Abstract
Background
Macrophages play a key role in iron homeostasis. In peripheral tissues, they are known to polarize into classically activated (or M1) macrophages and alternatively activated (or M2) macrophages. Little is known on whether the polarization program influences the ability of macrophages to store or recycle iron and the molecular machinery involved in the processes.
Design and Methods
Inflammatory/M1 and alternatively activated/M2 macrophages were propagated in vitro from mouse bone-marrow precursors and polarized in the presence of recombinant interferon-γ or interleukin-4. We characterized and compared their ability to handle radioactive iron, the characteristics of the intracellular iron pools and the expression of molecules involved in internalization, storage and export of the metal. Moreover we verified the influence of iron on the relative ability of polarized macrophages to activate antigen-specific T cells.
Results
M1 macrophages have low iron regulatory protein 1 and 2 binding activity, express high levels of ferritin H, low levels of transferrin receptor 1 and internalize – albeit with low efficiency -iron only when its extracellular concentration is high. In contrast, M2 macrophages have high iron regulatory protein binding activity, express low levels of ferritin H and high levels of transferrin receptor 1. M2 macrophages have a larger intracellular labile iron pool, effectively take up and spontaneously release iron at low concentrations and have limited storage ability. Iron export correlates with the expression of ferroportin, which is higher in M2 macrophages. M1 and M2 cells activate antigen-specific, MHC class II-restricted T cells. In the absence of the metal, only M1 macrophages are effective.
Conclusions
Cytokines that drive macrophage polarization ultimately control iron handling, leading to the differentiation of macrophages into a subset which has a relatively sealed intracellular iron content (M1) or into a subset endowed with the ability to recycle the metal (M2).
Keywords: macrophages, iron, inflammation
Introduction
Macrophages are heterogeneous populations that polarize, depending on microenvironmental cues, into classically activated (or M1) and alternatively activated (or M2) macrophages. The two populations are functionally different: M1 cells exhibit potent anti-microbial properties and promote Th1 responses while M2 cells support Th2-associated effector functions.1
Macrophages are widely distributed in peripheral tissues where they play an indispensable role in the defense against pathogens. This is at least partially achieved through the control of intracellular iron availability, which limits pathogen growth.2 Macrophages are also important in the maintenance of tissue homeostasis and in the resolution of inflammation. These functions are achieved through the macrophages ability to release trophic factors1,3 and their clearance and iron recycling capacities, which are important for tissue remodeling and repair.
Iron homeostasis requires the finely tuned expression of molecules involved in iron uptake, storage, export and heme degradation.4,5 The transcriptional and post-transcriptional control of many of the genes responsible for these functions depends on inflammatory cytokines, free radicals, and on the ability of iron regulatory proteins 1 and 2 (IRP1, IRP2) to bind to iron responsive elements (IRE) on target mRNA.6
In this study, we verified how the functional polarization of primary murine macrophages towards an inflammatory/M1 or an alternative/M2 phenotype controls the expression of iron related genes and their ability to manage iron in conditions of iron overload or deficiency.
Design and Methods
Materials
Hemin, ferric ammonium citrate (FAC) and ascorbic acid, were purchased from Sigma (St. Louis, MI, USA). Deferroxamine mesylate (DFO) was obtained from Biofutura Pharma (Milan, Italy). Recombinant murine (rm) macrophage colony-stimulating factor (M-CSF) and rm interleukin-4 (IL4) were from R&D Systems (Minneapolis, MN, USA); rm-interferon-gamma (IFNγ) was purchased from PeproTech (Rocky Hill, NJ, USA). All labeled monoclonal antibodies were from BD Biosciences Pharmigen (San Jose, CA, USA).
Macrophages
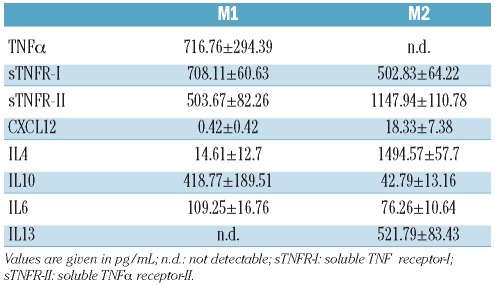
Bone marrow precursors from C57BL/6 female mice were isolated and propagated for 7 days in α-MEM (GIBCO, Invitrogen, Carlsbad, CA, USA) containing 10% fetal bovine serum (Lonza, Basel, Switzerland) in the presence of rm-M-CSF (100 ng/mL) to generate macrophages as described elsewhere.7 Cells were cultured for 2 additional days in the presence of rm-IFNγ (50 ng/mL) to generate M1 cells and for 4 additional days with rm-IL4 (10 ng/mL) and rm-M-CSF (10 ng/mL) to generate M2 cells. Macrophage polarization was verified by flow cytometry after staining with fluorochrome-conjugated antibodies.8 Briefly, 100,000 macrophages were incubated with fluorescent-specific antibodies or isotype control antibodies at 4°C for 20 min (final concentration 5 μg/mL) in phosphate-buffered saline containing 10% fetal bovine serum. APC-conjugated anti-CD11b antibodies were used to identify macrophages and FITC-conjugated anti-HMC class I and class II, CD86 and CD163 antibodies to discriminate between M1 (class I, class II and CD86 high, CD163 intermediate) and M2 macrophages (class I and class II intermediate, CD86 low, CD163 high). Macrophage polarization was also verified by evaluating the concentration of selected soluble molecules in the culture supernatant by enzyme-linked immunosorbent assay (ELISA) (DuoSet Kit, R&D System, Minneapolis, MN, USA). The molecules assayed were: tumor necrosis factor-alpha (TNFα), soluble TNFα receptors I and II (sTNFR-I and sTNFR-II), CXCL12, IL4, IL10, IL6 and IL13. Transferrin receptor (TfR) expression was assessed using a FITC-conjugated anti-CD71 antibody (BD Biosciences) as above. Labeled cells were washed and analyzed using a FACS-Calibur flow cytometer and FlowJo software (Tree Star Inc., Ashland, OR, USA). Results are expressed as relative fluorescence intensity (RFI), calculated by dividing the mean fluorescence intensity obtained in the experimental sample by the one obtained with the isotype-matched control antibody.
