Abstract
Background
Blastic plasmacytoid dendritic cell neoplasm is a rare malignancy that typically follows a highly aggressive clinical course in adults, whereas experience in children with this disease is very limited.
Design and Methods
This retrospective study analyzed the pathological and clinical findings of nine cases of blastic plasmactyoid dendritic cell neoplasm presenting in patients under the age of 18 years who were reviewed at our institution. We also identified 20 well-documented additional pediatric cases in the literature.
Results
In the combined analysis, the overall survival rate among the 25 patients with available follow-up, all having received chemotherapy, was 72% (follow-up ranging from 9 months to 13 years, with a median of 30 months). The event-free survival rate was 64%. Nine patients were alive 5 years after the original diagnosis, although only three of them had undergone hematopoietic stem cell transplantation – one in first complete remission and two in second remission. Of the seven patients who lacked cutaneous disease at presentation, 100% survived, including five who were alive more than 5 years after diagnosis, although only two had undergone stem cell transplantation. Among the 18 patients who presented with cutaneous disease and for whom follow-up data were available, only 11 survived (61%). Detailed immunophenotypic characterization and clinical features of all cases are presented. Unexpectedly, three of four cases of blastic plasmacytoid dendritic cell neoplasm tested showed focal positivity for S-100. S-100 was negative in 28 cases of acute myeloid leukemia evaluated for this marker.
Conclusions
In contrast to adult cases, in which long-term survival depends on stem cell transplantation in first complete remission, blastic plasmacytoid dendritic cell neoplasms in children are clinically less aggressive. Treatment with high-risk acute lymphoblastic leukemia-type chemotherapy appears to be effective, and stem cell transplantation may be reserved for children who relapse and achieve a second remission. Outcomes were more favorable in cases that lacked cutaneous disease at presentation, although a comparison of cutaneous and non-cutaneous cases might be confounded by differences in treatment regimens. Focal expression of S-100 may be seen in concert with other markers of plasmacytoid dendritic cells.
Keywords: blastic plasmacytoid dendritic cell neoplasm, CD4+/CD56+ hematodermic neoplasm, pediatric, acute myeloid leukemia, acute lymphoblastic leukemia, stem cell transplant, S-100, immunophenotyping, dendritic cells
Introduction
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is an extremely rare subtype of acute leukemia and was formerly known as blastic natural killer (NK)-cell lymphoma and CD4+/CD56+ hematodermic neoplasm.1–5 Once postulated to originate from NK-lineage precursors, accumulating phenotypic, functional, and genetic evidence has pointed to its derivation from hematopoietic precursors with commitment to the plasmacytoid dendritic cell lineage.6–11 While their role in antigen presentation has been debated, plasmacytoid dendritic cells serve as a principal source of type I interferons and have been implicated in a wide variety of immune functions, including antiviral immunity, antitumor immunity, and peripheral tolerance.12–13 The precise developmental pathway of plasmacytoid dendritic cells is somewhat controversial, but current evidence favors derivation from a common macrophage and dendritic cell precursor.14–15
Due to its rarity and only recent recognition as a distinct clinicopathological entity, no standardized therapeutic approach has been established for BPDCN. Reimer et al. published the most comprehensive retrospective study to date on the treatment and outcome of BPDCN, then called CD4+CD56+ malignancy, studying 91 previously published cases as well as six additional patients.16 The authors concluded that, with a median survival of only 13 months and generally poor prognosis, high-dose chemotherapy/radiotherapy followed by allogeneic stem cell transplantation (SCT) performed in first complete remission offered the highest curative potential. However, only four children were included in that study, as BPDCN predominantly affects older adults. A more recent study advocating the use of SCT for this disease included only one pediatric case among a total of 47 patients.17
The pediatric experience with BPDCN is especially limited, with only a few case reports and small collections of cases described in the literature.1,2,11,18–31 Historical review of cases is somewhat problematic since recently validated plasmacytoid dendritic cell markers were not routinely tested in the past. Recently identified key markers include CD123, the interleukin-3 receptor α chain, which is strongly expressed in BPDCN and is amenable to immunohistochemical detection on formalin-fixed tissue,7,8 T-cell leukemia/lymphoma 1 (TCL1),32,33 blood dendritic cell antigen-2 (CD303/BDCA-2),28,34 and CD2-associated protein (CD2AP).10 As CD56 expression may be non-specific, the former diagnostic category of blastic NK-cell lymphoma also encompassed cases of true precursor NK-cell malignancy and primitive hematopoietic malignancies of other lineages, such as acute monocytic leukemia, which are diseases that might behave differently.
