Abstract
Background
Hemoglobin concentrations slightly below the lower limit of normal are a common laboratory finding in the elderly, but scant evidence is available on the actual occurrence of mild anemia despite its potential effect on health. The objectives of this study were to estimate the prevalence and incidence of mild grade anemia and to assess the frequency of anemia types in the elderly.
Design and Methods
This was a prospective, population-based study in all residents 65 years or older in Biella, Italy.
Results
Blood test results were available for analysis from 8,744 elderly. Hemoglobin concentration decreased and mild anemia increased steadily with increasing age. Mild anemia (defined as a hemoglobin concentration of 10.0–11.9 g/dL in women and 10.0–12.9 g/dL in men) affected 11.8% of the elderly included in the analysis, while the estimated prevalence in the entire population was 11.1%. Before hemoglobin determination, most mildly anemic individuals perceived themselves as non-anemic. Chronic disease anemia, thalassemia trait, and renal insufficiency were the most frequent types of mild anemia. The underlying cause of mild anemia remained unexplained in 26.4% of the cases, almost one third of which might be accounted for by myelodysplastic syndromes. In a random sample of non-anemic elderly at baseline (n=529), after about 2 years, the annual incidence rate of mild anemia was 22.5 per 1000 person-years and increased with increasing age.
Conclusions
The prevalence and incidence of mild anemia increase with age and mild anemia affects more than one out of ten elderly individuals. Unexplained anemia is common and may be due to myelodysplastic syndromes in some cases.
Keywords: elderly, hemoglobin, incidence, mild anemia, prevalence, types of anemia
Introduction
Hemoglobin concentration is a continuously distributed variable. World Health Organization (WHO) criteria for the diagnosis of anemia,1 although universally used, are considered to a certain extent arbitrary as they are based on few data and do not account for significant ethnic differences.2 Anemia in the elderly is an under-diagnosed condition often not reported to the patient because it is mostly perceived as a mere consequence of aging or as a disease marker. However, recent studies have started to question this view reporting its association with several adverse outcomes in the elderly.3–7 We have previously reported independent associations of mild anemia with worse cognitive and quality of life outcomes and with increased risk of hospitalization and mortality in the general elderly population.8,9 Anemia can thus have a relevant effect on healthcare needs and become a significant healthcare burden.10,11
Several studies on the prevalence of anemia have been published,12 but the number of large-scale population-based surveys with a well-defined initial elderly population and with a high inclusion rate is low. Moreover, the prevalence of mild grade anemia has been reported by or can be drawn from only a few studies, while no data are available on its incidence. The aims of the present population-based study were to estimate the prevalence and incidence of anemia and mild anemia and to assess the frequency of anemia types in the elderly population. A secondary aim was to investigate the association of mild anemia with common pathological conditions in old age.
Design and Methods
The study population
“Salute e Anemia” (“Health and Anemia”) is a prospective population-based observational study of all elderly residents in the municipality of Biella, Piedmont, a town in the north-west of Italy situated at a mean altitude of 420 meters above sea level, with a population of about 46,000 inhabitants. North African and East European migration is recent and for the most part composed of young people (about 25 years old at the moment of transfer), thus the elderly population of Biella is almost exclusively of Italian origin, though composed not only of local natives but also of immigrant workers mainly from the north-eastern Venetian province of Vicenza (1911–1936) and southern Italian regions (1950–1970) attracted at the time by the flourishing textile industry in Biella. The population is predominantly well off and employment is mainly in the industrial and service sectors. The age structure of the Biella elderly is similar to that of the general Italian elderly population.
Lists of residents were obtained from the registry office of the municipality. The high prevalence of dementia, cognitive impairment, functional disability, and health problems in the oldest old prompted us to separate the investigation of the younger (65–84 years old) from the older subjects. All registered individuals of 65 to 84 years old residing in Biella on the prevalence day (May 12, 2003) were eligible for the study (n=10,082). Case ascertainment was made between May 2003 and April 2004. Subsequently the study was extended to all residents aged 85 years or older (n=1,526) on the prevalence day (May 7, 2007). Case ascertainment was made between May 2007 and July 2008.
No exclusion criteria other than age and residence were used. Elderly in nursing or residential homes were included. A letter describing the aims of the survey was sent to all eligible residents who were contacted by phone 1 to 2 weeks later to ascertain their intention to participate.
In consenting participants, arterial blood pressure (third reading) and heart and respiratory rates were measured and blood samples were taken by trained, registered nurses either at home or in an outpatient clinic at the elderly person’s choice and, for institutionalized individuals, in nursing homes. A questionnaire was also administered by the nurses in order to ascertain habits (smoking and alcohol use), present and past diseases, hospital admissions, and interventions.
To face the potential sources of non-participation due to the initial step-limiting request of a blood sample and the poor health condition of many elderly often already recently tested, complete blood count (CBC) results together with age and sex of all elderly residents aged 65 years or older who did not or could not participate, but had a CBC done in the same period and laboratory of the epidemiological study, were obtained in an anonymous way from the central laboratory of Biella Hospital, one of the teams involved in the present research. Biella has only one hospital which is public. Its laboratory is one of the very few in the area, and most residents go there for laboratory investigations.
In order to estimate the incidence of anemia and mild anemia, after an approximate time period of 2 years, a random sample of community-dwelling, non-anemic (together with all consenting mildly anemic) elderly free of severe organ insufficiency, stroke, neurodegenerative diseases, or terminal illness at the baseline evaluation, was contacted again during 2005–2006 to re-assess hemoglobin concentration.
Study procedures were conducted in accordance with the principles outlined in the Declaration of Helsinki of 1964 and its subsequent amendments. The local research Ethics Committee of the Azienda Sanitaria Ospedaliera di Novara approved the study and the informed consents. Written informed consent was obtained from each participant prior to blood sampling.