When indicated, macrophages were incubated over-night in complete medium in the presence or in the absence of hemin (100 μM), FAC (150 μM) plus ascorbic acid (150 μM) or DFO (150 μM).
Gene-expression profiling and data analysis
Gene expression profiling analysis was carried out as described with minor modifications.9 Total cellular RNA was extracted from M1 and M2 polarized macrophages using the RNeasy midi kit, following the manufacturer’s recommendations. All analyses were performed in quadruplicate. Disposable RNA chips (Agilent RNA 6000 Nano LabChip kit) were used to determine the concentration and purity/integrity of RNA samples using an Agilent 2100 bio-analyzer. cDNA synthesis, biotin-labeled target synthesis, HG-U133 plus 2.0 GeneChip (Affymetrix, Santa Clara, CA, USA) array hybridization, staining and scanning were performed according to the standard protocol supplied by Affymetrix. The GeneChip mouse expression set 430 2.0, which provides comprehensive coverage of the mouse transcriptome, was used. Raw data were acquired using the Affymetrix® GeneChip® Command Console® (AGCC) software. Data processing and appropriate statistical analysis (ANOVA, time course analysis, etc.) and all data quality controls were performed using R (Bioconductor) and Partek® Genomic Suite. The functional analyses were generated through the use of Ingenuity Pathways Analysis (Ingenuity® Systems, www.ingenuity.com).
RNA (1 μg) was used for qualitative polymerase chain reaction (qPCR) analysis for first-strand synthesis of cDNA with the High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Carlsbad, CA, USA) according to the manufacturer's instructions. qPCR was done using SYBR-green PCR Master Mix (Applied Biosystems). Each cDNA sample was amplified in triplicate on a real-time PCR system (7900HT Fast Real-Time PCR System, Applied Biosystems). The level of each RNA was normalized to the corresponding level of β-actin mRNA. The following primers were used: TfR1 forward: 5′-TGATTGTTAGAGCAGGGGAAA-3′, TfR1 reverse: 5′-ATGACTGAGATGGCGGAAAC-3′, β-actin forward: 5′-TGCTGTCCCTGTATGCCTCT-3′, β-actin reverse: 5′-GATGTCACGCACGATTTCC-3′, HO-1 forward: 5′-GACACCTGAGGTCAAGCACAG-3′, HO-1 reverse: 5′-CCACTGCCACTGTTGCCAAC-3′, FtH forward: 5′-GTCAGCTTAGCTCTCATCAC-3′, FtH reverse: 5′-ACGTCTATCTGTCTATGTCTTG-3′, ferroportin forward 5′-CCAGTGTCCCCAACTACCAA-3′, ferroportin reverse 5′-GTCACCGTCAAATCAAAGGA-3′.
RNA-protein gel retardation assay
Cells (2×106) were homogenized in Hepes 10 mM, pH 7.6, MgCl2 3 mM, KCl 40 mM, glycerol 5%, Nonidet P40 0.2% (Sigma), protease inhibitor mixture (Sigma) and dithiothreitol (DTT) 1 mM and centrifuged at 16,000 x g for 5 min at 4°C. The probe for the band-shift assay was transcribed from the linearized pSPT-fer plasmid containing the IRE of the human ferritin heavy (FtH) chain10 using T7 RNA polymerase in the presence of [32P]UTP in a commercially available kit (Promega Corp., Milan, Italy). Equal amounts of protein (2 μg, as determined using the BCA protein assay) from cell lysates were incubated with a molar excess of an iron-responsive elements probe and in the absence or presence of β-mercaptoethanol 2% and sequentially treated with RNase T1 and heparin. After separation on non-denaturing polyacrylamide gels (6%), RNA-protein complexes were visualized by autoradiography. IRP/IRE binding activity was measured by means of densitometric scanning of the autoradiograph, making sure that all signals were in the linear range.
Western blot analysis
Macrophage lysates were prepared in Tris 10 mM at pH 8.0, NaCl 150 mM, Nonidet P40 1%, sodium dodecylsulfate (SDS) 0.1%, EDTA 10 mM and protease inhibitors (Sigma). Lysates were centrifuged at 16,000 x g for 5 min at 4 °C. For western blot analyses, equal amounts of protein were resolved by SDS polyacrylamide gel electrophoresis (PAGE) and transferred onto Immobilon-P (Millipore). After Ponceau S staining, membranes were saturated in Tris-HCl 20 mM, pH 7.6, NaCl 150 mM (Tris-buffered saline) containing non-fat milk 5% and Tween 20 0.1%. Antigens were detected using either rabbit polyclonal anti-FtH, kindly provided by S. Levi (Milan),11 or mouse monoclonal anti-TfR1 (Invitrogen), rabbit polyclonal anti-HO-1 (Santa Cruz Biotechnology, H-105), rabbit anti-mouse ferroportin IgG (Alpha Diagnostic International, MTP11-A), or mouse monoclonal anti- β-actin (Sigma, clone AC15) antibodies. Primary antibodies were revealed with horseradish peroxidase-conjugated secondary antibodies (Amersham Biosciences, Milan, Italy) and a chemiluminescence kit (ECL, Amersham Biosciences).
Analysis of 55Fe-labeled ferritin
Macrophages were incubated overnight with ascorbic acid in the presence of [55Fe] ferric iron citrate (10 μCi/mL, 2.5 μM iron) or with 2.5 μM transferrin bound 55Fe. In selected experiments, the overall concentration of FAC was brought to 150 μM by addition of unlabeled FAC. Ferric iron citrate was prepared by mixing 55FeCl3 (PerkinElmer Life Sciences) with citric acid (1:2 molar ratio). Cells were then washed three times with phosphate-buffered saline and either lysed in Tris 20 mM, pH=7.5 containing 0.5% Triton X100 or chased for an additional 24 h in complete medium in the presence of bathophenantrolin (100 μM) before lysis. Lysates were centrifuged and aliquots of the supernatant used for protein determination or mixed with Ultima Gold (Packard Instrument Co.) to measure cellular 55Fe by liquid scintillation. To evaluate 55Fe incorporation into ferritin (Ft), equal amounts of proteins from supernatants were analyzed by non-denaturing PAGE and visualized by autoradiography.12 In selected experiments, to detect FtH, equal amount of proteins were separated on 7.5% native polyacrylamide gels and transferred onto Immobilon-P membrane. The membrane was probed with a rabbit polyclonal antibody raised against rm-FtH subunit, and Ft detected by chemiluminescence as before.
