-based methods (the so-called Sullivan method). However, Sullivan estimates of life expectancy with cognitive impairment using data from the first wave of the AHEAD study come relatively close to the estimate provided here (1.5 years with cognitive impairment vs. 1.3 in this study; Suthers et al., 2003). Previous analysis that has assumed no recovery from cognitive impairment may not accurately reflect the full spectrum of transitions in the population (Sauvaget et al., 2001), but our analysis suggests that population estimates of healthy life expectancy are not highly affected.
To compensate for the learning that accompanies cognitive testing, we included an adjustment practice effect in our cognitive score after the first measurement. We should note that the practice effect was somewhat greater for those with lower education (−.06 for each year of education) so that the average effect for those with 8 years of education was .9 and .4 for those with 16 years of education. However, if we eliminate the practice effect our results are not changed, as all life expectancy estimates are within the original confidence intervals. For instance, the life expectancy with cognitive impairment changes from 1.63 to 1.44 years for those with low education and from 1.02 to 0.97 for those with higher education.
It is important to understand more about those who are assessed as recovering cognitive function. Our analysis suggests that they are more likely to be males and among the more highly educated to have had a stroke than those who remain impaired. However, they are also more likely to be based on performance tests rather than proxy reports, introducing the possibility that some recovery may be due to measurement error. Some observed recovery may simply be due to intraindividual variability in cognitive performance, and it is possible that variability is higher among the less educated. Given that cognitive impairment is defined by a cutpoint, small movements around that threshold are observed as transitions in and out of cognitive impairment. However, in additional analysis, we find significant recovery even after increasing the cutpoint and considerable variability in the cognitive score increase among those who recovered. Small increases (such as from a score of 8 to 10) do not appear to account for the recovery observed here. Furthermore, it is useful to note that the total number of individuals who recover from cognitive impairment remains small relative to those who become impaired.
Unfortunately, without clinical data, it is difficult to determine the cause of cognitive impairment and how this might be related to differences in cognitively impaired life expectancy. Cognitive impairment may be due to a range of causes, although vascular dementia and Alzheimer’s disease are among the most common. It is likely that the distribution of type of impairment may differ by education, as vascular conditions are both more prevalent and have a higher incidence among older persons with less education (Crimmins, Hayward, & Seeman, 2004). Differences in both incidence of and recovery from cognitive impairment could be affected by this. It is also possible that some of the recovery observed here is due to treatment of clinical depression or delirium related to medication problems or nutrition deficiencies not identifiable in this sample.
Differences in health expectancies by education are the outcome of disparities in transition probabilities across cognitive states. The main factor driving educational differences in life expectancy without cognitive impairment are reduced or delayed onset of cognitive impairment among those with higher education. Educational differentials might result from conditions earlier in life, better health throughout life, more access to and use of health care, and better health behaviors among higher educated participants. These findings are consistent with the cognitive reserve hypothesis (Stern, 2002), which suggests that those with more education may process tasks more efficiently or use other compensatory mechanisms that either delay cognitive impairment or delay our ability to detect cognitive impairment in these individuals. The finding that people with higher education who developed cognitive impairment were less likely to recover and more likely to die relative to those with less education is also consistent with this hypothesis.
However, there are several limitations in our study. First, the sample only includes the community-dwelling population at baseline. The lack of the institutionalized population at baseline may lead to some underestimation of life expectancy with cognitive impairment; although, because our estimates are based on cognitive transitions, we feel that most of these have been represented in the sample. Persons who are in institutions are likely to already be cognitively impaired. Fortunately, this source of bias is partly compensated because those who moved to an institution after the first wave are interviewed. The lack of a representative sample of the institutionalized in the early years of the study is most likely to affect the mortality rate as the institutionalized have higher mortality but the sample mortality over the whole period is only 0.1 year lower at age 70 than that from the vital statistics.