Laboratory methods
Venous blood samples were collected from participants in a sitting position by venipuncture. The CBC was determined using a SISMEX SE-2100 electronic counter (Sysmex Corporation Kobe, Japan) by the central laboratory of Biella Hospital. When a hemoglobin concentration was below WHO reference criteria for anemia, further laboratory investigations were made: serum folic acid, vitamin B12, iron, ferritin and transferrin, and transferrin saturation (the laboratory tests are described in the Online Supplementary Appendix). These laboratory investigations were also assessed in an equal sample of non-anemic individuals matched for age and sex.
Definitions of anemia and anemia types
Anemia was defined according to the WHO criteria1 as a hemoglobin concentration lower than 12 g/dL in women and 13 g/dL in men. Along with most grading classification systems,13,14 mild grade anemia was defined as a hemoglobin concentration between 10.0 and 11.9 g/dL in women and between 10.0 and 12.9 g/dL in men.
Iron deficiency anemia was considered present if the elderly had low serum iron (lower than 50 μg/dL in women and 60 μg/dL in men), low ferritin (lower than 15 ng/mL), low transferrin saturation rate (lower than 16%) or increased total iron binding capacity (higher than 450 μg/dL). Anemia of chronic disease was defined as low circulating iron in the presence of increased iron stores (normal or increased ferritin higher than 100 ng/mL, transferrin saturation higher than 25% and lower than 50%) and decreased total iron binding capacity (lower than 250 μg/dL). Thalassemia trait was considered when the following conditions were present: low or very low mean corpuscular volume (MCV) and mean corpuscular hemoglobin (MCH), increased red blood cell (RBC) count, normal or increased circulating iron in the presence of normal or increased iron stores.15 Since HbA2 levels were not determined, it was not possible to distinguish alpha from beta thalassemia. Anemia associated with folate or vitamin B12 deficiency was defined as concentrations of folate lower than 3.0 ng/mL or vitamin B12 lower than 200 pg/mL and MCV higher than 95 fL. Subjects were classified as having anemia related to chronic renal disease when affected by renal insufficiency.
The classification of anemia based on the hematologic findings was supported by the clinical conditions and pharmacological therapies of the elderly. Anemias that could not be classified into any of the previous categories were considered to be of unexplained origin. The hematologic characteristics consistent with the possible presence of myelodysplastic syndromes were defined as macrocytosis (MCV higher than 100 fL) in the absence of folate or B12 deficiency, and leukopenia (white blood cell count lower than 3×109/L) or thrombocytopenia (platelet count lower than 150×109/L).
Based on these shared definitions, each study expert (ER, MGDP, and LP) classified the different types of anemia for all cases independent of the judgment of the others. Subsequently, the panel of the three physicians reviewed, discussed and reached a final consensus for each preliminary discrepant classification.
Statistical analysis
Hemoglobin concentration distribution was left skewed when all individuals were taken into consideration, so parametric bivariate analyses were always checked for significance using non-parametric tests: in each case the non-parametric tests gave P values very similar to their parametric counterparts. Moreover median hemoglobin values in the tested groups were almost identical to the mean values. When individuals with severe or moderate anemia were excluded, the hemoglobin distribution was almost perfectly Gaussian. The relation of hemoglobin concentration to age was tested using linear regression models, including the regressors in the following order: age, sex, and age by sex interaction. Ninety-five percent confidence limits for the prevalence proportions were computed using score intervals. Confidence intervals for incidences were calculated assuming that the number of cases of anemia and mild anemia followed a Poisson distribution. Hemoglobin concentration change after 2 years was estimated using the relative proportion of mildly anemic and non-anemic elderly in the population as a weight. All P values were two sided. Data analysis was performed using JMP v. 8.0.2 (SAS Institute Inc., Cary, NC, USA).
Results
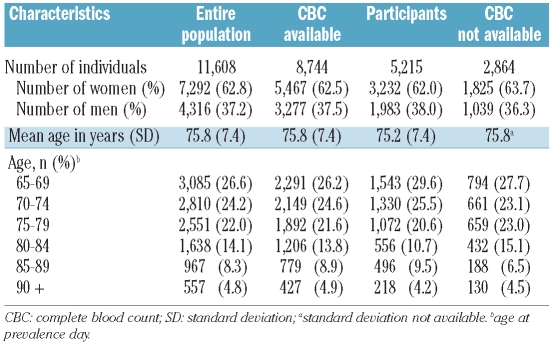
Of the original population of 11,608 Biella residents of 65 years or older (7,292 women and 4,316 men), 1,260 could not be contacted by phone, 263 died between the prevalence day and the time of contact, 4,870 refused to or could not donate a blood sample, and 5,215 agreed to participate in the study. A flow chart of the study is shown in Online Supplementary Figure S1. During the study period, among those who refused to or could not participate in the study, a further group of 3,529 individuals (2,968 out-patients and 561 in-patients) had a CBC test done in the same laboratory of the present investigation. Thus 8,744 individuals (75.3% of the initial population) could be included in the prevalence study analyses. Demographic characteristics of the entire population and of subgroups with and without blood tests are reported in Table 1. The mean ages, percentages of individuals in each age class, and proportions of men and women were similar between groups.
Table 1.
Comparison of demographic characteristics of the entire study population and subpopulations.
The characteristics of mildly anemic and non-anemic elderly are described in the Online Supplementary Table S1. Mildly anemic subjects were about 5 years older (P<0.0001), more likely to be men (P=0.0423), and had a slightly lower mean education (P=0.0012). After adjusting for age, sex and education, mild anemia was significantly associated with non-smoking (P=0.0026), alcohol abstinence (P<0.0001), lower body mass index (P=0.0015), lower mean systolic (P=0.0058) and diastolic (P<0.0001) blood pressure, and a higher prevalence of diabetes (P=0.0006), respiratory failure (P=0.0117) and renal insufficiency (P<0.0001), and a diagnosis of cancer during the preceding 5 years (P=0.0055).