Quantification of the labile iron pool
The labile iron pool was measured by loading cells with the iron-sensitive probe Calcein-AM (Molecular Probes) as previously described.13 Briefly, macrophages were incubated in 48-well plates with αMEM supplemented with 1 mg/mL bovine serum albumin and 0.25 μM Calcein-AM at 37°C for 15 min. After two cycle washes, cells were maintained in HBSS supplemented with 10 mM glucose and fluorescence was revealed during the following 20 min using the Victor3 Multilabel Counter (Wallac, Perkin Elmer) at 485 nm (excitation) and 535 nm (emission). The iron chelator deferiprone, kindly provided by Apopharma Inc. (Toronto, ON, Canada) was then added to reach 300 μM final concentration and fluorescence was re-determined. After 10 min, fluorescence increases induced by iron chelator were normalized on protein content, assessed by the BCA assay, and considered as labile iron pool (LIP) values.
Quantification of the total iron pool
Iron was determined by inductively coupled plasma mass spectrometry (ICP-MS) using a Perkin Elmer ELAN DRC II instrument (Perkin Elmer Sciex, Woodbridge, ON, Canada) and total quant technique with external calibration. For each sample, two runs were performed (two replicates each), with a dynamic reaction cell. The accuracy of the method was determined in natural water and bovine liver standard reference materials (NIST 1640 and MS1577b, respectively, National Institute of Standard and Technology, Gaithersburg, MD, USA) and resulted around 87.5%. The coefficients of variation ranged from 6% to 8% among series and from 6% to 12% between series. The instrument was calibrated using standard solution at a concentration of 10 μg/L (Multielement ICP-MS Calibration Standard 3, Matrix per Volume: 5% HNO3 per 100 mL, Perkin Elmer Plus). The limits of detection were determined on the basis of three standard deviations of the background signal, and a value of 0.005 was obtained.
Antigen presentation assay
Antigen presentation was carried out as described elsewhere,14 with minor modifications. Briefly, macrophages were incubated overnight in complete medium in the presence or in the absence of DFO (150 μM). Macrophages were then harvested, washed and incubated for 90 min at 37°C in 5% CO2 in the presence or absence of ovalbumin (1 mg/mL, Sigma-Aldrich) in RPMI 1640 medium (Euroclone) containing 10% fetal bovine serum (Lonza) and β-mercaptoethanol (50 μM, GIBCO, Invitrogen). Cell viability was routinely verified and was consistently greater than 98%. Class II-restricted BO97.10.2 T hybridoma cells, specific for the epitope between residues 327–339 of oval-bumin, were added to be stimulated overnight (using a 4:1 macrophages/T cells ratio). IL-2 secretion was assessed by ELISA (Duoset Muose IL2, R&D Systems).
Statistics
Data are expressed as means±standard error mean (SEM) from at least three independent experiments. The statistical analysis was performed using Student's t-test for unpaired data. P values less than 0.05 were considered statistically significant.
Results
Macrophage polarization shapes the expression of genes involved in iron metabolism
We relied on established protocols7 to polarize M-CSF-elicited macrophages into M1 and M2 cells. As expected,15 IFNγ-treated macrophages displayed a typical M1 gene signature and IL4-treated macrophages had a typical M2 gene signature, characterized by specific patterns of cytokines, chemokines and receptors;16 Table 1). Macrophage polarization was confirmed by flow cytometry and by evaluating the concentration of selected soluble molecules in the culture supernatant: M1 macrophages expressed high levels of molecules involved in T-cell activation and co-stimulation, such as MHC class I (H-2Kb) and class II (I-Ab) and CD86 and secreted pro-inflammatory molecules such as TNFα and IL6 (Online Supplementary Figure S1 and Table 2). M2 cells expressed high levels of scavenger receptors, such as the CD163 hemoglobin/haptoglobin receptor and secreted molecules involved in the regulation of the immune system such as TNFα soluble receptors, CXCL12, IL4, and IL13 (Online Supplementary Figure S1 and Table 2).
Table 1.
Expression profile of genes differentially expressed in M1 and M2 polarized macrophages.
Table 2.
Concentrations of soluble molecules in the supernatants of polarized M1 and M2 macrophages assessed by ELISA.
Polarization of macrophages skews the expression profile of genes involved in iron metabolism. In particular M1 macrophages showed high expression of the Ft heavy chain 1 (Fth1), of the natural resistance-associated macrophage protein 1 (Nramp1), involved in defense against intracellular pathogens, of β2-microglobulin (b2m), HIF1a (Hif-1a) and superoxide dismutase 2 (SOD2) and low expression of TfR1 (Tfr1) and Steap 3 (Steap3), which are involved in iron uptake. M1 macrophages expressed ceruloplasmin (Cp), had minimal expression of hepcidin (Hamp1) and low HO-1 (Hmox1) expression. On the other hand, M2 macrophages were characterized by lower expression of Fth1 and higher expression of Tfr1, DMT1 (Slc11A1), Irp1 and Irp2, Tf, Hfe, ferroportin 1 (Scl40a1) and Hmox1 as compared to M1. They expressed higher levels of aminolevulinic acid synthase 1 (Alas1) and frataxin (Fxn), which are involved in mitochondrial iron utilization. The expression of ferritin light chain (Ftl) was similar in both M1 and M2 cells (Table 1).
Macrophage polarization shapes the RNA binding activity of iron-regulatory proteins 1 and 2
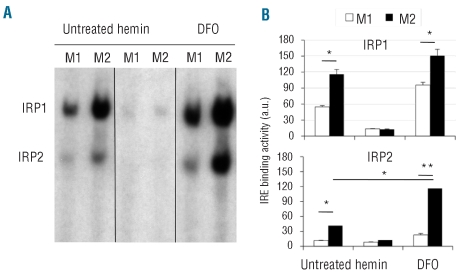
Because of the opposite behavior of FtH and TfR1 in M1 and M2 macrophages we verified the IRP-IRE binding activity in polarized macrophages. The results of band shift assays (Figure 1) indicated that RNA binding activity of IRP1 was significantly lower in M1 macrophages than in M2. IRP2 activity was very low in M1 macrophages. This is relevant in view of the greater importance in iron regulation of IRP2 compared to IRP1 in cultured mammalians cells.17
Figure 1.
