Fetal alloimmune thrombocytopenia is caused by maternal sensitization to paternally-derived antigens on fetal platelets, most commonly HPA-1a.1 It occurs in approximately 1 in 1000 live births and is the commonest cause of severe fetal and neonatal thrombocytopenia, and of intracranial hemorrhage in neonates born at term.2 Since there is currently no routine screening, first-time cases of fetal alloimmune thrombocytopenia are generally identified following the birth of a markedly thrombocytopenic neonate. Antenatal management is thus only possible in subsequent pregnancies.
Intracranial hemorrhage is the most devastating complication of fetal alloimmune thrombocytopenia and often occurs antenatally. Assessment of projected clinical severity is thus based on the development of intracranial hemorrhage in a previous sibling. If there is such a history of intracranial hemorrhage, the chance of this complication occurring again in the next pregnancy is extremely high in an untreated, antigen-positive sibling.3
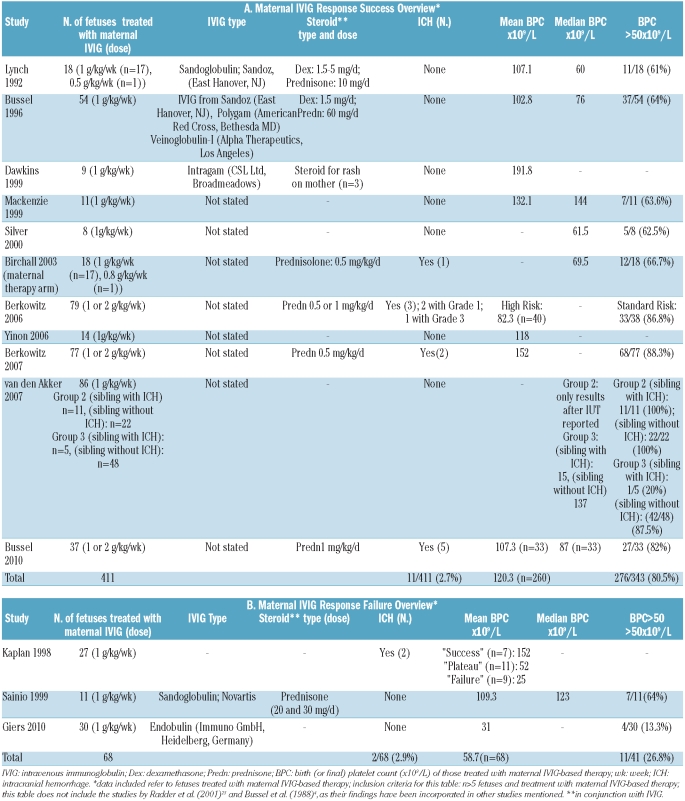
Administration of intravenous immunoglobulin (IVIG) to the mother, initially given in conjunction with dexamethasone, was first used to prevent recurrence of antenatal intracranial hemorrhage in 1988.4 This approach of providing IVIG-based medical therapy administered to the mother to increase the fetal platelet count has since been extensively investigated in hundreds of maternal-fetal pairs.5 The efficacy of IVIG-based therapy has been supported by numerous studies6–16 (Table 1A) but not by others17–19 (Table 1B). The studies presented in Tables 1A and 1B surprisingly report virtually identical percentages of cases of intracranial hemorrhage: 2.7% versus 2.9%, respectively. However, overall mean birth platelet counts differed markedly between the two groups. While platelet counts are considered to be surrogate markers of intracranial hemorrhage, fortunately, the likelihood of fetal and neonatal intracranial hemorrhage, in the absence of this complication having occurred in a previous sibling, is relatively low.
Table 1.
Maternal IVIG response success and failure overview.