We now report nine cases of pediatric BPDCN, the largest such series described to date. Older cases were reevaluated with CD123, CD303/BDCA-2, TCL1, and CD2AP immunohistochemistry for confirmation of the diagnosis. A comprehensive and critical literature search identified 20 additional cases of BPDCN presenting in children. The objectives of this study were to determine optimal therapy for this exceedingly rare disease based on the accumulated clinical data that are available, and also to compare cases with the typical cutaneous presentation to leukemic variants lacking cutaneous involvement at presentation.
Design and Methods
Case selection
Cases were identified from the hematopathology consultation files of the National Cancer Institute (NCI), Bethesda, MD, USA, between 1996 and 2009. This study was approved by the NIH Office of Human Subjects Research, and the NCI Institutional Review Board. Hematoxylin and eosin-stained sections from all cases were evaluated. Where available, flow cytometric and cytogenetic results were also reviewed.
In addition, an extensive literature search was performed for published cases of BPDCN, using its current name as well as former designations, blastic NK-cell lymphoma and CD4+/CD56+ hematodermic neoplasm, in children under the age of 18 years. Careful review of the reported immunophenotypes eliminated cases that had been negative for either CD4 or CD56 (unless positive for CD123), or reported positive for CD34 (which excludes BPDCN).1,28 Co-expression of CD33, CD7, CD2, and/or CD117 was acceptable, and has been previously described in several cases of BPDCN.1–4,28,35 The cases were evaluated with respect to clinical features, treatment, and outcome, and these data were combined with NCI data for comprehensive analysis.
Immunohistochemistry
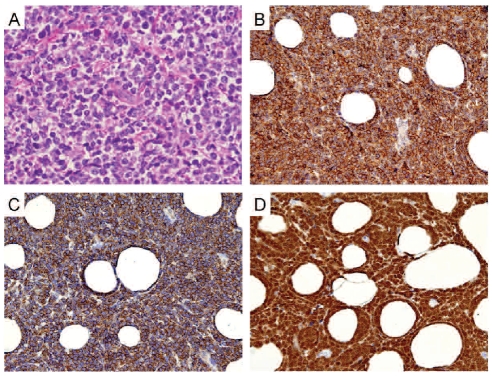
Immunostaining was performed on de-paraffinized, formalin-fixed tissue sections using a panel of antibodies (Table 1). Staining was conducted using an automated immunostainer (Ventana Medical Systems, Inc., Tucson, AZ, USA).
Table 1.
Immunohistochemistry – primary antibodies.
In situ hybridization
In situ hybridization for Epstein-Barr virus-encoded RNA was conducted on formalin-fixed paraffin sections using the Epstein-Barr Early RNA Probe Reagent-EBER 1–2 (Ventana INFORM EBER, Tucson, AZ, USA) according to the manufacturer’s recommendations, with appropriate positive and negative controls.
Molecular analysis
Polymerase chain reaction for T-cell receptor gene rearrangements was conducted using primers to the T-cell receptor gamma chain gene.36 Two separate reactions were carried out for each extract, with appropriate controls, and the products were analyzed by acrylamide gel electrophoresis, as previously described.37
Results
Histological and immunophenotypic features
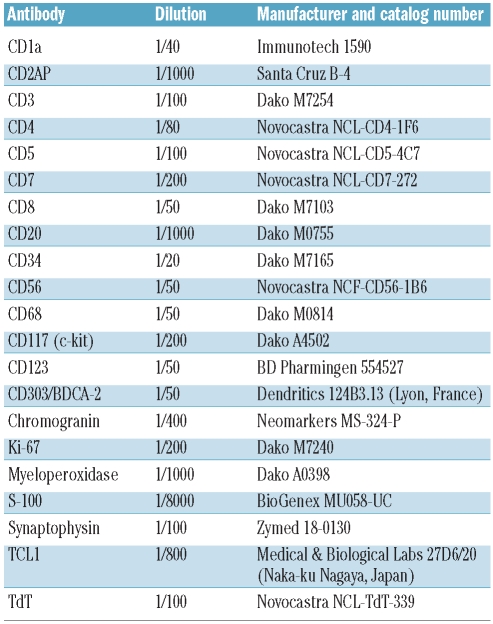
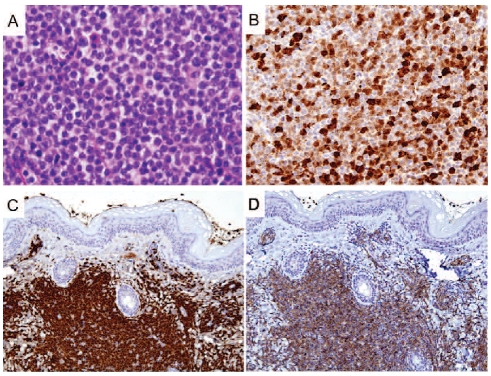
In eight of the nine NCI cases, the initial diagnostic biopsy was of skin. The neoplastic cells diffusely infiltrated the superficial dermis, sparing the Grenz zone and overlying epidermis. The malignant cells were small to medium-sized and fairly uniform, with ovoid to indented or slightly irregular nuclei containing finely dispersed chromatin and indistinct nucleoli (Figures 1A and 2A). Mitotic figures were observed but generally not abundant. The cytoplasm was indistinct. Figures 1 and 2 illustrate cases 2 and 4, respectively.