Hemoglobin distribution
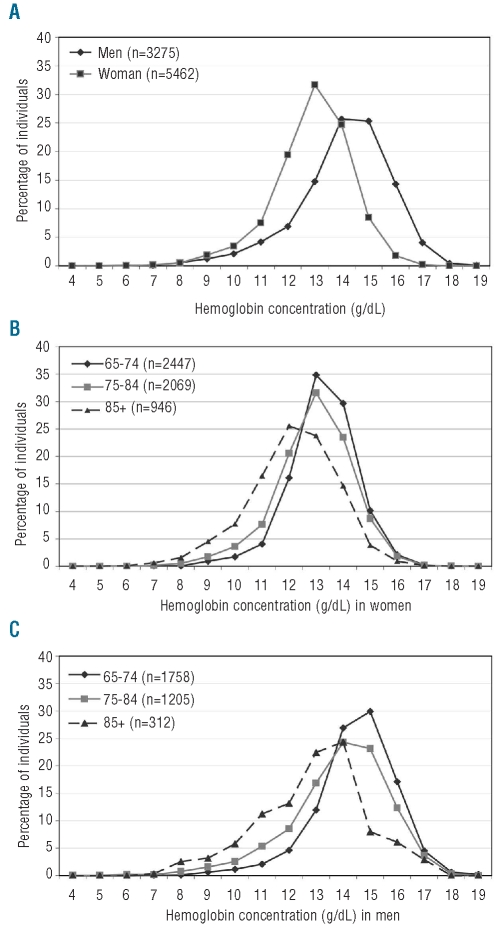
The hemoglobin distribution was nearly normal with a tail to the left (Figure 1A). As expected, women had a mean ± standard deviation (SD) hemoglobin concentration (13.4±1.4 g/dL) lower than that of the men (14.5±1.7 g/dL). Hemoglobin concentration, though weakly, was age-related in the whole population (P<0.0001) and in both genders separately (P<0.0001), with a cross-sectionally estimated mean yearly decline more pronounced in men than in women (−0.08 and −0.05 g/dL, respectively; P<0.0001). In fact, with increasing age the distribution curve shifted toward the lowest values in both genders, but more markedly in men (Figure 1B–C). In both men and women, all comparisons between the mean hemoglobin concentration of the three age classes are significantly different (P<0.0001 for all Tukey-Kramer honestly significant difference tests). Hemoglobin concentration decreased with increasing age also in the non-anemic (P for trend <0.0001) and anemic groups, both in thalassemia trait cases (P for trend =0.004) and in cases with the other types of anemia (P for trend =0.007).
Figure 1.
(A) Frequency distribution of hemoglobin concentration in the elderly population by sex. (B) Frequency distribution of hemoglobin concentration in women by age. (C) Frequency distribution of hemoglobin concentration in men by age.
Prevalence of anemia and mild grade anemia
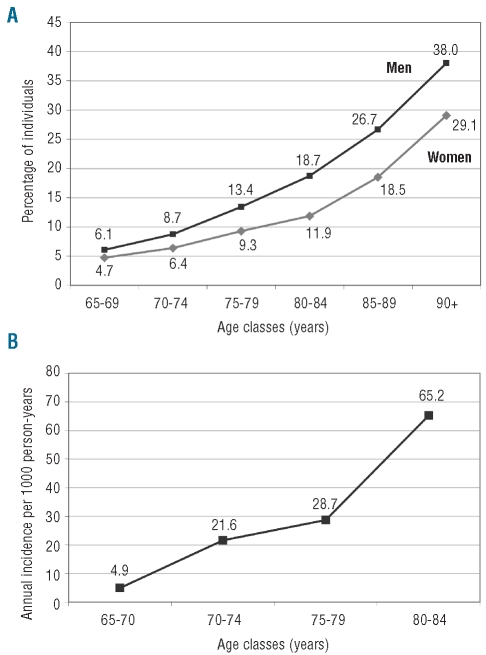
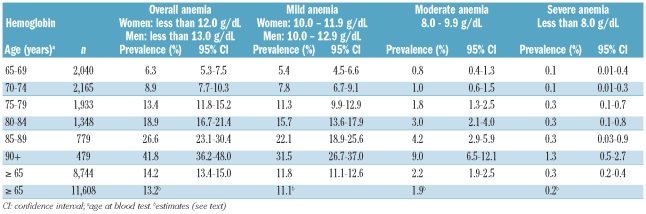
Based on WHO criteria, 1,243 out of 8,744 elderly were found to be anemic (14.2%; 95% CI: 13.4–15.0), with a significantly higher frequency (P=0.0001) in men (499/3,275; 15.2%, 95% CI: 13.9–16.6) than in women (744/5,462; 13.6%, 95% CI: 12.7–14.6). The prevalences of mild anemia (Figure 2A) and anemia increased with increasing age, both in men and in women (P<0.0001). Anemia was very common among hospitalized (291/561, 51.9%) and “deceased” elderly (108/191; 56.5%). Table 2 shows the prevalence of mild, moderate and severe anemia by age. Mild grade anemia accounted for most of the cases (1,032 out of 1,243; 83.0%) with a prevalence of 11.8 % (95% CI: 11.1–12.6). The frequency of moderate anemia was relatively high only among hospitalized cases (76/561 [13.5%] versus among the non-hospitalized 133/8,183 [1.6%]) and increased with increasing age. Severe anemia was rare, there being only 22 cases for a prevalence of 0.3%. Among institutionalized elderly, the prevalences of anemia and mild anemia were 31.1% and 27.4%, respectively.
Figure 2.
(A) Prevalence of mild anemia in the elderly population by sex. (B) Annual incidence of mild anemia in the elderly population.
Table 2.
Prevalence of mild, moderate, and severe anemia in the elderly by age class.
In the population under study, anemia and mild anemia were found to be associated with an increased risk of mortality (P<0.0001),9 and no significant difference in survival was seen between participants and elderly without CBC available (P=0.4910). Based on these findings, we hypothesized a similar prevalence of anemia in these last two groups to estimate the prevalences of anemia and mild anemia in the entire population. The overall estimated prevalences of anemia and mild anemia in the entire elderly population (n=11,608) were 13.2% (12.6% in women and 14.1% in men) and 11.1% (10.3% in women and 12.4% in men), respectively.