It is possible that some recovery is a result of those who transitioned from proxy response to performance-based tests, if they had enough problem behaviors to be identified as impaired via proxy reporting and a subsequent performance-based score identified no impairment. Even though this is a small percentage of recovering respondents overall, they are overrepresented in the recovered group, especially among the highly educated (see Table 4). Although measurement issues, such as the practice effect and use of proxy interviews, may have affected our estimates of recovery probabilities, they do not significantly affect our estimates of life expectancy with and without impairment because of the small probability of recovery. Finally, sample attrition may result in an overestimate of life expectancy without cognitive impairment, if those who are lost are in poorer health than those who participate.
Conclusion
Development of cognitive impairment is highly affected by education. Those with less than a high school education have a higher incidence of cognitive impairment and lower total life expectancies, leading to longer life with cognitive impairment and a reduced life expectancy without cognitive impairment relative to those with higher levels of education. However, those with 12 or more years of education who do become cognitively impaired appear to be in poorer health, leading to a reduced probability of recovery and increased probability of mortality relative to those with lower educational levels. One implication of these findings is that as education increases in the population, the length of time spent with cognitive impairment should be reduced.
Acknowledgments
The authors gratefully acknowledge support from the National Institute of Aging (T32 AG00037, R01 AG02334, P30 AG17265), the Institute for Longevity and Ageing, and the Robert Wood Johnson Foundation’s Health & Society Scholars Program.
Footnotes
Contributor Information
Agnès Lièvre, Institut National d’Etudes Démographiques, Paris.
Dawn Alley, University of Pennsylvania, Philadelphia.
Eileen M. Crimmins, University of Southern California, Los Angeles.
References
- Aguero-Torres H, Fratiglioni L, Guo Z, Viitanen M, Winblad B. Mortality from dementia in advanced age: A 5-year follow-up study of incident dementia cases. Journal of Clinical Epidemiology. 1999;52:737–742. doi: 10.1016/s0895-4356(99)00067-0. [DOI] [PubMed] [Google Scholar]
- Albert MS. How does education affect cognitive function? Annals of Epidemiology. 1995;5:76–78. doi: 10.1016/1047-2797(94)00044-t. [DOI] [PubMed] [Google Scholar]
- Alley D, Suthers K, Crimmins E. Education and cognitive decline in older Americans: Results from the AHEAD sample. Research on Aging. 2007;29:73–94. doi: 10.1177/0164027506294245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson MK, Ooi WL, Geva DL, Masur D, Blau A, Frishman W. Age-dependent incidence, prevalence, and mortality in the old. Archives of Internal Medicine. 1991;151:989–992. doi: 10.1001/archinte.151.5.989. [DOI] [PubMed] [Google Scholar]
- Bogner HR, Bruce ML, Reynolds CF, Mulsant BH, Cary MS, Morales K, et al. The effects of memory, attention, and executive dysfunction on outcomes of depression in a primary care intervention trial: The prospect study. International Journal of Geriatric Psychiatry. 2007;22:922–929. doi: 10.1002/gps.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer’s disease in the United States and the public health impact of delaying disease onset. American Journal of Public Health. 1998;88:1337–1342. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christenson BA, Johnson NE. Educational inequality in adult mortality: An assessment with death certificate date from Michigan. Demography. 1995;32:215–229. [PubMed] [Google Scholar]
