Abstract
Background
Tumor cells in chronic lymphocytic leukemia accumulate in the periphery through the proliferation of a minority of cells in lymph nodes. The proliferative and survival signals in these proliferation centers include interactions with T lymphocytes expressing CD40 ligand. We have demonstrated that the low toxicity combination of bezafibrate and medroxyprogesterone acetate induces mitochondrial superoxide-mediated apoptosis of non-CD40-liganded cells but not of cells exposed to CD40 ligand. Here, we assessed the ability of dasatinib and lycorine to restore bezafibrate- and medroxyprogesterone acetate- induced apoptosis in cells exposed to CD40 ligand. In parallel experiments we compared the ability of dasatinib to induce apoptosis of cells co-treated with fludarabine.
Design and Methods
Primary chronic lymphocytic leukemia and peripheral blood mononuclear cells were exposed to drug combinations for 72 hours on control and CD40 ligand-expressing fibroblast monolayers. Cells were harvested and analyzed for apoptosis and levels of mitochondrial superoxide using flow cytometry. In some experiments cells were removed from CD40 ligand at 48 hours, retreated and analyzed after a further 24 hours. The effect of CD40 ligand and drug treatments on mitochondrial superoxide levels were assessed.
Results
As previously described, dasatinib rendered cells sensitive to fludarabine but only when CD40 ligand was removed for the last 24 hours of culture. In contrast, lycorine restored the bezafibrate- and medroxyprogesterone acetate-induced apoptosis associated with mitochondrial superoxide even during continuous exposure to CD40 ligand. Furthermore, combined bezafibrate, medroxyprogesterone acetate and lycorine had little effect against normal peripheral blood mononuclear cells, whereas dasatinib with fludarabine induced high levels of apoptosis.
Conclusions
Our data indicate the potential of bezafibrate, medroxyprogesterone acetate and lycorine as novel therapy in chronic lymphocytic leukemia and have important implications for the reported potential of c-abl kinase inhibitors in this disease.
Keywords: bezafibrate, medroxyprogesterone acetate, lycorine, chronic lymphocytic leukemia, mitochondrial superoxide, CD40 ligand
Introduction
Chronic lymphocytic leukemia (CLL) is a disease of neoplastic B cells characterized by the progressive accumulation of CD5+ B lymphocytes in the peripheral blood, bone marrow and secondary lymphoid organs.1–4 In the western world it is the most common form of adult leukemia and has a widely variable clinical course with many patients never requiring therapy. There are 22–30/100,000 new cases per year worldwide and the majority of patients are over 65 years old.5 According to recent Surveillance, Epidemiology and End Results the median age at diagnosis is 72 years.6 In younger patients, the current front-line therapy is a combination of fludarabine, cyclophosphamide and rituximab7 but this regimen is associated with significant toxicity for elderly patients. CLL remains incurable for the majority of patients who become refractory to chemotherapy6,8,9 and there is a need to develop less toxic and novel therapies that can overcome drug resistance.8
The tumor microenvironment, in particular the lymph nodes, has been shown to be both the center for CLL cell proliferation and to protect cells against chemotherapeutics.10 The lymph node is rich in T cells that express CD40 ligand (CD40L) and engage CLL cells via CD40 to stimulate proliferation in proliferation centers and protect against apoptotic signals. Histopathology has confirmed that within the proliferation centers, proliferating CLL cells are directly exposed to CD40L signals.11 Furthermore, CLL cells are protected from spontaneous apoptosis in vitro when co-cultured with autologous T cells12 and many studies, including our own, have demonstrated that CLL cells are protected from drug-induced apoptosis by culture with CD40L and interleukin-4.13–16 Therapies that overcome this protective mechanism within proliferation centers, while also targeting the circulating neoplastic cells, are likely to provide better response rates and reduce the rate of relapse. Encouragingly, Hallaert et al.17 recently found that the protective effects of CD40L could be overcome by the use of the c-Abl kinase inhibitor, dasatinib. It was reported that this was due to a reduction in anti-apoptotic proteins and it was proposed that this agent could successfully sensitize CLL cells within the lymph nodes to chemotherapeutics such as fludarabine. However, the CD40L signal was not provided continuously and the apoptosis associated with dasatinib treatment was only apparent following removal of CD40L. It cannot, therefore, be categorically concluded that dasatinib would be able to overcome CD40L in vivo and sensitize CLL cells to therapeutics. In addition, it is questionable whether the dose of 100 μM fludarabine is clinically achievable, since the reported peak concentration of fludarabine in lymphocytes and plasma is between 3 and 19 μM.18–20
We have investigated, in vitro, the potential of combining bezafibrate and medroxyprogesterone acetate (MPA) against CLL, acute myeloid leukemia and Burkitt’s lymphoma,15,21,22 and also conducted a phase II clinical study of the combination (bezafibrate + MPA) in acute myeloid leukemia.23 This phase II study showed that the combination was of clinical benefit and did not have associated hematologic or systemic toxicities.23 These findings indicated the potential benefit of exploiting such therapy in CLL patients, especially those who are frail and not able to endure more toxic regimens. However, although we demonstrated that bezafibrate + MPA could induce apoptosis of non-CD40L-stimulated CLL cells and reduce their CD40L-induced proliferation,15 we did not observe that combined bezafibrate + MPA provoked apoptosis in CD40L-protected cells. The lack of apoptosis in the presence of CD40L was associated with protection against bezafibrate + MPA-induced mitochondrial superoxide production. In this study we examined whether the addition of a third agent could overcome the protective signal provided by CD40L and lead to the restoration of mitochondrial superoxide accumulation and the successful apoptosis of CLL cells within this environment.