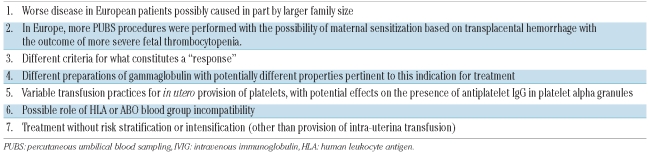
Over time, a number of considerations have emerged concerning the assessment of the efficacy of maternal IVIG-based therapy in fetal alloimmune thrombocytopenia (Table 2) in addition to the lack of a universally-accepted response criterion.20 A recent randomized study demonstrated that IVIG 1 g/kg/week alone does not work well in more severely affected patients (i.e., those with previous intracranial hemorrhage or whose initial fetal blood platelet count is ≤20×109/L).12 Among patients treated in the standard-risk arm (platelet count at pre-treatment fetal blood sampling > 20×109/L and no history of intracranial hemorrhage in a previous sibling), less intensive therapy was appropriate and prednisone 0.5 mg/kg/day was as good as IVIG. However, among high-risk fetuses, a satisfactory increase in platelets was seen in only 18% of cases treated with maternal IVIG 1 g/kg/week alone versus 82% of those treated with maternal IVIG 1 g/kg/week plus prednisone 1 mg/kg/day. Thus, IVIG 1 g/kg/week alone does not appear to be sufficiently effective for the approximately 50% of severely affected fetuses whose initial fetal platelet count is less than 20×109/L.21 Further studies suggested that IVIG x 2 infusions of 1 g/kg/week combined with 0.5–1.0 mg/kg/day prednisone is the most effective medical regimen for use in the most severely affected fetuses.16 These comparisons demonstrated that risk stratification was essential and that the severity and response to therapy of fetal alloimmune thrombocytopenia was not the same in all cases.
Table 2.
Possible reasons for observed differences in the results of antenatal management of alloimmune thrombocytopenia.
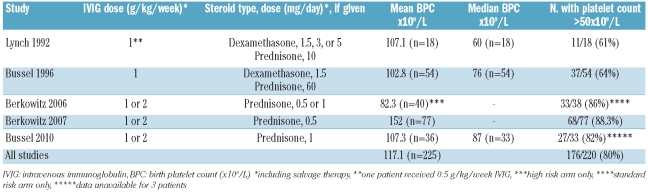
Another management strategy is the use of weekly fetal blood sampling associated with intra-uterine platelet transfusion. Since fetuses with alloimmune thrombocytopenia are vulnerable to compromised hemostasis due to severe thrombocytopenia, impaired platelet function, and endothelial dysfunction; the risks of fetal blood sampling are considerable and well-documented.10–12,14,15,17,18,22,23 Birchall and colleagues from Europe reported a number of procedure-related complications, including exsanguination and emergency Cesarean deliveries attributed to infection, needle dislodgement, severe fetal bradycardia, cord spasm and thrombosis.11 For these reasons, fetal blood sampling is generally coupled with intra-uterine platelet transfusion if platelet counts are low (e.g., < 50×109/L).7,22 In addition, serial fetal blood sampling and intra-uterine platelet transfusions may further sensitize the mother.20,24 Finally, if maternal platelets are used in an intra-uterine transfusion, antiplatelet antibodies may be transfused into the fetus with the platelets.20,25 Overall, as treatment results with maternal IVIG-based therapy have improved substantially6,7,12,14,16 (Table 3), while the morbidity and mortality from fetal blood sampling remain considerable, the consensus at many centers at present is to minimize its use, and, if possible, to avoid fetal blood sampling completely.9,13,15,23 This requires the use of “blind” treatment and, therefore, therapy that will be effective in fetuses with all degrees of severity since; for example, it is unknown whether the fetal platelet count is below or above 20×109/L without pre-treatment fetal blood sampling.
Table 3.
Outcomes of maternal therapy: platelet counts; multicenter US experience.