RNA binding activity of IRP1 and IRP2. (A) Cytoplasmic extracts of M1 and M2 macrophages, treated or not with hemin (100 μM) or DFO (150 μM), were incubated with an excess of a 32P-labeled iron responsive element probe. RNA-protein complexes were resolved on non-denaturing polyacrylamide gels and revealed by autoradiography. The result shown is representative of three independent experiments. (B) Radioactivity associated with RNA protein complexes was quantified and plotted (arbitrary units, a.u., y axis). * P<0.05; ** P<0.001, significantly different from control.
Next we explored whether iron regulation of IRP1 and IRP2 is maintained in both macrophage populations (Figure 1). In the presence of an excess of heme iron (100 μM) IRP1 and IRP2 binding activity was nearly abolished in both M1 and M2, as expected. Iron deficiency induced by treatment with DFO strongly enhanced the IRP1 activity in both macrophage populations. The effect of iron chelation on IRP2 activity was more pronounced in M2 macrophages. As expected, the IRP1 activity increased in both M1 and M2 macrophages in the presence of β-mercaptoethanol (Online Supplementary Figure S2).
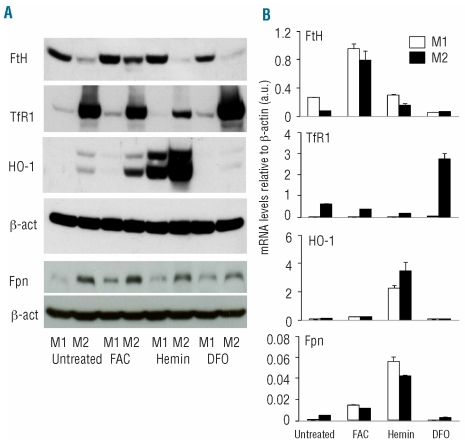
We then analyzed by western blotting (Figure 2A) and qPCR (Figure 2B) molecules involved in cellular iron home-ostasis. In agreement with their low IRP activity M1 macrophages expressed high levels of FtH (both at RNA and protein levels) and low levels of TfR1. However, Fpn protein (expected to be regulated as FtH) was low, since transcriptionally down-regulated, and HO-1 was virtually absent. M2 macrophages expressed low levels of FtH and high levels of TfR1, consistent with their high IRP-binding activities; compared to M1 macrophages, they expressed higher amounts of Fpn (Figure 2A). These expression patterns were unaffected by antibodies blocking IL4 and IFNγ, ruling out an effect on the regulation of iron-related genes of residual recombinant cytokines used to polarize macrophage precursors (data not shown).
Figure 2.
Expression of proteins involved in iron metabolism in M1 and M2 macrophages. (A) Western blot analysis of the expression of FtH, TfR1, HO-1 and Fpn in M1 and M2 macrophages, either untreated or incubated over night in the presence of FAC (150 μM), hemin (100 μM) and DFO (150 μM). Results shown are representative of three independent experiments. (B) qPCR analysis of FtH, TfR1, HO-1 and Fpn in M1 and M2 macrophages, either untreated or incubated overnight in the presence of FAC (150 μM), hemin (100 μM) or DFO (150μM).
Macrophage polarization influences the response to iron overload and depletion
To verify the response of M1 and M2 macrophages to cellular iron variations, we analyzed the expression of FtH, TfR1, HO-1 and Fpn in conditions of iron overload and depletion. Figure 2A shows that macrophage polarization shaped the response to iron availability in the microenvironment. Non-heme iron (FAC) enhanced FtH expression both at the translational and the transcriptional levels and, although to a lesser extent, Fpn in both M1 and M2 macrophages. FAC also enhanced HO-1 mRNA (from 0.05±0.003 to 0.2±0.03, P=0.001, in M1 cells and from 0.1±0.004 to 0.22±0.02, P=0.0009, in M2 cells) and protein expression in M2 macrophages. As expected, heme-iron strongly induced both mRNA and protein HO-1 levels, especially in M2. FtH was up-regulated in M1, but surprisingly not in M2 (Figure 2A), whereas TfR1 expression was significantly down-regulated in both macrophage populations (Figure 2 and Online Supplementary Figure S3). Heme-iron strongly induced Fpn mRNA in both M1 and M2 macrophages, although the effect was less evident at the protein level. Selective qPCR failed to detect the non-IRE Fpn isoform (data not shown), suggesting that both macrophage populations expressed only the IRE form.18
DFO treatment further enhanced TfR1 expression in M2 macrophages, but not in M1 ones (Figure 2A,B; Online Supplementary Figure S3) and reduced Fpn in both cell types. Moreover, iron chelation failed to suppress FtH expression in M1 macrophages. These data demonstrate that M1 macrophages have an iron storage phenotype, which is not influenced by the presence or absence of iron in the environment and that M2 macrophages have a phenotype reminiscent of iron deficiency.