Mankind has always used plants as a source of natural medicines. Indeed, extracts from the Narcissus genus of Amaryllidaceae were documented to have been used as cancer therapy by Hippocrates in the 4th century BC.24 In recent decades, the scientific community has investigated the therapeutic use of numerous plant-derived compounds and many have been studied as anti-leukemia therapies. These include PEP005, derived from Euphorbia peplus, in the treatment of acute myeloid leukemia,25 jasmonates, plant hormones found in all plants, in acute myeloid leukemia, CLL and B-cell lymphoma,26–29 and pan-cratistatin and lycorine from the Amaryllidaceae family in acute myeloid leukemia and acute promyelocytic leukemia.30–32
Lycorine is the most abundant of all the Amaryllidaceae alkaloids and has wide ranging biological activities. Studies this century have indicated that lycorine interferes with replication of the polio, small pox and SARS viruses,33–36 has anti-fungal activities37 and is anti-parasitic, including against malaria.38 In the last decade research has focused on the potential use of this compound to treat cells resistant to apoptotic stimuli,39 including leukemic cells.24,30–32,36 In vitro studies in such settings have shown that lycorine can induce apoptosis, specifically targeted against malignant cells.31 Its potential use as a therapeutic agent has recently led to studies into the production of the synthetic compound,40,41 highlighting it as a potential lead for drug development.24,42
In this study we investigated the potential of combining lycorine with bezafibrate + MPA to elicit an apoptotic response in the presence of CD40L and assessed the correlation between induced apoptosis and the generation of mitochondrial superoxide. We compared our findings of those of Hallaert et al. and looked at the importance of the continual provision of CD40L to truly mimic the lymph node environment.
Design and Methods
Patients and donors
The CLL cells used were from unselected patients diagnosed with B-cell CLL according to standard morphological, immunophenotypic and clinical criteria43 and attending the outpatient clinic at Birmingham Heartlands Hospital, UK. Patients provided informed written consent to the study which had received local ethical approval. Normal donors were recruited following informed consent. Primary mononuclear cells were prepared using Ficoll Paque-Plus (Anachem) as previously described.15
Cell culture using L cells
Murine L cells stably transfected with plasmids encoding CD40L, as described previously,44 as well as non-transfected L cells (both a gift from Prof. John Gordon) were maintained, treated with mitomyocin C (Sigma) and seeded for co-culture as described previously.15 Mononuclear cells were seeded on to the stromal L cells, at a ratio of 10:1 in RPMI 1640 with 10% fetal bovine serum, 1% penicillin-streptomycin and 1 ng/mL interleukin-4 (R&D systems), while treated as described in the text.
Removal of chronic lymphocytic leukemia cells from the stromal support
CLL cells were removed from the stroma as described previously.15 The CLL cells were either analyzed immediately following removal or washed in warm phosphate-buffered saline, and resuspended in 200 μL RPMI, supplemented as before, with 1 ng/mL interleukin-4 and the appropriate treatments and cultured for a further 24 h before analysis. Immediately prior to harvest cultures were viewed using an inverted microscope to ensure the integrity of the stromal support following the treatments.
Treatments
Stocks (1000 x) of 0.5 M bezafibrate in dimethylsulfoxide, 5 mM MPA in ethanol, 10 mM fludarabine, a 10,000 x stock of 100 mM lycorine (all from Sigma, Poole, UK) in dimethylsulfoxide and 50 mM dasatinib (LC Laboratories, MA, USA) in dimethylsulfoxide were stored at −20ºC. All treatments were set up in triplicate with controls containing the highest concentration of carrier solvent used in the corresponding treatments.
Assessment of apoptosis by annexin V
All analyses were carried out by flow cytometry on a BD FACS Calibur utilizing Cell Quest Pro software (BD). Following treatment, CLL cells from triplicate wells were pooled and incubated with annexin V and propidium iodide from an annexin V FITC kit (BD, Oxford, UK), according to manufacturer’s instructions.