- Clarfield AM. The decreasing prevalence of reversible dementias: An updated meta-analysis. Archives of Internal Medicine. 2003;163:2219–2229. doi: 10.1001/archinte.163.18.2219. [DOI] [PubMed] [Google Scholar]
- Crimmins E, Cambois E. Social inequalities in health expectancy. In: Robine JM, Jagger C, Mathers C, Crimmins E, Suzman R, editors. Determining health expectancies. West Sussex, UK: Wiley; 2003. pp. 111–125. [Google Scholar]
- Crimmins E, Hayward M, Saito Y. Changing mortality and morbidity rates and the health status and life expectancy of the older US population. Demography. 1994;31:159–175. [PubMed] [Google Scholar]
- Crimmins E, Hayward M, Saito Y. Differentials in active life expectancy in the older population of the United States. Journal of Gerontology: Social Sciences. 1996;51B:S111–S120. doi: 10.1093/geronb/51b.3.s111. [DOI] [PubMed] [Google Scholar]
- Crimmins E, Hayward M, Seeman T. Race/ethnicity, socioeconomic status, and health. In: Anderson N, Bulatao R, Cohen B, editors. Critical perspectives on race and ethnic differences in health in later life. Washington, DC: National Academies Press; 2004. pp. 310–352. [Google Scholar]
- Crimmins E, Saito Y. Trends in healthy life expectancy in the United States, 1970–1990: Gender, racial, and educational differences. Social Science & Medicine. 2001;52:1629–1641. doi: 10.1016/s0277-9536(00)00273-2. [DOI] [PubMed] [Google Scholar]
- Dubois MF, Hebert R. Cognitive-impairment-free life expectancy for Canadian seniors. Dementia and Cognitive Disorders. 2006;22:327–333. doi: 10.1159/000095593. [DOI] [PubMed] [Google Scholar]
- Evans DA, Beckett LA, Albert MS, Herbert LE, Scherr PA, Funkenstein HH, et al. Level of education and change in cognitive function in a community population of older persons. Annals of Epidemiology. 1993;3:71–77. doi: 10.1016/1047-2797(93)90012-s. [DOI] [PubMed] [Google Scholar]
- Farmer ME, Kittner SJ, Rae DS, Bartko JJ, Regier DA. Education and change in cognitive function: The epidemiologic catchment area study. Annals of Epidemiology. 1995;5:1–7. doi: 10.1016/1047-2797(94)00047-w. [DOI] [PubMed] [Google Scholar]
- Gabrel CS. Advance data from Vital and Health Statistics; No. 312. Hyattsville, MD: National Center for Health Statistics; 2000. Characteristics of elderly nursing home current residents and discharges: Data from the 1997 National Nursing Home Survey. [PubMed] [Google Scholar]
- Gallo JJ, Schoen R, Jones R. Cognitive impairment and syndromal depression in estimates of active life expectancy: The 13-year follow-up of the Baltimore Epidemiologic Catchment Area sample. Acta Psychiatrica Scandinavica. 2000;101:265–273. [PubMed] [Google Scholar]
- Haan MN, Wallace R. Can dementia be prevented? Brain aging in a population-based context. Annual Review of Public Health. 2004;25:1–24. doi: 10.1146/annurev.publhealth.25.101802.122951. [DOI] [PubMed] [Google Scholar]
- Hayward MD, Heron M. Racial inequality in active life among adult Americans. Demography. 1999;36:77–91. [PubMed] [Google Scholar]
- Helmer C, Joly P, Letenneur L, Commenges D, Dartigues JF. Mortality with dementia: Results from a French prospective community-based cohort. American Journal of Epidemiology. 2001;154:642–648. doi: 10.1093/aje/154.7.642. [DOI] [PubMed] [Google Scholar]
- Herzog AR, Rodgers WL. Cognitive performance measures in survey research on older adults. In: Schwarz N, Park DC, Knauper B, Sudman S, editors. Cognition, aging, and self-reports. Philadelphia: Psychology Press; 1999. pp. 327–340. [Google Scholar]
- Herzog AR, Wallace RB. Measures of cognitive functioning in the AHEAD study. Journals of Gerontology: Psychological Sciences. 1997;52:37–48. doi: 10.1093/geronb/52b.special_issue.37. [DOI] [PubMed] [Google Scholar]
- HRSonline. 2007 Retrieved March 10, 2008, from http://hrsonline.isr.umich.edu/intro/sho_uinfo.php?hfyle=sample_new_v3&xtyp=2.