Polarization influences the size of the labile iron pool and the ability of macrophages to internalize and release iron
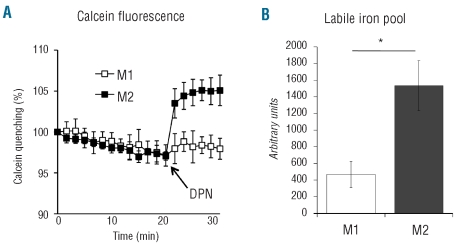
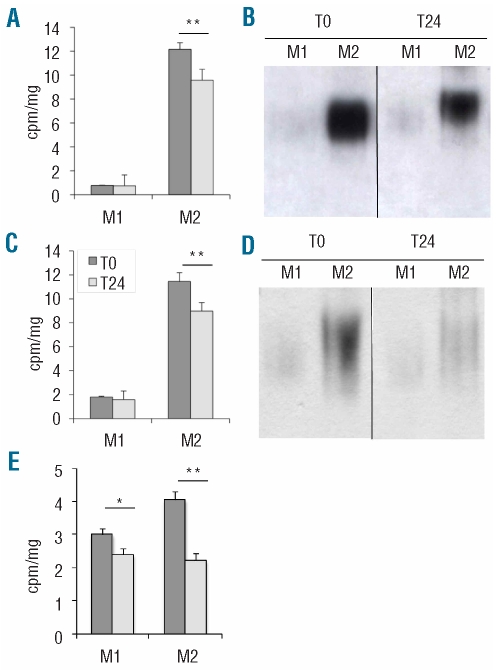
Under basal conditions, the total amount of iron of M1 and M2 macrophages was similar (0.026 and 0.028 ng/106 cells, respectively). To investigate the size of the labile iron pool (LIP) we used a metallo-sensor fluorescent probe (calcein). M1 macrophages did not have a detectable LIP, which, in contrast, was revealed in labeled M2 macrophages after addition of the iron chelator deferiprone (Figure 3A). The difference was highly statistically significant (Figure 3B). To investigate the ability of M1 and M2 cells to handle iron, we challenged polarized macrophages with radioactive 55Fe (2.5 μM FAC) and verified their ability to internalize, bind to Ft and release the metal during a 24 h chase period. M1 cells (Figure 4) failed to internalize 55Fe efficiently. In contrast M2 cells effectively took up the metal and displayed a large amount of cell-associated and Ft-bound 55Fe (Figure 4A,B). After a 24 h chase, cell-associated and Ft-bound 55Fe decreased only in M2 cells (Figure 4A,B), indicating active release of iron. Similar results were obtained when M1 and M2 macrophages were challenged with radioactive Tf-bound iron (Figure 4C,D). To investigate whether the amount of iron in the environment influences the efficiency of the response, we verified the ability of polarized macrophages to handle a 50-fold higher concentration of iron (150 μM final concentration: 55Fe-FAC 2.5 μM-unlabeled iron 147.5 μM), i.e. in conditions in which we observed Fpn induction in M1 macrophages (Figure 2). As shown in Figure 4E, in these conditions M1 internalized iron, although less effectively than M2 cells did and both M1 and M2 macrophages released a substantial fraction of the internalized iron upon a 24 h chase: M2 were again more effective than M1 cells at releasing 55Fe. These data suggest that M1 macrophages are able to take up and release iron when challenged with high concentrations of the metal, and confirm the ability of M2 macrophages to recycle iron.
Figure 3.
Labile iron pool in M1 and M2 macrophages. (A) Representative graph illustrating calcein fluorescence modulation in M1 and M2 macrophages before and after deferiprone addition. Data are presented as averages of four independent determinations for each experimental point. (B) Quantification of LIP. Data are presented as averages of three independent experiments, each done as in (A). Error bars indicate standard deviations. DPN: deferiprone. * P<1.5x10−5.
Figure 4.
Incorporation and release of 55Fe by M1 and M2 macrophages. M1 and M2 macrophages were incubated overnight with [55Fe] ferric iron citrate (2.5 μM iron) in the presence of ascorbic acid (A) and (B) or with 2.5 μM transferrin bound 55Fe (C and D), or with [55Fe] ferric iron citrate in the presence of ascorbic acid (150 μM iron, E), washed and lysed either immediately or after a 24 h chase. (A, C and E) Cell-associated radioactivity (cpm/mg of protein extract, y axis) was evaluated. Results represent the mean ± SD of quadruplicate samples. **P<0.01, significantly different from control. (B and D) Cell extracts were obtained at the times indicated and were resolved on a native polyacrylamide gel. Radioactive iron incorporation into Ft was evaluated by autoradiography.
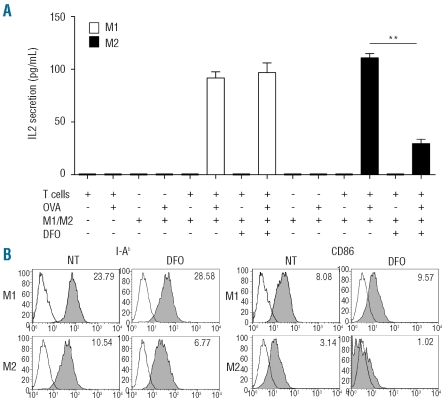
M2 macrophages depend on iron for T-cell activation
We verified the ability of M1 and M2 cells to present epitopes derived from nominal antigens to T lymphocytes: T lymphocyte activation was assessed by measuring IL2 secretion. Under basal conditions, M1 and M2 macrophages were almost equally effective at activating antigen-specific T cells. However, in condition of iron deficiency, T-cell activation by M2 cells abates (Figure 5) and the expression of molecules involved in antigen presentation, such as MHC class II (I-Ab), or in T-cell co-stimulation, such as CD86, was consensually reduced. In contrast, T-cell activation by M1 cells was maintained in conditions of iron chelation (Figure 5).
Figure 5.
Effects of iron availability on antigen presentation by M1 and M2 cells. (A) M1 (open bars) and M2 (filled bars) macrophages were used to activate MHC class II-restricted T hybridoma cells specific for the nominal antigen ovalbumin (OVA). When indicated, the iron chelator DFO was added. T-cell activation was assessed by IL2 secretion (pg/mL, y axis, see Design and Methods). T-cell activation, as expected, was detectable only when antigen-presenting cells (M1/M2), T cells and the antigen (OVA) were present. DFO did not influence T-cell activation by M1 macrophages, but significantly inhibited T-cell activation by M2 macrophages. (B): M1 and M2 macrophages, cultured in the presence or absence of DFO, were analyzed by flow cytometry after staining with antibodies directed against MHC class II molecules (I-Ab) or against the CD86 co-stimulatory molecule. Filled histograms represent the binding of specific antibodies, whereas open histograms represent the fluorescence background obtained in the presence of isotype-matched control antibodies. Numbers indicate the relative fluorescence intensity (RFI) values, calculated dividing the mean fluorescence intensity obtained in the experimental sample by the one obtained with the relevant control. The results shown are representative of three independent experiments. ** P<0.01, significantly different from control.
Discussion
Macrophages fulfill key functions in immunity. In particular, they are vital for the response to invading pathogens and for the regeneration of injured tissues. Specialized differentiation programs triggered in resting macrophages by environmental stimuli have been identified and are referred to as “classical” or “alternative” activation.