The study by Giers et al.19 in this issue of the journal introduces an entirely novel approach to management in which serial weekly fetal blood samples are taken and intra-uterine platelet transfusions are given only at the final sampling. Twenty-nine pregnancies were studied, 25 of which were from mothers with one to four previously affected children; sibling history was negative for intracranial hemorrhage in all cases. No intra-uterine platelet transfusions were performed except immediately prior to birth, despite the fact that the mean minimal fetal platelet count during pregnancy was 21.5×109/L (range, 4–60×109/L). Maternal therapy was weekly IVIG, 1 g/kg of Endobulin, without steroids (or intra-uterine platelet transfusion). Giers et al. concluded that their data support very limited efficacy of IVIG (mean fetal platelet count at initial intra-uterine platelet transfusion, 56.3×109/L [range, 4–130×109/L]; mean fetal platelet count at final sampling prior to intra-uterine platelet transfusion, 31.3×109/L [range 6–117×109/L]). This 6-year retrospective study included 219 fetal blood sampling procedures (median, 7 per fetus; range, 2–14). Not one procedure-related complication occurred in this study. Because the lack of adverse events associated with fetal blood sampling was attributed to one highly-skilled operator performing all procedures, as in a series of more than 5000 such procedures in France,26 the reproducibility of such a complication-free outcome may not be generalizable to other centers.19 Finally, the overall poor response to IVIG may stem from additional factors beyond the use of a dose of only 1 g/kg/week. These include the brand of IVIG (Endobulin) and the large number of fetal blood sampling procedures performed, as serial sampling may contribute to maternal sensitization, thus masking the therapeutic effects of maternal IVIG.20,24
Perspectives
The management of fetal alloimmune thrombocytopenia has progressed in recent years. Examples of this include implementation of non-invasive approaches, in which fetal blood sampling and intra-uterine platelet transfusion are minimized or eliminated completely, and treatment stratified according to estimated risk.
Non-invasive interventions
The current focus on non-invasive approaches9,13,15,23 serves both as a response to the high complication rate of invasive strategies10–12,14,15,17,18,22,23 and as a testament to the efficacy of maternal IVIG-based treatment. In one study Yinon et al. suggested that IVIG without fetal blood sampling is safe and effective in women who, like the subjects featured in the study conducted by Giers et al.,19 lacked a history of fetal or neonatal intracranial hemorrhage in a previous child. The 24 fetuses who underwent this treatment had significantly higher platelet counts at birth compared to their affected siblings and fetuses of mothers who refused treatment (118×109/L versus 25×109/L and 24×109/L, respectively; P<0.05).13 Furthermore, in a group treated with “blind” IVIG, preterm births were significantly less frequent than in groups treated with invasive approaches.23 While avoiding fetal blood sampling entirely is not without its own pitfalls (inability to assess the need for transfusion, determine aspects of severity, etc.), this approach may help to avoid fetal complications ranging from exsanguination to enhanced maternal sensitization and seems feasible in many cases.20,24,25
Risk stratification
Recent studies have demonstrated the need to acknowledge variations in severity in treatment protocols.12,16 Severity may be affected by a myriad of factors whose roles in fetal alloimmune thrombocytopenia are not yet understood, such as HLA and, possibly, ABO incompatibility.27,28 It has become clear that fetuses at different risk levels should not be treated identically, and that IVIG 1 g/kg/week alone is not sufficient to treat high-risk cases. Finally, there is now compelling support for the role of a risk-based approach to maternal IVIG-based therapy in the prevention of recurrent intracranial hemorrhage among affected fetuses, again indicating that treatment stratification based on sibling history is appropriate.16
Screening and biomarkers
Screening all pregnancies for fetal alloimmune thrombocytopenia is also a novel approach whose discussion is beyond the scope of this perspective review. Its appropriate implementation remains to be clarified.
Finally, development of biomarkers of severity would be extremely useful. Analogy could be made to the use of middle cerebral artery Doppler studies to predict the severity of fetal anemia.
Summary
Non-invasive approaches and the implementation of risk stratification (including combination therapy of IVIG with steroids and/or the use of more than 1 g/kg/week of IVIG) are appropriate in the management of fetal alloimmune thrombocytopenia. The data are equally compelling that fetal blood sampling cannot be considered routine in most centers and that Giers et al.19 were exceptional and fortunate in their skilled performance of this technically-demanding procedure.
Footnotes
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are available with the full text of this paper at www.haematologica.org.