Figure 1.
Histological and immunohistochemical features of case 2 (cutaneous mass), with staining for (A) hematoxylin and eosin (400x), (B) S-100 (200x), (C) TCL1 (100x), and (D) CD303/BDCA-2 (100x).
Figure 2.
Histological and immunohistochemical features of case 4 (subcutaneous mass), with staining for (A) hematoxylin and eosin (400x), (B) CD303/BDCA-2 (200x), (C) CD2AP (200x), and (D) TCL1 (200x).
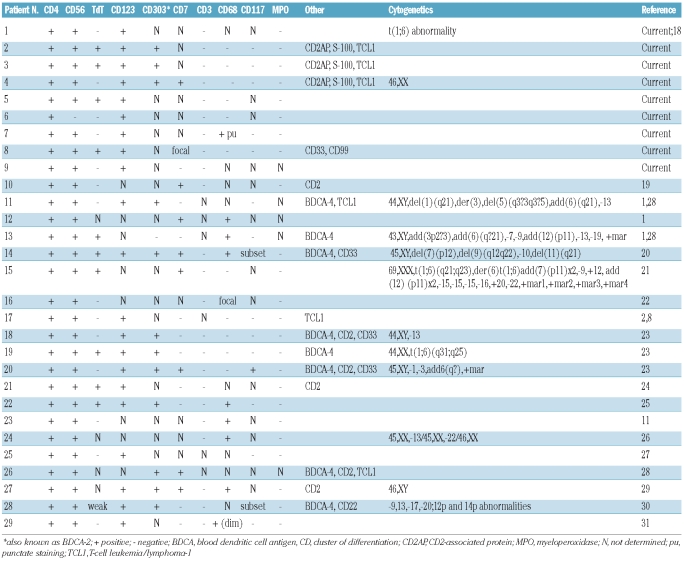
Table 2 summarizes the results of our immunohistochemical studies, as well as immunohistochemical, flow cytometric, and cytogenetic results reported for the 20 cases identified in the literature. All nine NCI cases were positive for CD4 and CD123, and all but one (case 6) were positive for CD56. CD68 was negative in all cases, with the exception of case 7, which showed focal punctate staining.
Table 2.
Immunophenotypic and cytogenetic features of pediatric BPDCN.
Myeloperoxidase was negative in cases 1 through 8 (case 9 not tested). All cases were negative for CD3, CD20, and CD34. In situ hybridization for Epstein-Barr virus-encoded RNA was negative in all cases studied (n =4), as were molecular studies for T-cell clonality (n = 3).
Interestingly, three of the four pediatric BPDCN cases from the NCI (75%) that were tested for S-100 expression showed S-100 positivity in the neoplastic cells, typically in a focal or zonal distribution (Figure 1B). Langerin and CD1a stains were negative, arguing against Langerhans’ cell histiocytosis. A retrospective review of a total of 20 additional BPDCN cases from adults, reviewed at the NCI, demonstrated S-100 expression in five (25%).5 In contrast, none of 28 cases of acute myeloid leukemia expressed S-100. The biopsies from these 28 cases were taken from the skin (n=7), bone marrow (n=18), central nervous system (n=1), lymph node (n=1), and mesentery (n=1) (Online Supplementary Table S1).
