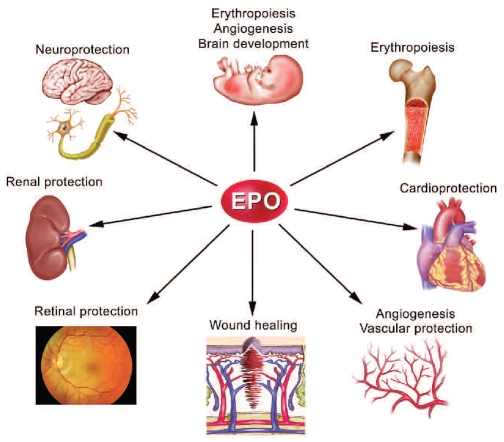
Erythropoietin (EPO) regulates red blood cell production by binding to its cell surface receptor, EPO-R, expressed on erythroid progenitor cells. Although EPO was originally believed to be an erythroid-specific hematopoietic cytokine, for over a decade, a substantial body of scientific evidence has accumulated to demonstrate that the biological effects of EPO are not limited to the erythron (Figure 1). In this issue of the journal, Lifshitz and colleagues1 report on their most recent contribution to this field of research by demonstrating that, within the hematopoietic system, EPO may exhibit modulatory effects on macrophage number and function. The authors examined in vivo effects of EPO on splenic macrophages and inflammatory peritoneal macrophages, as well as in vitro effects of EPO on bone marrow-derived macrophages in culture. The experimental data show that splenic macrophage numbers were increased in mice in response to systemic EPO treatment. In transgenic mice engineered to constitutively over-express endogenous EPO, an even more significant increase in the number of splenic macrophages was observed, possibly as part of an adaptive mechanism leading to increased erythro-phagocytosis in severely polycythemic mice.2 Inflammatory macrophages isolated from murine peritoneum displayed enhanced activation and phagocytic function, both following exogenous EPO treatment and in association with the over-expression of endogenous EPO, but without an increase in the number of macrophages migrating into the peritoneal cavity. The in vivo activity of EPO observed in these studies may be associated with direct effects on macrophages, indirect effects of EPO on other cell types that modulate macrophage number and function, or a combination of direct and indirect effects. Additional experiments by the investigators using cultured murine primary bone marrow-derived macrophages revealed enhanced activation and phagocytic function of the cells following EPO treatment. These direct EPO effects were associated with increased macrophage nitric oxide and interleukin (IL)-12 secretion, whereas IL-10 production was decreased, consistent with the generation of a pro-inflammatory phenotype and classical Th1 immune response.
Figure 1.
Schematic representation of the biological effects of erythropoietin.
The investigation of non-erythroid biological effects of EPO raises the question of the role of the erythroid receptor EPO-R, which is ubiquitously expressed at relatively low levels in many non-hematopoietic tissues. Lifshitz and colleagues addressed this issue in part by demonstrating that the newly discovered effects of EPO on macrophages were associated with the expression of EPO-R mRNA in cultured murine bone marrow-derived macrophages. The investigators further demonstrated the ability of EPO to mediate the increased phosphorylation of STAT proteins, as well as the induction of AKT and ERK2 phosphorylation and the nuclear translocation of p65 NFκB in macrophages. Although the direct effects of EPO on intracellular signal transduction and the induced changes in macrophage phenotype and function are presumably mediated in part by EPO-R, further studies will be necessary to delineate the structure of the cell surface receptor that mediates the effects of EPO in macrophages. Previous studies investigating non-erythropoietic EPO activities suggested that, in some experimental models, the tissue protective activity of EPO and of some EPO derivatives without erythropoietic activity may be mediated by a heteroreceptor complex between EPO-R and the common β receptor (βC-R) – a signal-transducing component of the cellular receptors for granulocyte-macrophage colony-stimulating factor, IL-3 and IL-5.3,4 Other studies reported, however, that the βC-R may not be required for EPO-induced signal transduction and its cellular effects in some non-hematopoietic cells.5,6 The detection of low levels of cell surface EPO-R on non-hematopoietic cells has been made possible by using a novel radiolabeled-EPO binding assay to demonstrate as few as 50 EPO binding sites on the cell surface, a receptor number that was nevertheless sufficient to mediate cellular effects of EPO in tumor cell lines of neural origin.5 Whether βC-R may be involved in EPO signaling in macrophages, the mechanisms of EPO-induced effects on macrophages and the role of EPO-R remain to be determined.