Classically activated/M1 macrophages have inflammatory functions: they produce effector molecules and inflammatory cytokines, participate as inducer and effector cells in polarized Th1 responses and mediate resistance against intracellular microbes and tumors. Alternatively activated/M2 macrophages participate in polarized Th2 reactions, promote killing and encapsulation of parasites and are present in established tumors where they promote progression. Moreover, they are involved in wound healing and have immunoregulatory functions.1 Cytokines, IFNγ and IL4, in particular, drive macrophage polarization. The effect of the cytokines is not restricted to single genes. They involve specific well-defined gene signatures, which allow polarized macrophages to exert their biological functions.16,19
The limited ability of M1 macrophages to recycle iron is possibly involved in the ability of these cells to control bacterial pathogenicity. Limited intracellular iron availability has been clearly demonstrated to impair intracellular pathogen growth thus facilitating the microbicidal function of macrophages.2,20,21 It is tempting to speculate that this function is preferentially associated with macrophages polarized towards an M1 phenotype, which are characterized by a restricted LIP. Further studies are warranted to address this issue.
M1 macrophages express high levels of FtH and low levels of Fpn, CD163, TfR1 and HO-1. The culture conditions we used were not suitable for the detection of the transient autocrine synthesis of hepcidin that has been identified in stimulated human monocytes;22 particularly so since to drive M1 polarization we used recombinant IFN γ alone in the absence of microbial co-stimuli. The basal expression of Fpn was negligible. M1 macrophages, which directly deal with microbes at sites of infection, down-regulate Fpn, thus limiting release of iron which could favor invading pathogens. On the other hand, by up-regulating FtH expression and limiting the size of the LIP, M1 cells possibly protect themselves against oxidative damage23 and further limit the availability of the molecule to internalized microbes.
Macrophages that phagocytose senescent red blood cells represent the major cellular system responsible for supplying iron required for erythropoiesis.6,24–26 Ingested heme is degraded within the phagolysosomes by the enzyme HO-127,28 and iron is released to the extracellular environment. Iron that is not released is mostly incorporated into Ft, the central protein for iron storage.29,30 Therefore, macrophages in charge of recycling iron are expected to have a relatively high ability to internalize senescent cells, to endocytose transferrin-bound iron via TfR, and to release but not to store iron. M2 macrophages satisfy these requirements: they are professional scavengers of senescent and apoptotic cells.31,32 Furthermore we found that they express high levels of membrane molecules involved in iron internalization, such as TfR1 and the CD163 hemoglobin/haptoglobin receptor, and low levels of FtH.
Accordingly, they effectively internalize and release radioactive iron. The expression of Fpn, which is further enhanced in the presence of heme and non-heme iron, is critical for the transport of the metal since Fpn is the sole mammalian iron exporter so far characterized. Its over-expression causes, for example, enhanced release of iron derived from the processing of phagocytosed erythrocytes.26 Hemin has a role in the control of Fpn transcription. Heme binds Fpn promoter33 and the heme protoporphyrin ring effectively increases Fpn transcription in immortalized murine macrophages.34 FtH and Fpn protein levels are under the control of the iron-dependent IRP-IRE regulation. Effective Fpn translation due to IRP–IRE interactions is possibly limited due to the relatively low amount of iron released from heme, as reflected by the poor induction of FtH. Accordingly Fpn protein levels were not substantially modified in hemin-treated cells as compared to untreated cells.
A reduction of circulating iron also stimulates T-cell-dependent anti-microbial acquired immunity, favoring the shift towards Th1 responses.35–38 M1 macrophages differentiate under the control of cytokines produced by Th1 cells, and in turn sustain establishment and maintenance of Th1 responses.3,39 Here we observed that M1 cells maintained the ability to present antigens to memory T cells even after iron depletion: this feature possibly allows them to keep activating pathogen-specific T cells in conditions scarcely permissive for microbe spreading. In contrast, the ability of M2 macrophages to activate T cells abated in the absence of iron. Interestingly, low environmental iron was recently reported to result in defective production of cytokines by activated macrophages.40 Further studies are warranted to better characterize the effect of polarized iron handling by macrophages on acquired immune functions.
M2 cells play a pivotal role in sustaining angiogenesis, wound repair and tumor growth.41,42 These functions are associated with their ability to provide nutrients, including iron, to the healing tissues. Interestingly, M2 macrophages are necessary for the regeneration of acutely injured skeletal muscles,43 i.e. a condition in which large amounts of the metal are essential to build new, functional myofibers.
Our results indicate that M1 and M2 macrophages maintained iron-IRP regulation, although their iron sensing was reset according to their role. M1 had increased FtH and low TfR1, but to escape iron control they suppressed Fpn transcription. M1 were still sensitive to increased iron, but, in keeping with their storage phenotype, they were almost insensitive to DFO.
In contrast M2 had a clear iron-deficient phenotype. They actively took up iron that they were unable to store. Even in the presence of high iron concentrations they did not suppress TfR1 and did not activate FtH. Still M2 macrophages could respond to DFO further increasing TfR1 expression.
Via the HIF1α pathway hypoxia elicits a coordinated response for controlling iron metabolism and oxygen transport, mediated via hepcidin/ferroportin.44 Our study suggests a differential expression of the transcription factor in the M1 and M2 populations, and modulations at this level are possibly involved in macrophage polarization.45 Confirmation of this possibility will require further experiments.
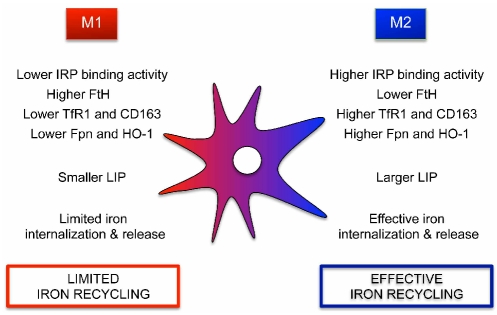
Our results agree well with those recently published by Recalcati et al. using human monocyte-derived macrophages.46 The conservation of the pathway between mouse and human clearly indicates that a dichotomy in iron handling represents a general feature of the functional polarization of macrophages. Resting macrophages appear to have the potential to differentiate towards subpopulations of cells with distinct patterns of iron handling (Figure 6), which may be instrumental for their homeostatic roles in conditions as diverse as the inflammatory response to invading microbes or the repair of injured tissues. Moreover, our results reveal an instructive role of environmental cytokines in determining iron homeostasis.
Figure 6.
Main characteristics of iron handling by M1 and M2 macrophages. LIP, labile iron pool.