- Hummer RA, Rogers RG, Eberstein IW. Socioeconomic differentials in adult mortality: A review of analytic approaches. Population & Development Review. 1998;24:553–578. [Google Scholar]
- Hurria A, Lachs M. Is cognitive dysfunction a complication of adjuvant chemotherapy in the older patient with breast cancer? Breast Cancer Research and Treatment. 2007;103:259–268. doi: 10.1007/s10549-006-9383-9. [DOI] [PubMed] [Google Scholar]
- Insel KC, Badger TA. Deciphering the 4 D’s: Cognitive decline, delirium, depression and dementia: A review. Journal of Advanced Nursing. 2001;38:360–368. doi: 10.1046/j.1365-2648.2002.02196.x. [DOI] [PubMed] [Google Scholar]
- Jacqmin-Gadda H, Fabrigoule C, Commenges D, Dartigues JF. A 5-year longitudinal study of the Mini-Mental State Examination in normal aging. American Journal of Epidemiology. 1997;145:498–506. doi: 10.1093/oxfordjournals.aje.a009137. [DOI] [PubMed] [Google Scholar]
- Kempen GI, Brilman EI, Ranchor AV, Ormel J. Morbidity and quality of life and the moderating effects of level of education in the elderly. Social Science & Medicine. 1999;49:143–149. doi: 10.1016/s0277-9536(99)00129-x. [DOI] [PubMed] [Google Scholar]
- Laditka SB, Hayward MD. The evolution of demographic methods to calculate health expectancies. In: Robine JM, Jagger C, Mathers CD, Crimmins EM, Suzman RM, editors. Determining health expectancies. West Sussex, UK: Wiley; 2003. pp. 221–234. [Google Scholar]
- Land KC, Guralnik JM, Blazer DG. Increment-decrement life tables with multiple covariates from panel data: The case of active life expectancy. Demography. 1994;31:297–319. [PubMed] [Google Scholar]
- Langa KM, Chernew ME, Kabeto MU, Herzog AR, Ofstedal MB, Willis RJ, et al. National estimates of the quantity and cost of informal caregiving for the elderly with dementia. Journal of General Internal Medicine. 2001;16:770–778. doi: 10.1111/j.1525-1497.2001.10123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrieu S, Letenneur L, Orgogozo JM, Fabrigoule C, Amieva H, Le Carret N, et al. Incidence and outcome of mild cognitive impairment in a population-based prospective cohort. Neurology. 2002;59:1594–1599. doi: 10.1212/01.wnl.0000034176.07159.f8. [DOI] [PubMed] [Google Scholar]
- Lee S, Kawachi I, Berkman LF, Grodstein F. Education, other socioeconomic indicators, and cognitive function. American Journal of Epidemiology. 2003;157:712–720. doi: 10.1093/aje/kwg042. [DOI] [PubMed] [Google Scholar]
- Liang J, Borawski-Clark E, Liu X, Sugisawa H. Transitions in cognitive status among the aged in Japan. Social Science & Medicine. 1996;43:325–337. doi: 10.1016/0277-9536(95)00381-9. [DOI] [PubMed] [Google Scholar]
- Lièvre A, Brouard N, Heathcote CR. The estimation of health expectancies from cross-longitudinal surveys. Mathematical Population Studies. 2003;10:211–248. [Google Scholar]
- Manton KG, Corder LS, Clark R. Estimates and projections of dementia-related service expenditures. In: Manton KG, Singer BH, Suzman RM, editors. Forecasting the health of elderly populations. New York: Springer-Verlag; 1993. pp. 207–238. [Google Scholar]
- Manton KG, Stallard E, Corder L. The dynamics of dimensions of age-related disability from 1982 to 1994 in the U.S. elderly population. Journal of Gerontology. 1998;53:B59–B70. doi: 10.1093/gerona/53a.1.b59. [DOI] [PubMed] [Google Scholar]
- National Center for Health Statistics. U.S Life Tables. 1997;Vol. 47(No. 28):40. (PHS) 2000-1120. Retrieved March 10, 2008, from http://www.cdc.gov/nchs/products/pubs/pubdlftbls/life/1966.htm. [PubMed]