The studies by Lifshitz and colleagues are likely to pave the way to new avenues of research investigating the role of EPO in the biology of macrophages – key effector cells of the immune system which influence inflammatory responses, microbial defenses, wound healing, angiogenesis, tumor biology, as well as physiological erythropoiesis within erythroblastic islands in the bone marrow.7–9 EPO derivatives without erythropoietic activity have been explored recently in pre-clinical studies of wound healing.10 Another study investigated EPO expression and function in macrophages in the context of atherosclerosis and the inhibitory effect of EPO on the formation of foam cells – the hallmark of early-stage atherosclerosis due to uptake of modified low-density lipoprotein (LDL) leading to cholesterol accumulation in the cells.11 Treatment of macrophages with exogenous EPO and oxidized LDL increased cholesterol efflux from cells by EPO-induced upregulation of major transporters of cholesterol efflux from foam cells to mitigate lipid accumulation. Bone marrow-derived macrophages from transgenic mice that over-expressed EPO exhibited decreased lipid accumulation in response to oxidized LDL, an effect that was abolished by treatment with EPO antibody. Furthermore, endogenous EPO protein was found to be elevated in the aortic atherosclerotic lesions of apoE−/− mice and treatment of macrophages with oxidized LDL led to increased EPO expression and secretion from cells suggesting an autocrine involvement of EPO in modulating this process.11 In view of the possibility that macrophages may produce functional EPO, further work will be required to delineate the effects of EPO as a paracrine factor in influencing the various physiological and pathological processes that macrophages are involved in, including regulation of erythropoiesis in bone marrow erythroblastic islands.9
Several previous studies investigated the role of EPO as an immunomodulatory cytokine, showing that EPO may attenuate inflammatory responses in some experimental models. For instance, EPO treatment was reported to improve neurological recovery in a mouse model of autoimmune encephalomyelitis, an effect that was associated with significant reduction of inflammatory glial cell and macrophage infiltration in the spinal cord, delayed appearance of tumor necrosis factor and decreased levels of IL-6.12 In the ischemic brain in a rodent stroke model, EPO treatment was reported to reduce astrocyte activation and the recruitment of leukocytes and microglia in the infarct site, associated with a reduction of levels of inflammatory cytokines such as tumor necrosis factor and IL-6, although EPO did not directly inhibit cytokine release by astrocytes in culture.13 In another example, anti-inflammatory effects were observed in an experimental rat model of autoimmune myocarditis in which EPO treatment resulted in a reduction in the area of myocarditis associated with decreased expression of the inflammatory cytokines tumor necrosis factor and IL-6.14 Among other reported immunomodulatory effects, in a rodent model of multiple myeloma the administration of EPO resulted in an anti-tumor effect that was dependent on a T-cell mediated mechanism15 and in patients with multiple myeloma, EPO therapy was associated with decreased levels of serum IL-6 and normalization of the CD4:CD8 T-lymphocyte ratio.16 The mechanisms by which EPO exerts its observed immunomodulatory effects, whether EPO may elicit pro-versus anti-inflammatory responses in different organs and the discovery of macrophages as a target for EPO will undoubtedly constitute subjects for future investigation to better understand the direct and/or indirect role that the action of EPO on macrophages might play in modulating EPO-regulated functions including erythropoiesis, as well as the various non-hematopoietic activities of EPO.
The data reported by Lifshitz and colleagues contribute to previous work by many investigators indicating that EPO exerts biological effects in non-erythroid cells. More than two decades following the cloning of EPO-R and the availability of recombinant human EPO for the treatment of patients with anemia associated with chronic kidney disease, there is still much to learn about the full spectrum of EPO effects and mechanisms of EPO-R signaling.17 The adverse effects of EPO therapy observed in randomized clinical trials involving patients with chronic kidney disease and cancer, such as increased thromboembolic complications, cardiovascular mortality, tumor progression and impaired survival may potentially be related to non-erythropoietic actions of EPO.18 The biological consequences of EPO signaling in non-erythroid cells and organs is an important area of research that will contribute to the optimization of the current, safe use of recombinant EPO in the clinic and to better understanding of the risks involved in potential tissue-protective applications of EPO and novel EPO derivatives without erythropoietic activity.19,20
Footnotes
Financial and other disclosures provided by the author using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are available with the full text of this paper at www.haematologica.org.