Acknowledgments
this work was supported by the Ministero della Salute (FIRB-IDEAS to PR-Q), by the EU (Endostem to SB and PR-Q), by the AFM (to SB, PR-Q and LS) and by the Fondazione Cariplo (to CC). We thank Sonia Levi for anti-FtH antibodies, Gaetano Cairo who kindly provided the pSPT-fer plasmid, and Luciano Adorini for the generous gift of the antigen-specific T-cell hybridomas.
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Mantovani A, Sica A, Locati M. New vistas on macrophage differentiation and activation. Eur J Immunol. 2007;37(1):14–6. doi: 10.1002/eji.200636910. [DOI] [PubMed] [Google Scholar]
- 2.Paradkar PN, De Domenico I, Durchfort N, Zohn I, Kaplan J, Ward DM. Iron depletion limits intracellular bacterial growth in macrophages. Blood. 2008;112(3):866–74. doi: 10.1182/blood-2007-12-126854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–83. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 4.De Domenico I, McVey Ward D, Kaplan J. Regulation of iron acquisition and storage: consequences for iron-linked disorders. Nat Rev Mol Cell Biol. 2008;9(1):72–81. doi: 10.1038/nrm2295. [DOI] [PubMed] [Google Scholar]
- 5.Theurl I, Aigner E, Theurl M, Nairz M, Seifert M, Schroll A, et al. Regulation of iron homeostasis in anemia of chronic disease and iron deficiency anemia: diagnostic and therapeutic implications. Blood. 2009;113(21):5277–86. doi: 10.1182/blood-2008-12-195651. [DOI] [PubMed] [Google Scholar]
- 6.Weiss G. Iron metabolism in the anemia of chronic disease. Biochim Biophys Acta. 2009;1790(7):682–93. doi: 10.1016/j.bbagen.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Bacci M, Capobianco A, Monno A, Cottone L, Di Puppo F, Camisa B, et al. Macrophages are alternatively activated in patients with endometriosis and required for growth and vascularisation of lesions in a mouse model of disease. Am J Pathol. 2009;175(2):547–56. doi: 10.2353/ajpath.2009.081011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lolmede K, Campana L, Vezzoli M, Bosurgi L, Tonlorenzi R, Clementi E, et al. Inflammatory and alternatively activated human macrophages attract vessel-associated stem cells, relying on separate HMGB1- and MMP-9-dependent pathways. J Leukoc Biol. 2009;85(5):779–87. doi: 10.1189/jlb.0908579. [DOI] [PubMed] [Google Scholar]
- 9.Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L, et al. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol. 2007;9(3):255–67. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]
- 10.Mullner EW, Neupert B, Kuhn LC. A specific mRNA binding factor regulates the iron-dependent stability of cytoplasmic transferrin receptor mRNA. Cell. 1989;58(2):373–82. doi: 10.1016/0092-8674(89)90851-9. [DOI] [PubMed] [Google Scholar]
- 11.Santambrogio P, Cozzi A, Levi S, Rovida E, Magni F, Albertini A, et al. Functional and immunological analysis of recombinant mouse H- and L-ferritins from Escherichia coli. Protein Expr Purif. 2000;19(1):212–8. doi: 10.1006/prep.2000.1212. [DOI] [PubMed] [Google Scholar]
- 12.Corna G, Santambrogio P, Minotti G, Cairo G. Doxorubicin paradoxically protects cardiomyocytes against iron-mediated toxicity: role of reactive oxygen species and ferritin. J Biol Chem. 2004;279(14):13738–45. doi: 10.1074/jbc.M310106200. [DOI] [PubMed] [Google Scholar]
- 13.Epsztejn S, Kakhlon O, Glickstein H, Breuer W, Cabantchik I. Fluorescence analysis of the labile iron pool of mammalian cells. Anal Biochem. 1997;248(1):31–40. doi: 10.1006/abio.1997.2126. [DOI] [PubMed] [Google Scholar]
- 14.Rovere P, Sabbadini MG, Vallinoto C, Fascio U, Rescigno M, Crosti MC, et al. Dendritic cell presentation of antigens from apoptotic cells in a pro-inflammatory context: role of opsonizing anti-beta 2 glyco-protein I antibodies. Arthritis Rheum. 1999;42(7):1412–20. doi: 10.1002/1529-0131(199907)42:7<1412::AID-ANR15>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 15.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–61. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 16.Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol. 2006;177(10):7303–11. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- 17.Meyron-Holtz EG, Ghosh MC, Rouault TA. Mammalian tissue oxygen levels modulate iron-regulatory protein activities in vivo. Science. 2004;306(5704):2087–90. doi: 10.1126/science.1103786. [DOI] [PubMed] [Google Scholar]
- 18.Zhang DL, Hughes RM, Ollivierre-Wilson H, Ghosh MC, Rouault TA. A ferroportin transcript that lacks an iron-responsive element enables duodenal and erythroid precursor cells to evade translational repression. Cell Metab. 2009;9(5):461–73. doi: 10.1016/j.cmet.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3(1):23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 20.Chlosta S, Fishman DS, Harrington L, Johnson EE, Knutson MD, Wessling-Resnick M, et al. The iron efflux protein ferroportin regulates the intracellular growth of Salmonella enterica. Infect Immun. 2006;74(5):3065–7. doi: 10.1128/IAI.74.5.3065-3067.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nairz M, Theurl I, Ludwiczek S, Theurl M, Mair SM, Fritsche G, et al. The coordinated regulation of iron homeostasis in murine macrophages limits the availability of iron for intracellular Salmonella typhimurium. Cell Microbiol. 2007;9(9):2126–40. doi: 10.1111/j.1462-5822.2007.00942.x. [DOI] [PubMed] [Google Scholar]
- 22.Theurl I, Theurl M, Seifert M, Mair S, Nairz M, Rumpold H, et al. Autocrine formation of hepcidin induces iron retention in human monocytes. Blood. 2008;111(4):2392–9. doi: 10.1182/blood-2007-05-090019. [DOI] [PubMed] [Google Scholar]