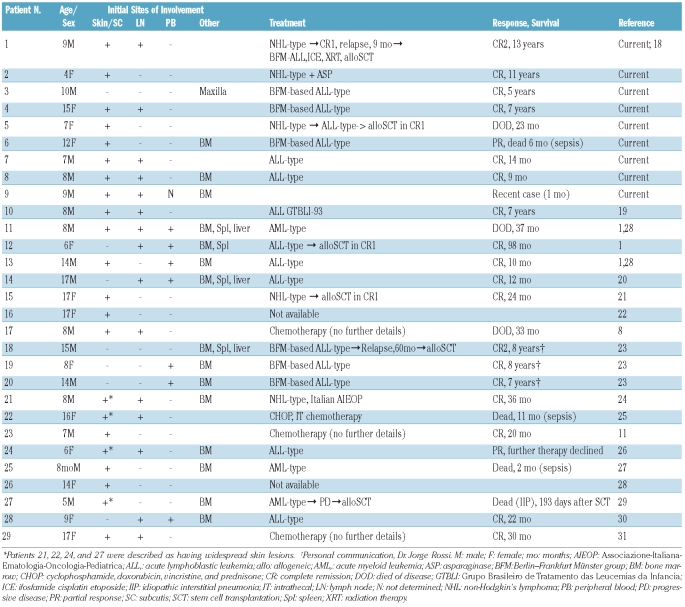
Clinical features of the National Cancer Institute cases and previously published cases
The clinical findings, including initial sites of involvement, treatment modalities, and outcome data, are summarized in Table 3. The nine NCI cases are described individually in detail in the Online Supplementary Appendix. Clinical follow-up was available for 25 of 29 cases (all patients except for 9, 16, 24, and 26) and ranged between 9 months and 13 years (median, 30 months). Seven of the 29 cases (24%) lacked cutaneous disease at presentation, with disease confined to the bone marrow, peripheral blood, lymph nodes, spleen, and/or liver. All seven of these patients survived, with follow-up ranging between 12 and 98 months (100% survival; median follow-up, 60 months). The other 22 of the 29 (76%) had skin involvement at the time of presentation, and among the 18 with available clinical follow-up of at least 9 months, seven patients had died (61% survival rate). All patients who lacked cutaneous disease received acute lymphoblastic leukemia (ALL)-type chemotherapy regimens. Of the eight patients with initial cutaneous disease who had been treated with ALL-type therapy and for whom follow-up information was available, only two died (patients 5 and 6), one of whom from therapy-related complications.
Table 3.
Clinical presentation, therapy, and outcome of pediatric BPDCN cases.
Overall, 14 patients in this series initially received ALL-type regimens; 12 achieved remission and two had a partial response. Two of these patients (cases 12 and 18) underwent stem cell transplantation (SCT), one in first complete remission and one in second complete remission, and both were disease-free at their latest follow-up. The children initially treated with high-risk ALL-type therapy but without SCT also fared well, with only one reported death (patient 6), occurring in a child with relatively advanced disease in the form of a 12 cm cutaneous mass at presentation; this girl died of therapy-related complications (described in detail in the Online Supplementary Appendix).
Non-Hodgkin’s lymphoma-type therapy was limited to six cases (patients 1, 2, 5, 15, 21, and 22), four of whom survived. Three of these underwent SCT, one in first complete remission (patient 15), one in first complete remission after receiving ALL therapy (patient 5), and one in second complete remission after receiving ALL therapy (patient 1). Two of the three patients who underwent SCT survived, and two of the three who did not undergo SCT survived. All three patients who received acute myeloid leukemia-type chemotherapy died; one of progressive disease (patient 11) and two of therapy-related complications (patients 25 and 27).
Among all children with BPDCN who underwent SCT, the overall survival was 67% (4 of 6 cases), and two of these survivors had been transplanted in second complete remission. Importantly, the two patients who died in spite of SCT had not received initial ALL-type therapy - one of the cases of post-transplant mortality was disease-related, occurring in a patient who had undergone SCT in first complete remission (patient 5), the other was attributable to transplant-related complications (patient 27).
Discussion
BPDCN is a rare hematopoietic precursor cell malignancy that has only recently been established as a distinct pathologic entity.38 With a median age at diagnosis of approximately 65 years, it generally affects older adults and often follows a rapidly fatal course in spite of a transient initial response to therapy.1,2,16 Approximately 85% of BPDCN cases show cutaneous involvement at presentation, with about half the cases confined to skin at the time of initial staging, although leukemic variants lacking cutaneous involvement have also been documented based on rigorous immunophenotypic profiling.1,4,20,23,30 The vast majority of adult patients who achieve complete remission subsequently relapse at a median of 9 – 11 months, according to the largest studies.1,2 Those patients who relapse typically develop rapid dissemination of disease to bone marrow and other sites, with central nervous system involvement in 33% of relapsed cases.1 While initial response to therapy may be accomplished with a variety of therapy regimens, sustained remission in adults has only been described in those who underwent SCT.16,17,39 Hence, the optimal treatment approach in adults appears to justify SCT in first complete remission and should include central nervous system prophylaxis given the high risk of relapse at this site.
To improve understanding of BPDCN in the pediatric age group, we compiled data on nine childhood cases evaluated at our institution, and in addition critically reviewed the features of 20 previously published cases. In the cumulative series, 24% of the pediatric cases lacked cutaneous involvement at presentation, which is a slightly higher rate than described in adults, with disease confined to bone marrow, peripheral blood, lymph nodes, spleen, and/or liver. Interestingly, all seven of these patients survived (100% survival; median follow-up, 60 months), although only two had undergone SCT. In contrast, only 11 of the 18 children with cutaneous involvement at presentation and with follow-up data available survived (61%) – only two of these 11 survivors had undergone SCT, and another two died despite SCT (1 of disease, 1 of transplant-related complications).