References
- 1.Lifshitz L, Tabak G, Mittelman M, Gassmann M, Neumann D. Macrophages as novel targets for erythropoietin. Haematologica. 2010;95(11):1823–31. doi: 10.3324/haematol.2010.025015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bogdanova A, Mihov D, Lutz H, Saam B, Gassmann M, Vogel J. Enhanced erythro-phagocytosis in polycythemic mice overexpressing erythropoietin. Blood. 2007;110(2):762–9. doi: 10.1182/blood-2006-12-063602. [DOI] [PubMed] [Google Scholar]
- 3.Brines M, Grasso G, Fiordaliso F, Sfacteria A, Ghezzi P, Fratelli M, et al. Erythropoietin mediates tissue protection through an erythropoietin and common beta-subunit heteroreceptor. Proc Natl Acad Sci USA. 2004;101(41):14907–12. doi: 10.1073/pnas.0406491101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sautina L, Sautin Y, Beem E, Zhou Z, Schuler A, Brennan J, et al. Induction of nitric oxide by erythropoietin is mediated by the β common receptor and requires interaction with VEGF receptor 2. Blood. 2010;115(4):896–905. doi: 10.1182/blood-2009-04-216432. [DOI] [PubMed] [Google Scholar]
- 5.Um M, Gross AW, Lodish HF. A “classical” homodimeric erythropoietin receptor is essential for the antiapoptotic effects of erythropoietin on differentiated neuroblastoma SH-SY5Y and pheochromocytoma PC-12 cells. Cell Signal. 2007;19(3):634–45. doi: 10.1016/j.cellsig.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 6.Kanellakis P, Pomilio G, Agrotis A, Gao X, Du XJ, Curtis D, et al. Darbepoetin-mediated cardioprotection after myocardial infarction involves multiple mechanisms independent of erythropoietin receptor-common beta-chain heteroreceptor. Br J Pharmacol. 2010;160(8):2085–96. doi: 10.1111/j.1476-5381.2010.00876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327(5966):656–61. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141(1):39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chasis JA, Mohandas N. Erythroblastic islands: niches for erythropoiesis. Blood. 2008;112(3):470–8. doi: 10.1182/blood-2008-03-077883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erbayraktar Z, Erbayraktar S, Yilmaz O, Cerami A, Coleman T, Brines M. Nonerythropoietic tissue protective compounds are highly effective facilitators of wound healing. Mol Med. 2009;15(7–8):235–41. doi: 10.2119/molmed.2009.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu KY, Ching LC, Su KH, Yu YB, Kou YR, Hsiao SH, et al. Erythropoietin suppresses the formation of macrophage foam cells: role of liver X receptor alpha. Circulation. 2010;121(16):1828–37. doi: 10.1161/CIRCULATIONAHA.109.876839. [DOI] [PubMed] [Google Scholar]
- 12.Agnello D, Bigini P, Villa P, Mennini T, Cerami A, Brines ML, et al. Erythropoietin exerts an anti-inflammatory effect on the CNS in a model of experimental autoimmune encephalomyelitis. Brain Res. 2002;952(1):128–34. doi: 10.1016/s0006-8993(02)03239-0. [DOI] [PubMed] [Google Scholar]
- 13.Villa P, Bigini P, Mennini T, Agnello D, Laragione T, Cagnotto A, et al. Erythropoietin selectively attenuates cytokine production and inflammation in cerebral ischemia by targeting neuronal apoptosis. J Exp Med. 2003;198(6):971–5. doi: 10.1084/jem.20021067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitsuma W, Ito M, Kodama M, Fuse K, Okamura K, Minagawa S, et al. Cardioprotective effects of recombinant human erythropoietin in rats with experimental autoimmune myocarditis. Biochem Biophys Res Commun. 2006;344(3):987–94. doi: 10.1016/j.bbrc.2006.03.230. [DOI] [PubMed] [Google Scholar]
- 15.Mittelman M, Neumann D, Peled A, Kanter P, Haran-Ghera N. Erythropoietin induces tumor regression and antitumor immune responses in murine myeloma models. Proc Natl Acad Sci USA. 2001;98(9):5181–6. doi: 10.1073/pnas.081275298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prutchi-Sagiv S, Golishevsky N, Oster HS, Katz O, Cohen A, Naparstek E, et al. Erythropoietin treatment in advanced multiple myeloma is associated with improved immunological functions: could it be beneficial in early disease? Br J Haematol. 2006;135(5):660–72. doi: 10.1111/j.1365-2141.2006.06366.x. [DOI] [PubMed] [Google Scholar]
- 17.Becker V, Schilling M, Bachmann J, Baumann U, Raue A, Maiwald T, et al. Covering a broad dynamic range: information processing at the erythropoietin receptor. Science. 2010;328(5984):1404–8. doi: 10.1126/science.1184913. [DOI] [PubMed] [Google Scholar]
- 18.Unger EF, Thompson AM, Blank MJ, Temple R. Erythropoiesis-stimulating agents–time for a reevaluation. N Engl J Med. 2010;362(3):189–92. doi: 10.1056/NEJMp0912328. [DOI] [PubMed] [Google Scholar]
- 19.Ehrenreich H, Weissenborn K, Prange H, Schneider D, Weimar C, Wartenberg K, et al. Recombinant human erythropoietin in the treatment of acute ischemic stroke. Stroke. 2009;40(12):e647–56. doi: 10.1161/STROKEAHA.109.564872. [DOI] [PubMed] [Google Scholar]
- 20.Pankratova S, Kiryushko D, Sonn K, Soroka V, Kohler LB, Rathje M, et al. Neuroprotective properties of a novel, non-haematopoietic agonist of the erythropoietin receptor. Brain. 2010;133(Pt 8):2281–94. doi: 10.1093/brain/awq101. [DOI] [PubMed] [Google Scholar]