Frequency of types of anemia
Anemia was mostly normocytic (72.3%), while microcytosis (MCV less than 80 fL) was present in 16.9% of the anemic individuals versus 1.5% in the non-anemic subjects. Mean serum vitamin B12 and folate concentrations were very similar in the two groups (Online Supplementary Table S1). The frequency of macrocytosis (MCV higher than 100 fL) was comparable in anemic (10.8%) and non-anemic (12.4%) elderly, but for more elevated MCV values, the percentage of macrocytosis was apparently higher in the anemic than in the non-anemic individuals: 2.3% versus 0.7% for MCV higher than 110 fL and 1.2% versus 0.2% for MCV higher than 115 fL.
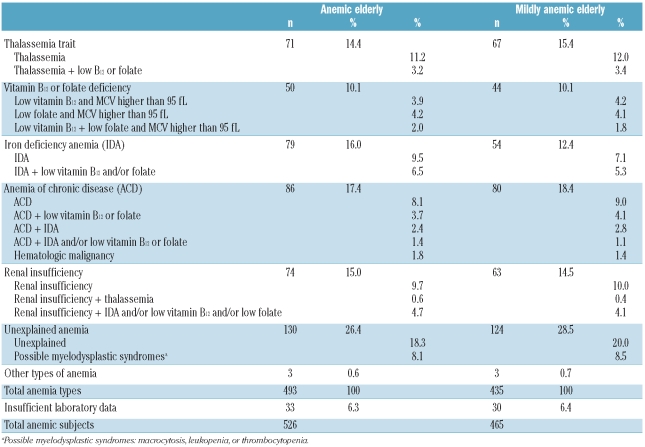
Estimates of the frequency of anemia types in participants (n=5,215) for whom complete information was available are reported in Table 3. The percentage of anemic elderly classified as having a thalassemia trait was considerable in this population (about 15%), with a much higher frequency among the younger-elderly (65–84 years: 21%) than the older-elderly (85-plus: 4%). In 83% of the cases the elderly thalassemics originated from regions with a high prevalence of thalassemia. Comparatively, elderly with other types of anemia or who were non-anemic from the same regions were, respectively, 27% and 26%. Anemia of chronic disease and iron deficiency accounted for about 30% of the cases. The diseases associated with anemia of chronic disease in the present study population are reported in the Online Supplementary Table S2. The underlying cause of mild anemia was unexplained in 26.4% of the cases. Almost one third of unexplained mild anemia cases had hematologic abnormalities consistent with the presence of myelodysplastic syndromes.
Table 3.
Types of anemia and mild anemia in the elderly population.
Self-perception as being mildly anemic
At the time of the nurse’s visit, before receiving the blood test results, 63.5% of the mildly anemic individuals perceived themselves as not being anemic, while among the non-anemic individuals only 5.8% thought they were anemic. The mean hemoglobin concentrations of mildly anemic individuals self-perceiving themselves as being anemic or non-anemic were 11.4±0.7 g/dL and 11.7±0.7 g/dL, respectively (P<0.0001).
Incidence of anemia and mild grade anemia
After a mean of 2.2 (SD= 0.1) years, of the initial random sample of 650 elderly free of anemia, 17 individuals had died, 11 could not be traced and 93 refused to participate, while 529 consented to donate a blood sample (response rate 83.6%), for a total of 1,158 person-years available for the analysis. Among participants, a total of 28 individuals (15 women and 13 men) became anemic (26 mild and two moderate). Overall, the annual incidence rates of anemia and mild anemia were 24.2 per 1000 person-years (95% CI: 16.0–35.0) and 22.5 per 1,000 person-years (95% CI: 14.7–32.9) respectively. Incidence rates of anemia did not differ between women and men (P=0.8851), while they increased with increasing age (P=0.0001), passing from 4.9 per 1,000 person-years (95% CI: 0.5–17.8) in the age-class 65–69 to 72.4 per 1,000 person-years (95% CI: 34.8–133.3) in the age-class 80–84. Figure 2B shows the age-specific annual incidence rates of mild anemia. In the whole sample of mildly anemic and non-anemic elderly participants (n=692), after about 2 years the mean weighted hemoglobin concentration declined from 14.04 g/dL to 13.97 g/dL (mean annual decline: −0.035 g/dL).
Discussion
The present population-based study was specifically aimed at thoroughly investigating the epidemiology of mild anemia in an unselected population of elderly. Blood samples were prospectively collected for the purposes of the study and rates of inclusion were high. Findings from this large survey indicate that more than one out of ten elderly persons are anemic and that most of the cases are of a mild grade. Hemoglobin concentration decreases and incidence and prevalence of mild anemia steadily increase with increasing age, affecting more than two out of ten people over 80 years old.
Associations of mild anemia with other clinical conditions
Previous findings on the relationship of anemia with several conditions persisted in the current study also when the analysis was restricted to the mildly anemic group. Mild anemia was found to be significantly associated with smoking habit, low body mass index, lower mean blood pressure, diabetes, respiratory and renal insufficiency, and cancer also when comparisons were adjusted for age, sex, and education. Elevation of hemoglobin concentration among smokers results from exposure to carbon monoxide and probably leads to an underestimation of the prevalence of anemia among smokers.16 Several hypotheses have been advanced, but why anemia is associated with lower body mass index remains unclear.17 The pathogenesis of anemia in diabetes is multifactorial and includes diabetic nephropathy, chronic inflammation, and “functional erythropoietin deficiency”.18,19 The anemia associated with chronic obstructive pulmonary disease has also been related to the systemic inflammatory response often occurring in this condition.20,21
Hemoglobin distribution and prevalences of anemia and mild anemia
As expected,22,23 the frequency distribution of hemoglobin was shifted more to the lower values in women than in men. The WHO criteria of anemia have been challenged recently and new lower limits of normal for hemoglobin concentration in old age have been suggested.2 Minor changes in the anemia criteria would result in significant increase or decrease in the prevalence rate of anemia. Using the proposed cut-off of hemoglobin concentration (lower than 12.2 g/dL in women and 13.2 g/dL in men),2 the overall prevalence of anemia in the 8,744 elderly with CBC available would increase from 14.2% to 16.8% (95% CI: 15.9–17.7), 16.4% (95% CI: 16.1–19.0) in women and 17.5% (95% CI: 15.3–17.5) in men.