Although cases without initial cutaneous manifestations of BPDCN were associated with a favorable outcome, direct comparison of cutaneous and extracutaneous-only cases might be confounded by treatment modalities employed, since all of the patients who lacked cutaneous disease received ALL-type chemotherapy regimens, which may be more effective. The biological significance of variants with or without cutaneous involvement does, therefore, require further study. It is important to note that CD123, CD4, and CD56 positivity, the most commonly used BPDCN immunophenotypic profile, is not specific and that these markers can be expressed in both acute myeloid leukemia and ALL. However, cases without cutaneous involvement also expressed other BPDCN markers, including BDCA-4, CD303/BDCA-2, CD2AP, and TCL1. CD68 is generally negative, but focal punctate staining is seen in a minority of cases. Strong staining for CD68 should raise the suspicion of acute or chronic leukemia with monocytic differentiation.
A variety of regimens have been employed in the treatment of BPDCN, and some patients have received multiple therapies, making it difficult to define an optimal management approach. ALL-type therapies have been most commonly utilized and are associated with the best-reported results. Unlike adult cases, the pediatric cases in our review who received high-risk ALL-type therapy had favorable outcomes, irrespective of SCT. All patients who received acute myeloid leukemia-type therapy died (n = 3). Patients who received non-Hodgkin’s lymphoma-type therapy appeared to have a favorable response; however, the number of patients treated with this approach was small. Incorporation of prednisone in the treatment regimen may be critical, as previous studies have suggested that initial responsiveness to prednisone may serve as a predictor of favorable prognosis.23 Furthermore, Rossi et al. hypothesized that intensive methotrexate dosing may avert subsequent relapse, as the one patient who relapsed in their series of three cases had received a less intensive maintenance regimen (patient 18 in our meta-analysis).23 Still, this patient appears cured after undergoing SCT in second complete remission (Dr. Rossi, personal communication).
In determining the role of SCT in first complete remission for children with BPDCN, we reviewed outcome data with respect to this treatment modality. Among all pediatric patients with BPDCN who underwent SCT, the overall survival was 67% (4 of 6 cases), and two of these survivors were transplanted in second complete remission. Importantly, only one of the post-transplant deaths was disease-related and occurred in a patient who had undergone SCT in first complete remission and had not received ALL-type therapy; the other death was attributable to transplant-related complications. The overall survival of patients who did not undergo SCT was 74% (14 of 19 cases). Thus, the role of SCT in pediatric cases of BPDCN is unclear. Based on our review, our recommendation for treatment of children with BPDCN would include high-risk ALL therapy with central nervous system prophylaxis, reserving SCT for second complete remission, or to cases in which initial treatment does not induce a rapid or complete remission.
This study represents the largest series of pediatric BPDCN cases reported to date. We conclude that in contrast to the poor outcome typically observed in adults with BPDCN, this disease is often associated with a relatively favorable outcome in children. The basis for the apparent disparity in clinical behavior between adult and pediatric cases is not certain. A subtle histological difference might be related to the fairly uniform and “lymphoblast”-like appearance of neoplastic cells among the pediatric cases studied, compared to a variability of morphological features seen in adult cases. Cota et al. recently described a series of adult BPDCN cases with pleomorphic cells, including elongated, twisted, or hyperchromatic cells.39 Furthermore, although the immunophenotypic profiles are quite similar between pediatric and adult cases, we did note a difference in S-100 expression. S-100 was detected in 75% of pediatric cases tested but in only 25% of adult cases reviewed at the NIH.5 All three pediatric cases with S-100 expression, including patient 2, who did not receive ALL-type therapy, had a favorable outcome (greater than 5-year survival without SCT). Importantly, expression of plasmacytoid dendritic cell lineage markers CD303/BDCA-2, CD2AP, and TCL1, in addition to CD4, CD56, and CD123, was demonstrated in these three cases, solidifying the diagnosis of BPDCN. The functional significance of S-100 positivity, which is normally present in cells derived from the neural crest as well as in some macrophages and dendritic cells, is uncertain, but the zonal distribution with variable expression may signify divergent subclones. Interestingly, gene expression profiling studies conducted by Dijkman et al. identified a set of genes expressed in BPDCN which was not described in other hematopoietic cells but highly expressed in neuronal cells and implicated in neurogenesis.11
Additional studies of larger numbers of cases are required in order to better define prognostic markers in BPDCN. Although TdT expression has been proposed to predict a relatively favorable outcome in what was formerly called blastic NK-cell lymphoma,40 a clear pattern did not emerge from our analysis. A significant correlation between S-100 and TdT expression was not apparent from this study. Future studies, including gene expression profiling, may provide insight into the pathobiology of BPDCN in the pediatric population.