References
- 1.Schulman NR, Marder VJ, Hiller MC, Collier EM. Platelet and leucocyte isoantigens and their antibodies. Prog Haematol. 1964;4:222–304. [PubMed] [Google Scholar]
- 2.Bussel JB, Zacharoulis S, Kramer K, McFarland JG, Pauliny J, Kaplan C. Clinical and diagnostic comparison of neonatal alloimmune thrombocytopenia to non-immune cases of thrombocytopenia. Pediatr Blood Cancer. 2005;45(2):176–83. doi: 10.1002/pbc.20282. [DOI] [PubMed] [Google Scholar]
- 3.Herman JH, Jumbelic MI, Ancona RJ, Kickler TS. In utero cerebral hemorrhage in alloimmune thrombocytopenia. Am J Pediatr Hematol Oncol. 1986;8(4):312–7. doi: 10.1097/00043426-198624000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Bussel JB, Berkowitz RL, McFarland JG, Lynch L, Chitkara U. Antenatal treatment of neonatal alloimmune thrombocytopenia. New Engl J Med. 1988;319(21):1374–8. doi: 10.1056/NEJM198811243192103. [DOI] [PubMed] [Google Scholar]
- 5.Rayment R, Brunskill SJ, Stanworth S, Soothill PW, Roberts DJ, Murphy MF. Antenatal interventions for fetomaternal alloimmune thrombocytopenia. Cochrane Database Syst Rev. 2005;(1):CD004226. doi: 10.1002/14651858.CD004226.pub2. [DOI] [PubMed] [Google Scholar]
- 6.Lynch L, Bussel JB, McFarland JG, Chitkara U, Berkowitz RL. Antenatal treatment of alloimmune thrombocytopenia. Obstet Gynecol. 1992;80(1):67–71. [PubMed] [Google Scholar]
- 7.Bussel JB, Berkowitz RL, Lynch L, Lesser ML, Paidas MJ, Huang CL, et al. Antenatal management of alloimmune thrombocytopenia with intravenous gamma-globulin. Am J Obstet Gynecol. 1996;174(5):1414–23. doi: 10.1016/s0002-9378(96)70582-3. [DOI] [PubMed] [Google Scholar]
- 8.Dawkins B, Minchinton RM. Fetomaternal alloimmune thrombocytopenia treated with intragam. Med J Aust. 1999;170(9):451–2. doi: 10.5694/j.1326-5377.1999.tb127826.x. [DOI] [PubMed] [Google Scholar]
- 9.Mackenzie F, Brennand J, Peterkin M, Cameron A. Management of fetal alloimmune thrombocytopenia-a less invasive option? J Obstet Gynaecol. 1999;19(2):119–21. doi: 10.1080/01443619965372. [DOI] [PubMed] [Google Scholar]
- 10.Silver RM, Porter TF, Branch DW, Esplin MS, Scott JR. Neonatal alloimmune thrombocytopenia: antenatal management. Am J Obstet Gynecol. 2000;182(5):1233–8. doi: 10.1067/mob.2000.104841. [DOI] [PubMed] [Google Scholar]
- 11.Birchall JE, Murphy MF, Kaplan C, Kroll H. European collaborative study of the antenatal management of feto-maternal alloimmune thrombocytopenia. Br J Haematol. 2003;122(2):275–88. doi: 10.1046/j.1365-2141.2003.04408.x. [DOI] [PubMed] [Google Scholar]
- 12.Berkowitz RL, Kolb EA, McFarland JG, Wissert M, Primani A, Lesser M, et al. Parallel randomized trials for fetal alloimmune thrombocytopenia. Obstet Gynecol. 2006;107(1):91–6. doi: 10.1097/01.AOG.0000192404.25780.68. [DOI] [PubMed] [Google Scholar]
- 13.Yinon Y, Spira M, Solomon O, Weisz B, Chayen B, Schiff E, et al. Antenatal noninvasive treatment of patients at risk for alloimmune thrombocytopenia without a history of intracranial hemorrhage. Am J Obstet Gynecol. 2006;195(4):1153–7. doi: 10.1016/j.ajog.2006.06.066. [DOI] [PubMed] [Google Scholar]
- 14.Berkowitz RL, Lesser ML, McFarland JG, Wissert M, Primiani A, Hung C, et al. Antepartum treatment without early cordocentesis for standard-risk alloimmune thrombocytopenia: a randomized controlled trial. Obstet Gynecol. 2007;110(2 Pt 1):249–55. doi: 10.1097/01.AOG.0000270302.80336.dd. [DOI] [PubMed] [Google Scholar]