In line with most cross-sectional studies and the findings of three longitudinal investigations,24–26 hemoglobin concentration decreased and prevalence of anemia increased with increasing age. Annual rates of decline of hemoglobin concentration estimated cross-sectionally and longitudinally were similar and consistent with those reported in longitudinal surveys of 8- and 40-year duration.25,26
Several estimates of the prevalence of anemia in the elderly have been reported in the literature, but few were the result of very large-scale population-based studies with a well-defined initial population from which samples were drawn and with a high inclusion rate. The prevalence of anemia was unusually high in elderly residents of Holmsted County: 20.5% in men and 15.9% in women;27 however, this was a retrospective analysis of all hemoglobin results in the elderly who received medical attention at the Mayo Clinic during a lengthy period of 3 to 5 years. In fact, among the new cases identified during one of the study years, a good 36% had an anemia due to acute blood loss as a result of surgery or injuries.28 A rather low prevalence of anemia was reported in northern European women: 3.3% in Finland,29 3.8% in Denmark (30–60 years old),30 5.0% in Norway,17 and 7.3% in Sweden,31 while a high anemia prevalence (between 18% and 39%) has been consistently reported in African Americans.22,23,32–35 The prevalence of anemia appears relatively high also in Japanese (14.5% in men and 17.1% in women aged 51 to 80 years, and 17.9% in women 60 years or older)25,36 and Taiwanese (18.8%) elderly populations.37 In a Korean population aged 60 to 95 years, the prevalence of anemia was 13.6%, but higher in women (14.7%) than in men (9.9%).38
In the present study, the prevalence of anemia in the elderly was similar to that found in other population-based studies:22,23,39–44 13.2% overall (about 11–12% not considering thalassemia), 12.6% in women and 14.1% in men. The high prevalence of anemia estimated in the very old (over 85 years old, 31.3%) is consistent with that recently reported the Newcastle 85+ cohort for (29.8%).45 Grouping together the results of 12 studies,46 weighted regional estimates of anemia prevalence in the elderly in northern America (12%) and Europe (12%) were close to that found in the present study. According to recent estimates, the worldwide prevalence of anemia in the elderly population is 23.9%.47
Data on mild anemia (hemoglobin concentration between 10.0 g/dL and WHO lower limits of normal) can be drawn only from few, mainly small and not so recent, population- or community-based studies (Online Supplementary Table S3).17,22,23,39–43,48 In the present study, mild grade anemia accounted for some 84% of the cases; the majority of these subjects were unaware of being anemic. This seems to confirm that mild anemia in the elderly often goes unscreened and undiagnosed or is disregarded and not reported to the patient by the physician.49
The very high prevalence of anemia, with a large proportion of moderate-severe cases, among hospitalized elderly suggests that results from hospital-based studies are poorly representative of the anemia prevalence in the general population. As in previous studies, also in the present survey the proportion of anemia cases among institutionalized elderly was high. Further prospective studies are thus needed to better understand the determinants of this finding.
Frequency of types of anemia
Although criteria used to classify types of anemia in an epidemiological setting cannot be regarded, strictly speaking, as diagnostic, the present survey is one of the largest population-based studies in which a characterization of anemia types in the elderly has been attempted. The frequency of microcytosis associated with anemia was higher (16.9%) than in previous surveys22,50 (about 9–12%) because of the substantial presence of elderly with a thalassemia trait in the present study population. This appreciable presence of thalassemia and its higher frequency among the younger-elderly than in the older-elderly possibly reflects past migrations from Italian regions where malaria was endemic. As in the EPESE study,22 the frequency of microcytosis in non-anemic elderly was very low while that of macrocytosis was almost the same in anemic and non-anemic individuals. Multiple factors are likely to underlie many of the cases of anemia in the elderly. Apart from thalassemia trait, in line with previous population-based studies,23,28 in the current study chronic disease and iron deficiency were the most common causes of anemia. The frequency of anemia associated with vitamin B12 or folate deficiency (10.1%) was slightly lower than that found in the NHANES III study (14.3%, but MCV was not a criterion),23 but higher than the frequencies reported in clinic,51,52 general practice,53,54 and population28 settings (between 1 and 8%). However, beyond possible regional variations, comparisons between studies are also made difficult by the different criteria used to define and classify the various types of anemia.
Though important in view of a possible specific treatment, the underlying cause of anemia in the elderly fails to be identified in a large proportion of fully examined cases.51,55 This is particularly true in epidemiological surveys: the prevalence of anemia of unknown or unclear cause was rather high in the present study (26.4%, 30.8% not considering thalassemia) as in other community-based surveys in the elderly (30–34%).23,48,53
Some cases of unexplained anemia can be accounted for by myelodysplastic syndromes, a heterogeneous group of disorders mainly found in the elderly population and characterized by peripheral cytopenia, followed by a progressive impairment in the ability of myelodysplastic stem cells to differentiate and an increasing risk of evolution into acute myeloid leukemia.23,56,57 In the present epidemiological survey, a substantial proportion of cases of unexplained anemia had blood test characteristics consistent with myelodysplastic syndromes. Further investigations also including bone marrow examination are needed for more accurate estimates. Recent clinical findings suggest that the prevalence of myelodysplastic syndromes may be higher than previously postulated.58
Incidence of anemia and mild grade anemia
To our knowledge, there are only four other studies which have reported the incidence rates of anemia and none reporting that of mild anemia in elderly populations. In a general practice in south London, over a 5-year period (1950–1955), Fry53 estimated an annual incidence of anemia (defined as a hemoglobin concentration below 11.8 g/dL both in women and men) of approximately 11 per 1,000 person-years in subjects 60 years or older. In a retrospective analysis, the overall incidence of anemia in the Olmsted County population aged 65 years or more receiving medical care from the Mayo Clinic in 1986 was much higher (61.8 per 1,000 person-years), but in 75% of the cases anemia was detected in conjunction with a hospital admission.28 Among independent older community-dwelling Koreans, over a 3-year study period the annual incidence rate of anemia was about 24 per 1,000 person-years.59 The annual incidence rate of anemia in the Leiden 85-plus study (74‰)60 is in line with that in the slightly younger 80–84 age-class (65.2‰) of the present study. Consistent with the findings of these studies, in the present survey incidence rates of anemia increased with age. Mild anemia accounted for more than 90% of the new cases.