Supplementary Appendix
Individual case reports are provided in the Online Supplementary Appendix. Details of the cases of acute myeloid leukemia studied for S-100 are presented in Online Supplementary Table S1.
Acknowledgments
the authors would like to thank the following physicians for sharing with us their patients’ clinical histories and specimens: Dr. Denize Azambuja, Rio de Janeiro, Brazil; Dr. Salvatore Bertolone, Louisville, KY, USA; Dr. Robin Hanson, St. Louis, MO, USA; Dr. Paul Jubinsky, New Haven, CT, USA; Dr. Frank Keller, Atlanta, GA, USA; Dr. Dennis Kuo, Paterson, NJ, USA; Dr. Anne-Marie Langevin, Houston, USA, TX; Dr. Elaine Morgan, Chicago, IL, USA; Dr. Anne Nepo, Albany, NY, USA; Dr. John Torrey Sandlund, Memphis, TN, USA; and Dr. Michael Weintraub, Jerusalem, Israel.
Footnotes
Funding: this work was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, NIH.
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Feuillard J, Jacob MC, Valensi F, Maynadie M, Gressin R, Chaperot L, et al. Clinical and biologic features of CD4(+)CD56(+) malignancies. Blood. 2002;99(5):1556–63. doi: 10.1182/blood.v99.5.1556. [DOI] [PubMed] [Google Scholar]
- 2.Petrella T, Bagot M, Willemze R, Beylot-Barry M, Vergier B, Delaunay M, et al. Blastic NK-cell lymphomas (agranular CD4+CD56+ hematodermic neoplasms): a review. Am J Clin Pathol. 2005;123(5):662–75. [PubMed] [Google Scholar]
- 3.Reichard KK, Burks EJ, Foucar MK, Wilson CS, Viswanatha DS, Hozier JC, et al. CD4(+) CD56(+) lineage-negative malignancies are rare tumors of plasmacytoid dendritic cells. Am J Surg Pathol. 2005;29(10):1274–83. doi: 10.1097/01.pas.0000172194.32918.5c. [DOI] [PubMed] [Google Scholar]
- 4.Herling M, Jones D. CD4+/CD56+ hematodermic tumor: the features of an evolving entity and its relationship to dendritic cells. Am J Clin Pathol. 2007;127(5):687–700. doi: 10.1309/FY6PK436NBK0RYD4. [DOI] [PubMed] [Google Scholar]
- 5.Jegalian AG, Facchetti F, Jaffe ES. Plasmacytoid dendritic cells: physiologic roles and pathologic states. Adv Anat Pathol. 2009;16(6):392–404. doi: 10.1097/PAP.0b013e3181bb6bc2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lucio P, Parreira A, Orfao A. CD123hi dendritic cell lymphoma: an unusual case of non-Hodgkin lymphoma. Ann Intern Med. 1999;131(7):549–50. doi: 10.7326/0003-4819-131-7-199910050-00035. [DOI] [PubMed] [Google Scholar]
- 7.Chaperot L, Bendriss N, Manches O, Gressin R, Maynadie M, Trimoreau F, et al. Identification of a leukemic counterpart of the plasmacytoid dendritic cells. Blood. 2001;97(10):3210–7. doi: 10.1182/blood.v97.10.3210. [DOI] [PubMed] [Google Scholar]
- 8.Petrella T, Comeau MR, Maynadie M, Couillault G, De Muret A, Maliszewski CR, et al. 'Agranular CD4+ CD56+ hematodermic neoplasm' (blastic NK-cell lymphoma) originates from a population of CD56+ precursor cells related to plasmacytoid monocytes. Am J Surg Pathol. 2002;26(7):852–62. doi: 10.1097/00000478-200207000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Chaperot L, Perrot I, Jacob MC, Blanchard D, Salaun V, Deneys V, et al. Leukemic plasmacytoid dendritic cells share phenotypic and functional features with their normal counterparts. Eur J Immunol. 2004;34 (2):418–26. doi: 10.1002/eji.200324531. [DOI] [PubMed] [Google Scholar]
- 10.Marafioti T, Paterson JC, Ballabio E, Reichard KK, Tedoldi S, Hollowood K, et al. Novel markers of normal and neoplastic human plasmacytoid dendritic cells. Blood. 2008;111(7):3778–92. doi: 10.1182/blood-2007-10-117531. [DOI] [PubMed] [Google Scholar]
- 11.Dijkman R, van Doorn R, Szuhai K, Willemze R, Vermeer MH, Tensen CP. Gene-expression profiling and array-based CGH classify CD4+CD56+ hematodermic neoplasm and cutaneous myelomonocytic leukemia as distinct disease entities. Blood. 2007;109(4):1720–7. doi: 10.1182/blood-2006-04-018143. [DOI] [PubMed] [Google Scholar]