- 23.Kakhlon O, Cabantchik ZI. The labile iron pool: characterization, measurement, and participation in cellular processes(1) Free Radic Biol Med. 2002;33(8):1037–46. doi: 10.1016/s0891-5849(02)01006-7. [DOI] [PubMed] [Google Scholar]
- 24.Kawane K, Fukuyama H, Kondoh G, Takeda J, Ohsawa Y, Uchiyama Y, et al. Requirement of DNase II for definitive erythropoiesis in the mouse fetal liver. Science. 2001;292(5521):1546–9. doi: 10.1126/science.292.5521.1546. [DOI] [PubMed] [Google Scholar]
- 25.Krieser RJ, MacLea KS, Longnecker DS, Fields JL, Fiering S, Eastman A. Deoxyribonuclease IIalpha is required during the phagocytic phase of apoptosis and its loss causes perinatal lethality. Cell Death Differ. 2002;9(9):956–62. doi: 10.1038/sj.cdd.4401056. [DOI] [PubMed] [Google Scholar]
- 26.Knutson MD, Oukka M, Koss LM, Aydemir F, Wessling-Resnick M. Iron release from macrophages after erythrophagocytosis is up-regulated by ferroportin 1 overexpression and down-regulated by hepcidin. Proc Natl Acad Sci USA. 2005;102(5):1324–8. doi: 10.1073/pnas.0409409102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poss KD, Tonegawa S. Heme oxygenase 1 is required for mammalian iron reutilization. Proc Natl Acad Sci USA. 1997;94(20):10919–24. doi: 10.1073/pnas.94.20.10919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bach FH. Heme oxygenase-1: a therapeutic amplification funnel. FASEB J. 2005;19(10):1216–9. doi: 10.1096/fj.04-3485cmt. [DOI] [PubMed] [Google Scholar]
- 29.Moura E, Noordermeer MA, Verhoeven N, Verheul AF, Marx JJ. Iron release from human monocytes after erythrophagocytosis in vitro: an investigation in normal subjects and hereditary hemochromatosis patients. Blood. 1998;92(7):2511–9. [PubMed] [Google Scholar]
- 30.Hentze MW, Muckenthaler MU, Andrews NC. Balancing acts: molecular control of mammalian iron metabolism. Cell. 2004;117(3):285–97. doi: 10.1016/s0092-8674(04)00343-5. [DOI] [PubMed] [Google Scholar]
- 31.Leidi M, Gotti E, Bologna L, Miranda E, Rimoldi M, Sica A, et al. M2 macrophages phagocytose rituximab-opsonized leukemic targets more efficiently than M2 cells in vitro. J Immunol. 2009;182(7):4415–22. doi: 10.4049/jimmunol.0713732. [DOI] [PubMed] [Google Scholar]
- 32.Michlewska S, Dransfield I, Megson IL, Rossi AG. Macrophage phagocytosis of apoptotic neutrophils is critically regulated by the opposing actions of pro-inflammatory and anti-inflammatory agents: key role for TNF-alpha. FASEB J. 2009;23(3):844–54. doi: 10.1096/fj.08-121228. [DOI] [PubMed] [Google Scholar]
- 33.Muckenthaler M. 6th International Conference on Heme Oxygenase- Heme Oxygenases in Biology and Medicine; September 30th – October 4th, 2009; Miami Beach, Florida. 2009. [Google Scholar]
- 34.Marro S, Chiabrando D, Messana E, Stolte J, Turco E, Tolosano E, et al. Heme controls ferroportin1 (FPN1) transcription involving Bach1, Nrf2 and a MARE/ARE sequence motif at position -7007 of the FPN1 promoter. Haematologica. 2010;95(8):1261–8. doi: 10.3324/haematol.2009.020123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Omara FO, Blakley BR. The effects of iron deficiency and iron overload on cell-mediated immunity in the mouse. Br J Nutr. 1994;72(6):899–909. doi: 10.1079/bjn19940094. [DOI] [PubMed] [Google Scholar]
- 36.Weiss G, Wachter H, Fuchs D. Linkage of cell-mediated immunity to iron metabolism. Immunol Today. 1995;16(10):495–500. doi: 10.1016/0167-5699(95)80034-4. [DOI] [PubMed] [Google Scholar]
- 37.Mencacci A, Cenci E, Boelaert JR, Bucci P, Mosci P, Fe d'Ostiani C, et al. Iron overload alters innate and T helper cell responses to Candida albicans in mice. J Infect Dis. 1997;175(6):1467–76. doi: 10.1086/516481. [DOI] [PubMed] [Google Scholar]
- 38.Weiss G, Thuma PE, Mabeza G, Werner ER, Herold M, Gordeuk VR. Modulatory potential of iron chelation therapy on nitric oxide formation in cerebral malaria. J Infect Dis. 1997;175(1):226–30. doi: 10.1093/infdis/175.1.226. [DOI] [PubMed] [Google Scholar]
- 39.Montaner LJ, da Silva RP, Sun J, Sutterwala S, Hollinshead M, Vaux D, et al. Type 1 and type 2 cytokine regulation of macrophage endocytosis: differential activation by IL-4/IL-13 as opposed to IFN-gamma or IL-10. J Immunol. 1999;162(8):4606–13. [PubMed] [Google Scholar]
- 40.Wang L, Harrington L, Trebicka E, Shi HN, Kagan JC, Hong CC, et al. Selective modulation of TLR4-activated inflammatory responses by altered iron homeostasis in mice. J Clin Invest. 2009;119(11):3322–8. doi: 10.1172/JCI39939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 42.Brunelli S, Rovere-Querini P. The immune system and the repair of skeletal muscle. Pharmacol Res. 2008;58(2):117–21. doi: 10.1016/j.phrs.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 43.Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204(5):1057–69. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peyssonnaux C, Zinkernagel AS, Schuepbach RA, Rankin E, Vaulont S, Haase VH, et al. Regulation of iron homeostasis by the hypoxia-inducible transcription factors (HIFs) J Clin Invest. 2007;117(7):1926–32. doi: 10.1172/JCI31370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weigert A, Brune B. Nitric oxide, apoptosis and macrophage polarization during tumor progression. Nitric Oxide. 2008;19(2):95–102. doi: 10.1016/j.niox.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 46.Recalcati S, Locati M, Marini A, Santambrogio P, Zaninotto F, De Pizzol M, et al. Differential regulation of iron homeostasis during human macrophage polarized activation. Eur J Immunol. 2010;40(3):824–35. doi: 10.1002/eji.200939889. [DOI] [PubMed] [Google Scholar]