The findings of the present study show that mild anemia is common and frequently undiagnosed in the elderly. Anemia is a public health problem affecting some 164 million elderly people worldwide.47 Increased disability, morbidity, and mortality are associated with mild grade anemia in the elderly. A better understanding of the causes of the decline of hemoglobin with age and, in particular, of unexplained anemia would represent an important step towards effective strategies for anemia control.
Acknowledgments
the authors are grateful to all the elderly participants of Biella, the residential homes and organizations of Biella that made this investigation possible and to the “Health and Anemia” Study Group. A full list of acknowledgements is given in the Online Supplementary Appendix.
Footnotes
Funding: this study was supported by a research grant from Amgen Italy. The sponsor of the study had no role in the conception and design of the study; collection, management, analysis, and interpretation of data; preparation and writing of the report or in the decision to submit the manuscript for publication. Francesca Gandini and Angela Recchia were supported by fellowships of the Fondazione “Franco Gallini”, Aviano, Italy.
The online version of this article has a Supplementary Appendix.
Authorship and Disclosure
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.World Health Organization. Nutritional Anemia: Report of a WHO Scientific Group. Tech Rep Ser. 1968;405:1–40. [PubMed] [Google Scholar]
- 2.Beutler E, Waalen J. The definition of anemia: what is the lower limit of normal of the blood hemoglobin concentration? Blood. 2006;107(5):1747–50. doi: 10.1182/blood-2005-07-3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carmel R. Anemia and aging: an overview of clinical, diagnostic and biological issues. Blood Reviews. 2001;15(1):9–18. doi: 10.1054/blre.2001.0146. [DOI] [PubMed] [Google Scholar]
- 4.Nissenson AR, Goodnough LT. Anemia. Not just an innocent bystander? Arch Intern Med. 2003;163(12):1400–4. doi: 10.1001/archinte.163.12.1400. [DOI] [PubMed] [Google Scholar]
- 5.Lipschitz D. Medical and functional consequences of anemia in the elderly. J Am Geriatr Soc. 2003;51(3 suppl):S10–S3. doi: 10.1046/j.1532-5415.51.3s.6.x. [DOI] [PubMed] [Google Scholar]
- 6.Woodman R, Ferrucci L, Guralnik J. Anemia in older adults. Curr Opin Hematol. 2005;12(2):123–8. doi: 10.1097/01.moh.0000154030.13020.85. [DOI] [PubMed] [Google Scholar]
- 7.Spivak JL. Anemia in the elderly. Time for new blood in old vessels? Arch Intern Med. 2005;165(19):2187–9. doi: 10.1001/archinte.165.19.2187. [DOI] [PubMed] [Google Scholar]
- 8.Lucca U, Tettamanti M, Mosconi P, Apolone G, Gandini F, Nobili, et al. Association of mild anemia with cognitive, functional, mood and quality of life out-comes in the elderly: the “Health and Anemia” study. PloS ONE. 2008;3(4):e1920. doi: 10.1371/journal.pone.0001920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riva E, Tettamanti M, Mosconi P, Apolone G, Gandini F, Nobili A, et al. Association of mild anemia with hospitalization and mortality in the elderly: the Health and Anemia population-based study. Haematologica. 2009;94(1):22–8. doi: 10.3324/haematol.13449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robinson B. Cost of anemia in the elderly. J Am Geriatr Soc. 2003;51(3suppl):S14–S7. doi: 10.1046/j.1532-5415.51.3s.5.x. [DOI] [PubMed] [Google Scholar]
- 11.Ershler WB, Chen K, Reyes EB, Dubois R. Economic burden of patients with anemia in selected diseases. Value Health. 2005;8 (6):629–38. doi: 10.1111/j.1524-4733.2005.00058.x. [DOI] [PubMed] [Google Scholar]
- 12.Beghé C, Wilson A, Ershler WB. Prevalence and outcomes of anemia in geriatrics: a systematic review of the literature. Am J Med. 2004;116(suppl 7A):3S–10S. doi: 10.1016/j.amjmed.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 13.Groopman JE, Itri LM. Chemotherapy-induced anemia in adults: incidence and treatment. J Natl Cancer Inst. 1999;91 (19):1616–34. doi: 10.1093/jnci/91.19.1616. [DOI] [PubMed] [Google Scholar]
- 14.Wilson A, Yu H-T, Goodnough LT, Nissenson AR. Prevalence and outcomes of anemia in rheumatoid arthritis: a systematic review of the literature. Am J Med. 2004;116(suppl 7A):50S–7S. doi: 10.1016/j.amjmed.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 15.Lichtman MA, Beutler E, Kipps TJ, Seligsohn U, Kaushansky K, Prchal JT, editors. Williams Hematology. 7th ed. New York: McGraw-Hill; 2006. [Google Scholar]
- 16.Nordenberg D, Yip R, Binkin NJ. The effect of cigarette smoking on hemoglobin levels and anemia screening. JAMA. 1990;264 (12):1556–9. [PubMed] [Google Scholar]
- 17.Skjelbakken T, Langbakk B, Dahl IMS, Løchen M-L. Haemoglobin and anaemia in a gender perspective: the Tromsø study. Eur J Haematol. 2005;74(5):381–8. doi: 10.1111/j.1600-0609.2004.00392.x. [DOI] [PubMed] [Google Scholar]