- 15.Van den Akker E, Oepkes D, Lopriore E, Brand A, Kanhai H. Noninvasive antenatal management of fetal and neonatal alloimmune thrombocytopenia: safe and effective. BJOG. 2007;114(4):469–73. doi: 10.1111/j.1471-0528.2007.01244.x. [DOI] [PubMed] [Google Scholar]
- 16.Bussel JB, Berkowitz RL, Hung C, Kolb EA, Wissert M, Primiani A, et al. Intracranial hemorrhage in alloimmune thrombocytopenia: stratified management to prevent recurrence in the subsequent affected fetus. Am J Obstet Gynecol. 2010;203(2):135.e1–14. doi: 10.1016/j.ajog.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan C, Murphy MF, Kroll H, Waters AH. Feto-maternal alloimmune thrombocytopenia: antenatal therapy with IvIgG and steroids--more questions than answers. European Working Group on FMAIT. Br J Haematol. 1998;100(1):62–5. doi: 10.1046/j.1365-2141.1998.00533.x. [DOI] [PubMed] [Google Scholar]
- 18.Sainio S, Teramo K, Kekomäki R. Prenatal treatment of severe feto-maternal alloimmune thrombocytopenia. Transfus Med. 1999;9(4):321–30. doi: 10.1046/j.1365-3148.1999.00216.x. [DOI] [PubMed] [Google Scholar]
- 19.Giers G, Wenzel F, Stockschlader M, Riethmacher R, Lorenz H, Tutschek B. Fetal alloimmune thrombocytopenia and maternal intravenous immunoglobin infusions. Haematologica. 2010;95(11):1921–6. doi: 10.3324/haematol.2010.025106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bussel JB, Skupski DW, McFarland JG. Fetal alloimmune thrombocytopenia: consensus and controversy. J Matern Fetal Med. 1996;5(5):281–92. doi: 10.1002/(SICI)1520-6661(199609/10)5:5<281::AID-MFM6>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 21.Bussel JB, Zabusky MR, Berkowitz RL, McFarland JG. Fetal alloimmune thrombocytopenia. N Engl J Med. 1997;337(1):22–6. doi: 10.1056/NEJM199707033370104. [DOI] [PubMed] [Google Scholar]
- 22.Paidas MJ, Berkowitz RL, Lynch L, Lockwood CJ, Lapinski R, McFarland JG, et al. Alloimmune thrombocytopenia: fetal and neonatal losses related to cordocentesis. Am J Obstet Gynecol. 1995;172(2 Pt 1):475–9. doi: 10.1016/0002-9378(95)90559-6. [DOI] [PubMed] [Google Scholar]
- 23.Radder CM, Brand A, Kanhai HH. A less invasive treatment strategy to prevent intracranial hemorrhage in fetal and neonatal alloimmune thrombocytopenia. Am J Obstet Gynecol. 2001;185(3):683–8. doi: 10.1067/mob.2001.116727. [DOI] [PubMed] [Google Scholar]
- 24.Bowman JM, Pollock JM, Peterson LE, Harman CR, Manning FA, Menticoglou SM. Fetomaternal hemorrhage following funipuncture: increase in severity of maternal red-cell alloimmunization. Obstet Gynecol. 1994;84(5):839–43. [PubMed] [Google Scholar]
- 25.Vietor HE, Kanhai HHH, Brand A. Induction of additional red cell alloantibodies after intrauterine transfusions. Transfusion. 1994;34 (11):970–4. doi: 10.1046/j.1537-2995.1994.341195065035.x. [DOI] [PubMed] [Google Scholar]
- 26.Hohlfeld P, Forestier F, Kaplan C, Tissot JD, Daffos F. Fetal thrombocytopenia: a retrospective survey of 5,194 fetal blood samplings. Blood. 1994;84(6):1851–6. [PubMed] [Google Scholar]
- 27.Murphy MF, Metcalfe P, Waters AH, Ord J, Hambley H, Nicolaides K. Antenatal management of severe feto-maternal alloimmune thrombocytopenia: HLA incompatibility may affect responses to fetal platelet transfusions. Blood. 1993;81(8):2174–9. [PubMed] [Google Scholar]
- 28.Boehlen F, Kaplan C, de Moerloose P. Severe neonatal alloimmune thrombocytopenia due to anti-HPA-3a. Vox Sang. 1998;74(3):201–4. [PubMed] [Google Scholar]