- 12.Villadangos JA, Young L. Antigen-presentation properties of plasmacytoid dendritic cells. Immunity. 2008;29(3):352–61. doi: 10.1016/j.immuni.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5(12):1219–26. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- 14.Merad M, Manz MG. Dendritic cell home-ostasis. Blood. 2009;113(15):3418–27. doi: 10.1182/blood-2008-12-180646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu K, Victora GD, Schwickert TA, Guermonprez P, Meredith MM, Yao K, et al. In vivo analysis of dendritic cell development and homeostasis. Science. 2009;324(5925):392–7. doi: 10.1126/science.1170540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reimer P, Rudiger T, Kraemer D, Kunzmann V, Weissinger F, Zettl A, et al. What is CD4+CD56+ malignancy and how should it be treated? Bone Marrow Transplant. 2003;32(7):637–46. doi: 10.1038/sj.bmt.1704215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dalle S, Beylot-Barry M, Bagot M, Lipsker D, Machet L, Joly P, et al. Blastic plasmacytoid dendritic cell neoplasm: is transplantation the treatment of choice? Br J Dermatol. 2010;162(1):74–9. doi: 10.1111/j.1365-2133.2009.09373.x. [DOI] [PubMed] [Google Scholar]
- 18.Shaw PH, Cohn SL, Morgan ER, Kovarik P, Haut PR, Kletzel M, et al. Natural killer cell lymphoma: report of two pediatric cases, therapeutic options, and review of the literature. Cancer. 2001;91(4):642–6. doi: 10.1002/1097-0142(20010215)91:4<642::aid-cncr1047>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 19.Falcao RP, Garcia AB, Marques MG, Simoes BP, Fonseca BA, Rodrigues ML, et al. Blastic CD4 NK cell leukemia/lymphoma: a distinct clinical entity. Leuk Res. 2002;26 (9):803–7. doi: 10.1016/s0145-2126(02)00014-0. [DOI] [PubMed] [Google Scholar]
- 20.Anargyrou K, Paterakis G, Boutsis D, Politou M, Papadhimitriou SI, Siakandaris M, et al. An unusual case of CD4+ CD7+ CD56+ acute leukemia with overlapping features of type 2 dendritic cell (DC2) and myeloid/NK cell precursor acute leukemia. Eur J Haematol. 2003;71(4):294–8. doi: 10.1034/j.1600-0609.2003.00098.x. [DOI] [PubMed] [Google Scholar]
- 21.Karube K, Ohshima K, Tsuchiya T, Yamaguchi T, Suefuji H, Suzumiya J, et al. Non-B, non-T neoplasms with lymphoblast morphology: further clarification and classification. Am J Surg Pathol. 2003;27 (10):1366–74. doi: 10.1097/00000478-200310000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Kim Y, Kang MS, Kim CW, Sung R, Ko YH. CD4+CD56+ lineage negative hematopoietic neoplasm: so called blastic NK cell lymphoma. J Korean Med Sci. 2005;20(2):319–24. doi: 10.3346/jkms.2005.20.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rossi JG, Felice MS, Bernasconi AR, Ribas AE, Gallego MS, Somardzic AE, et al. Acute leukemia of dendritic cell lineage in childhood: incidence, biological characteristics and outcome. Leuk Lymphoma. 2006;47(4):715–25. doi: 10.1080/10428190500353216. [DOI] [PubMed] [Google Scholar]
- 24.Ruggiero A, Maurizi P, Larocca LM, Arlotta A, Riccardi R. Childhood CD4+/CD56+ hematodermic neoplasm: case report and review of the literature. Haematologica. 2006;91(12 Suppl):ECR48. [PubMed] [Google Scholar]
- 25.Pilichowska ME, Fleming MD, Pinkus JL, Pinkus GS. CD4+/CD56+ hematodermic neoplasm (“blastic natural killer cell lymphoma”): neoplastic cells express the immature dendritic cell marker BDCA-2 and produce interferon. Am J Clin Pathol. 2007;128(3):445–53. doi: 10.1309/W9Q5AGYDE5LANN39. [DOI] [PubMed] [Google Scholar]
- 26.Eguaras AV, Lo RW, Veloso JD, Tan VG, Enriquez ML, Del Rosario ML. CD4+/CD56+ hematodermic neoplasm: blastic NK cell lymphoma in a 6-year-old child: report of a case and review of literature. J Pediatr Hematol Oncol. 2007;29(11):766–9. doi: 10.1097/MPH.0b013e318159a4e6. [DOI] [PubMed] [Google Scholar]