- 18.Thomas MC. Anemia in diabetes: marker or mediator of microvascular disease? Nat Clin Pract Nephrol. 2007;3(1):20–30. doi: 10.1038/ncpneph0378. [DOI] [PubMed] [Google Scholar]
- 19.Singh DK, Winocour P, Farrington K. Erythropoietic stress and anemia in diabetes mellitus. Nat Rev Endocrinol. 2009;5(4):204–10. doi: 10.1038/nrendo.2009.17. [DOI] [PubMed] [Google Scholar]
- 20.John M, Hoernig S, Doehner W, Okonko DD, Witt C, Anker SD. Anemia and inflammation in COPD. Chest. 2005;127(3):825–9. doi: 10.1378/chest.127.3.825. [DOI] [PubMed] [Google Scholar]
- 21.Similowski T, Agusti A, MacNee W, Schönhofer B. The potential impact of anaemia of chronic disease in COPD. Eur Respir J. 2006;27(2):390–6. doi: 10.1183/09031936.06.00143704. [DOI] [PubMed] [Google Scholar]
- 22.Salive ME, Cornoni-Huntley J, Guralnik JM, Phillips CL, Wallace RB, Ostfeld AM, et al. Anemia and hemoglobin levels in older persons: relationship with age, gender, and health status. J Am Geriatr Soc. 1992;40 (5):489–96. doi: 10.1111/j.1532-5415.1992.tb02017.x. [DOI] [PubMed] [Google Scholar]
- 23.Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG, Woodman RC. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004;104(8):2263–8. doi: 10.1182/blood-2004-05-1812. [DOI] [PubMed] [Google Scholar]
- 24.Nilsson-Ehle H, Jagenburg R, Landahl S, Svanborg A. Blood haemoglobin declines in the elderly: implications for the reference intervals from age 70 to 88. Eur J Haematol. 2000;65(6):297–305. doi: 10.1034/j.1600-0609.2000.065005297.x. [DOI] [PubMed] [Google Scholar]
- 25.Yamada M, Wong FL, Suzuki G. Longitudinal trends of hemoglobin levels in a Japanese population – RERF’s Adult Health Study subjects. Eur J Haematol. 2003;70(3):129–35. doi: 10.1034/j.1600-0609.2003.00031.x. [DOI] [PubMed] [Google Scholar]
- 26.Ershler WB, Sheng S, McKelvey J, Artz AS, Denduluri N, Tecson J, et al. Serum erythropoietin and aging: a longitudinal analysis. J Am Geriatr Soc. 2005;53(8):1360–5. doi: 10.1111/j.1532-5415.2005.53416.x. [DOI] [PubMed] [Google Scholar]
- 27.Anía BJ, Suman VJ, Fairbanks VF, Melton LJ., III Prevalence of anemia in medical practice: community versus referral patients. Mayo Clin Proc. 1994;69(8):730–5. doi: 10.1016/s0025-6196(12)61089-1. [DOI] [PubMed] [Google Scholar]
- 28.Anía BJ, Suman VJ, Fairbanks VF, Rademacher DM, Melton LJ., III Incidence of anemia in older people: an epidemiological study in a well defined population. J Am Geriatr Soc. 1997;45(7):825–31. doi: 10.1111/j.1532-5415.1997.tb01509.x. [DOI] [PubMed] [Google Scholar]
- 29.Takkunen H, Seppänen R. Iron deficiency in the elderly population in Finland. Scand J Soc Med Suppl. 1977;14:151–62. [PubMed] [Google Scholar]
- 30.Milman N, Byg K-E, Mulvad G, Pedersen HS, Bjerregaard P. Haemoglobin concentrations appear to be lower in indigenous Greenlanders than in Danes: assessment of haemoglobin in 234 Greenlanders and in 2804 Danes. Eur J Haematol. 2001;67(1):23–9. doi: 10.1034/j.1600-0609.2001.067001023.x. [DOI] [PubMed] [Google Scholar]
- 31.Atti AR, Palmer K, Volpato S, Zuliani G, Winbland B, Fratiglioni L. Anaemia increases the risk of dementia in cognitively intact elderly. Neurobiol Aging. 2006;27(2):278–84. doi: 10.1016/j.neurobiolaging.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 32.Zakai NA, Katz R, Hirsch C, Shlipak MG, Chaves PHM, Newman AB, et al. A prospective study of anemia status, hemoglobin concentration, and mortality in an elderly cohort. The Cardiovascular Health Study. Arch Intern Med. 2005;165(19):2214–20. doi: 10.1001/archinte.165.19.2214. [DOI] [PubMed] [Google Scholar]
- 33.Denny SD, Kuchibhatla MN, Cohen HJ. Impact of anemia on mortality, cognition, and function in community-dwelling elderly. Am J Med. 2006;119(4):327–34. doi: 10.1016/j.amjmed.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 34.Patel KV, Harris TB, Faulhaber M, Angleman SB, Connelly S, Bauer DC, et al. Racial variation in the relationship of anemia with mortality and mobility disability among older adults. Blood. 2007;109(11):4663–70. doi: 10.1182/blood-2006-10-055384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dong XQ, Mendes de Leon C, Artz A, Tang YX, Shah R, Evans D. A population-based study of hemoglobin, race, and mortality in elderly persons. J Gerontol. 2008;63A:873–8. doi: 10.1093/gerona/63.8.873. [DOI] [PubMed] [Google Scholar]
- 36.Hayashi F, Yoshiike N, Yoshita K, Kawahara K. Trends in the prevalence of anaemia in Japanese adult women, 1989–2003. Public Health Nutrition. 2008;11(3):252–7. doi: 10.1017/S1368980007000274. [DOI] [PubMed] [Google Scholar]
- 37.Wang J-L, Shaw N-S. Iron status of the Taiwanese elderly: the prevalence of iron deficiency and elevated iron stores. Asia Pac J Clin Nutr. 2005;14(3):278–84. [PubMed] [Google Scholar]