- Perenboom RJM, Boshuizen HC, Breteler MMB, Ott A, Van de Water HPA. Dementia-free life expectancy (DemFLE) in the Netherlands. Social Science & Medicine. 1996;43:1703–1707. doi: 10.1016/s0277-9536(96)00058-5. [DOI] [PubMed] [Google Scholar]
- Portrait F, Lindeboom M, Deeg D. Life expectancies in specific health states: Results from a joint model of health status and mortality of older persons. Demography. 2001;38:525–536. doi: 10.1353/dem.2001.0038. [DOI] [PubMed] [Google Scholar]
- Rabbitt P, Diggle P, Holland F, McInness L. Practice and drop-out effects during a 17-year longitudinal study of cognitive aging. Journal of Gerontology: Psychological Sciences. 2004;59B:P84–P97. doi: 10.1093/geronb/59.2.p84. [DOI] [PubMed] [Google Scholar]
- Ritchie K, Polge C. Mental health expectance. In: Robine JM, Jagger C, Mathers CD, Crimmins EM, Suzman RM, editors. Determining health expectancies. West Sussex, UK: Wiley; 2003. pp. 175–182. [Google Scholar]
- Robine JM, Jagger C, Mathers CD, Crimmins EM, Suzman RM. Determining health expectancies. West Sussex, UK: Wiley; 2003. [Google Scholar]
- Rodgers W, Ofstedal M, Herzog AR. Trends in scores on tests of cognitive ability in the elderly U.S. population: 1993–2000. Journals of Gerontology: Social Sciences. 2003;58B:S338–S346. doi: 10.1093/geronb/58.6.s338. [DOI] [PubMed] [Google Scholar]
- Sauvaget C, Jagger C, Arthur A. Active and cognitive impairment-free life expectancies: Results from the Melton Mowbray 75+ health checks. Age and Ageing. 2001;30:509–515. doi: 10.1093/ageing/30.6.509. [DOI] [PubMed] [Google Scholar]
- Saxena SK, Ng TP, Koh G, Yong D, Fong NP. Is improvement in impaired cognition and depressive symptoms in post-stroke patients associated with recovery in activities of daily living? Acta Neurologica Scandinavica. 2007;115:339–346. doi: 10.1111/j.1600-0404.2006.00751.x. [DOI] [PubMed] [Google Scholar]
- Seeman TE, Crimmins E, Huang MH, Singer B, Bucur A, Gruenewald T, et al. Cumulative biological risk and socio-economic differences in mortality: MacArthur studies of successful aging. Social Science & Medicine. 2004;58:1986–1997. doi: 10.1016/S0277-9536(03)00402-7. [DOI] [PubMed] [Google Scholar]
- Steenland K, Hu S, Walker J. All-cause and cause-specific mortality by socioeconomic status among employed persons in 27 US states, 1984–1997. American Journal of Public Health. 2004;94:1037–1042. doi: 10.2105/ajph.94.6.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. Journal of the International Neuropsychological Society. 2002;8:448–460. [PubMed] [Google Scholar]
- Suthers K, Kim J, Crimmins E. Life expectancy with cognitive impairment in the older population of the United States. Journal of Gerontology: Social Science. 2003;58B:S179–S185. doi: 10.1093/geronb/58.3.s179. [DOI] [PubMed] [Google Scholar]
- Toseland RW, McCallion P, Gerber T, Banks S. Predictors of health and human services use by persons with dementia and their family caregivers. Social Science & Medicine. 2002;55:1255–1266. doi: 10.1016/s0277-9536(01)00240-4. [DOI] [PubMed] [Google Scholar]
- Vacca V. Diagnosis and treatment of idiopathic normal pressure hydrocephalus. Journal of Neuroscience Nursing. 2007;2:107–111. doi: 10.1097/01376517-200704000-00007. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Beckett LA, Bienias JL, Evans DA, Bennett DA. Terminal decline in cognitive function. Neurology. 2003;60:1782–1787. doi: 10.1212/01.wnl.0000068019.60901.c1. [DOI] [PubMed] [Google Scholar]
- Zhu L, Fratiglioni L, Guo Z, Aguero-Torres H, Winblad B, Vittanen M. Association of stroke with dementia, cognitive impairment, and functional disability in the very old: A population-based study. Stroke. 1998;10:2094–2099. doi: 10.1161/01.str.29.10.2094. [DOI] [PubMed] [Google Scholar]