- 27.Hu SC, Tsai KB, Chen GS, Chen PH. Infantile CD4+/CD56+ hematodermic neoplasm. Haematologica. 2007;92(9):e91–3. doi: 10.3324/haematol.11307. [DOI] [PubMed] [Google Scholar]
- 28.Garnache-Ottou F, Feuillard J, Ferrand C, Biichle S, Trimoreau F, Seilles E, et al. Extended diagnostic criteria for plasmacytoid dendritic cell leukaemia. Br J Haematol. 2009;145:624–36. doi: 10.1111/j.1365-2141.2009.07679.x. [DOI] [PubMed] [Google Scholar]
- 29.Hama A, Kudo K, Itzel BV, Muramatsu H, Nishio N, Yoshida N, et al. Plasmacytoid dendritic cell leukemia in children. J Pediatr Hematol Oncol. 2009;31(5):339–43. doi: 10.1097/MPH.0b013e31819b7215. [DOI] [PubMed] [Google Scholar]
- 30.Reineks EZ, Osei ES, Rosenberg A, Auletta J, Meyerson HJ. CD22 expression on blastic plasmacytoid dendritic cell neoplasms and reactivity of anti-CD22 antibodies to peripheral blood dendritic cells. Cytometry B Clin Cytom. 2009;76B(4):237–48. doi: 10.1002/cyto.b.20469. [DOI] [PubMed] [Google Scholar]
- 31.Tsagarakis NJ, Kentrou NA, Papadimitriou KA, Pagoni M, Kokkini G, Papadaki H, et al. Acute lymphoplasmacytoid dendritic cell (DC2) leukemia: results from the Hellenic Dendritic Cell Leukemia Study Group. Leuk Res. 2010;34(4):438–46. doi: 10.1016/j.leukres.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 32.Herling M, Teitell MA, Shen RR, Medeiros LJ, Jones D. TCL1 expression in plasmacytoid dendritic cells (DC2s) and the related CD4+ CD56+ blastic tumors of skin. Blood. 2003;101(12):5007–9. doi: 10.1182/blood-2002-10-3297. [DOI] [PubMed] [Google Scholar]
- 33.Petrella T, Meijer CJ, Dalac S, Willemze R, Maynadie M, Machet L, et al. TCL1 and CLA expression in agranular CD4/CD56 hematodermic neoplasms (blastic NK-cell lymphomas) and leukemia cutis. Am J Clin Pathol. 2004;122(2):307–13. doi: 10.1309/0QPP-AVTU-PCV9-UCLV. [DOI] [PubMed] [Google Scholar]
- 34.Dzionek A, Fuchs A, Schmidt P, Cremer S, Zysk M, Miltenyi S, et al. BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J Immunol. 2000;165(11):6037–46. doi: 10.4049/jimmunol.165.11.6037. [DOI] [PubMed] [Google Scholar]
- 35.Garnache-Ottou F, Chaperot L, Biichle S, Ferrand C, Remy-Martin JP, Deconinck E, et al. Expression of the myeloid-associated marker CD33 is not an exclusive factor for leukemic plasmacytoid dendritic cells. Blood. 2005;105(3):1256–64. doi: 10.1182/blood-2004-06-2416. [DOI] [PubMed] [Google Scholar]
- 36.McCarthy KP, Sloane JP, Kabarowski JH, Matutes E, Wiedemann LM. A simplified method of detection of clonal rearrangements of the T-cell receptor-gamma chain gene. Diagn Mol Pathol. 1992;1(3):173–9. [PubMed] [Google Scholar]
- 37.Dojcinov SD, Venkataraman G, Raffeld M, Pittaluga S, Jaffe ES. EBV positive mucocu-taneous ulcer--a study of 26 cases associated with various sources of immunosuppression. Am J Surg Pathol. 2010;34(3):405–17. doi: 10.1097/PAS.0b013e3181cf8622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. In: WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Bosman FT, Jaffe ES, Lakhani SR, Ohgaki H, editors. Lyon, France: International Agency for Research on Cancer; 2008. [Google Scholar]
- 39.Cota C, Vale E, Viana I, Requena L, Ferrara G, Anemona L, et al. Cutaneous manifestations of blastic plasmacytoid dendritic cell neoplasm-morphologic and phenotypic variability in a series of 33 patients. Am J Surg Pathol. 2010;34(1):75–87. doi: 10.1097/PAS.0b013e3181c5e26b. [DOI] [PubMed] [Google Scholar]
- 40.Bekkenk MW, Jansen PM, Meijer CJ, Willemze R. CD56+ hematological neoplasms presenting in the skin: a retrospective analysis of 23 new cases and 130 cases from the literature. Ann Oncol. 2004;15(7):1097–108. doi: 10.1093/annonc/mdh268. [DOI] [PubMed] [Google Scholar]