- 38.Choi CW, Lee J, Park KH, Yoon SY, Choi IK, Oh SC, et al. Prevalence and characteristics of anemia in the elderly: cross-sectional study of three urban Korean population samples. Am J Hematol. 2004;77(1):26–30. doi: 10.1002/ajh.20140. [DOI] [PubMed] [Google Scholar]
- 39.Hobson W, Blackburn EK. Haemoglobin levels in a group of elderly persons living at home alone or with spouse. BMJ. 1953;1(4811):647–9. doi: 10.1136/bmj.1.4811.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parsons PL, Withey JL, Kilpatrick GS. The prevalence of anaemia in the elderly. Practitioner. 1965;195(169):656–60. [PubMed] [Google Scholar]
- 41.Elwood PC, Shinton NK, Wilson CID, Sweetnam P, Frazer AC. Haemoglobin, vitamin B12 and folate levels in the elderly. Brit J Haematol. 1971;21(5):557–63. doi: 10.1111/j.1365-2141.1971.tb02717.x. [DOI] [PubMed] [Google Scholar]
- 42.Milne JS, Williamson J. Hemoglobin, hematocrit, leukocyte count, and blood grouping in older people. Geriatrics. 1972;27(9):118–26. [PubMed] [Google Scholar]
- 43.Hill RD. The prevalence of anaemia in the over-65s in a rural practice. Practitioner. 1976;217(1302):963–7. [PubMed] [Google Scholar]
- 44.Ble A, Fink JC, Woodman RC, Klausner MA, Windham BG, Guralnik JM, et al. Renal function, erythropoietin, and anemia of older persons. Arch Intern Med. 2005;165(19):2222–7. doi: 10.1001/archinte.165.19.2222. [DOI] [PubMed] [Google Scholar]
- 45.Collerton J, Davies K, Jagger C, Kingston A, Bond J, Eccles MP, et al. Health and disease in 85 year olds: baseline findings from the Newcastle 85+ cohort study. BMJ. 2009;339:b4904. doi: 10.1136/bmj.b4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DeMaeyer E, Adiels-Tegman M. The prevalence of anaemia in the world. World Health Stat Q. 1985;38(3):302–16. [PubMed] [Google Scholar]
- 47.de Benoist B, McLean E, Egli I, Cogswell M, editors. WHO Global database on anaemia. World Health Organization; 2008. Worldwide prevalence of anaemia 1993–2005. [Google Scholar]
- 48.McLennan WJ, Andrews GR, Macleod C, Caird FI. Anaemia in the elderly. Q J Med. 1973;42(165):1–13. [PubMed] [Google Scholar]
- 49.de Craen AJM, Gussekloo J, Teng YKO, Macfarlane PW, Westendorp RGJ. Prevalence of five common clinical abnormalities in very elderly people: population based cross sectional study. BMJ. 2003;327(7407):131–2. doi: 10.1136/bmj.327.7407.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Izaks GJ, Westendorp RGJ, Knook DL. The definition of anemia in older persons. JAMA. 1999;281(18):1714–7. doi: 10.1001/jama.281.18.1714. [DOI] [PubMed] [Google Scholar]
- 51.Joosten E, Pelemans W, Hiele M, Noyen J, Verhaeghe R, Boogaerts MA. Prevalence and causes of anaemia in a geriatric hospitalized population. Gerontology. 1992;38(1–2):111–7. doi: 10.1159/000213315. [DOI] [PubMed] [Google Scholar]
- 52.Guyatt GH, Patterson C, Ali M, Singer J, Levine M, Turpie I, et al. Diagnosis of iron-deficiency anemia in the elderly. Am J Med. 1990;88(3):205–9. doi: 10.1016/0002-9343(90)90143-2. [DOI] [PubMed] [Google Scholar]
- 53.Fry J. Clinical patterns and course of anaemias in general practice. BMJ. 1961;2(5269):1732–6. doi: 10.1136/bmj.2.5269.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kirkeby OJ, Fossum S, Risøe C. Anaemia in elderly patients. Incidence and causes of low haemoglobin concentration in a city general practice. Scand J Prim Health Care. 1991;9(3):167–71. doi: 10.3109/02813439109018513. [DOI] [PubMed] [Google Scholar]
- 55.Nilsson-Ehle H, Jagenburg R, Landahl S, Svanborg A, Westin J. Haematological abnormalities and reference intervals in the elderly. A cross-sectional comparative study of three urban Swedish population samples aged 70, 75 and 81 years. Acta Med Scand. 1988;224(6):595–604. [PubMed] [Google Scholar]
- 56.Makipour S, Kanapuru B, Ershler WB. Unexplained anemia in the elderly. Semin Hematol. 2008;45(4):250–4. doi: 10.1053/j.seminhematol.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Steensma DP, Bennett JM. The myelodysplastic syndromes: diagnosis and treatment. Mayo Clin Proc. 2006;81(1):104–30. doi: 10.4065/81.1.104. [DOI] [PubMed] [Google Scholar]
- 58.Buckstein R, Jang K, Friedlich J, Zhang L, Reis M, Chesney A, et al. Estimating the prevalence of myelodysplastic syndromes in patients with unexplained cytopenias: a retrospective study of 322 bone marrows. Leuk Res. 2009;33(10):1313–8. doi: 10.1016/j.leukres.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 59.Choi CW, Lee J, Park KH, Choi IK, Kim SJ, Seo JH, et al. Incidence of anemia in older Koreans: Community-based cohort study. Arch Gerontol Geriatr. 2005;41(3):303–9. doi: 10.1016/j.archger.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 60.den Elzen WP, Willems JM, Westendorp RGJ, de Craen AJM, Assendelft WJJ, Gussekloo J. Effect of anemia and comorbidity on functional status and mortality in old age: results from the Leiden 85-plus study. CMAJ. 2009;181(3–4):151–7. doi: 10.1503/cmaj.090040. [DOI] [PMC free article] [PubMed] [Google Scholar]