Assessment of accumulation of mitochondrial superoxide
MitoSOX Red (Molecular Probe) was used to assess the presence of mitochondrial superoxide in pooled triplicate wells, as described previously.15
Assessment of protein expression
CLL cells were cultured in 6-well plates with non-CD40L stroma or CD40L stroma and were treated for 24 h or 48 h, respectively. The cells were removed from the stromal support as described above, washed once in phosphate-buffered saline and the pellet snap-frozen in liquid nitrogen. Whole cell pellets were lysed in 1x Laemmli dye loading buffer and boiled for 10 min. CLL lysates, together with the lysate from Huh cells (used as a positive control), were separated by 12.5% sodium-dodecylsulfate polyacrylamide gel electrophoresis prior to semi-dry transfer to polyvinylidene fluoride Immobilon-P membrane (Millipore Corp, Watford, Herts., UK). Membranes were blocked for 1 h with 5% (w/v) non-fat milk powder (Marvel, Wisbech, Cambs., UK) in Tris-buffered saline Tween-20 (TBS-T), briefly washed and probed with a 1 in 1000 dilution of anti-SODII antibody (BD) in 5% (w/v) non-fat milk powder in Tris-buffered saline Tween-20 overnight. Following washing in TBS-T, membranes were probed with anti-mouse antibody (Sigma) diluted 1 in 1000 as above, for 45 min. The signal was developed using Supersignal West Pico Chemiluminescent substrate (Pierce, Cramlington, Northumberland, UK). Autorads were exposed for 1 min. Equal loading was assessed using anti-β-actin antibody (Sigma) diluted 1 in 25 000 for 45 min and an anti-mouse secondary antibody diluted 1 in 25 000 for 45 min and developed as above.
Statistical analysis
Data are reported as mean values and their standard deviation or standard error of the mean as stated. P values were calculated using the non-parametric Wilcoxon’s test in SPSS version 14.
Results
When the protective CD40-ligand is maintained, the dasatinib plus fludarabine combination no longer induces apoptosis of chronic lymphocytic leukemia cells
Using 5 μM of fludarabine, a dose that more closely reflects an achievable plasma and intra-lymphocyte concentration18–20 we were able to reproduce the observations reported by Hallaert et al. that dasatinib did not induce apoptosis alone, but that the combination of dasatinib and fludarabine did, following continuous drug exposure for 72 h with the final 24 h of treatment being in the absence of CD40L (Figure 1A). However, using this methodology bezafibrate + MPA also induced apoptosis and to a similar level as dasatinib + fludarabine (P=0.51). In contrast, and as we described previously,15 treatment of CLL cells with bezafibrate + MPA for 72 h in the continuous presence of CD40L failed to induce apoptosis. (Figure 1B) (P=0.52). Additionally, dasatinib did not sensitize the CD40L-protected cells to bezafibrate + MPA (data not shown). Most interesting was the similar lack of apoptosis induced by dasatinib + fludarabine under these conditions (P=0.22) (Figure 1B). The drug combinations studied by Hallaert et al. can, therefore, only induce CLL cell apoptosis following release from CD40L protection, as is also seen with bezafibrate + MPA. These data indicate that the dasatinib + fludarabine combination represents no advantage over bezafibrate + MPA and further indicate that dasatinib does not overcome the previously suggested protection of CLL cells against fludarabine by CD40L.
Figure 1.

The sensitization of CLL cells to fludarabine (F-ara-A) by dasatinib (DSN) is dependent on the removal of CD40L protection. (A) Removal of CD40L and drug re-treatment; CLL cells were cultured in a 10:1 ratio on stromal cells expressing CD40L and were treated with solvent control, 30 μM DSN, 5 μM F-ara-A, DSN+F-ara-A or bezafibrate (BEZ)+MPA. At 48 h the CLL cells were removed from the stroma, washed and re-plated without CD40L and re-treated for a further 24 h prior to analysis for the binding of annexin V and exclusion of propidium iodide; Plots show total annexin V for six CLL samples ±s.e.m. (B) Drug treatment in the continuous presence of CD40L; CLL cells were cultured in a 10:1 ratio on stromal cells expressing CD40L and were treated with solvent control, 30 μM DSN, 5 μM F-ara-A, DSN+F-ara-A or BEZ+MPA for 72 h. CLL cells were removed from the stroma and immediately analyzed for the binding of annexin V and exclusion of propidium iodide; graph shows total annexin V (as described in Figure 2) for the same six CLL samples ±s.e.m.
Lycorine alone does not induce apoptosis of primary chronic lymphocytic leukemia cells in the presence of CD40-ligand
We hypothesized that the addition of an appropriate third agent may be able to overcome the protective effects of CD40L against bezafibrate + MPA, and result in the desired induction of apoptosis. Following recent publications on the use of lycorine in causing apoptosis of acute promyelocytic leukemia and acute myeloid leukemia cell lines,30,36,45 we investigated whether this agent has potential in CLL. A dose titration of lycorine, starting at the highest dose shown to be effective against HL-60 and K562 cells by Lui et al.,32 was conducted in CLL samples, from six patients, in the presence of CD40L. None of the doses tested induced significant apoptosis in primary CLL cells (P=0.1 for all treatments) (Figure 2B). However, the addition of lycorine to bezafibrate + MPA proved to be effective at inducing the death of CLL cells even when continuously exposed to CD40L (Figure 2A and B). Even the lowest dose tested (0.6 μM) combined with bezafibrate + MPA potentiated apoptosis (36%) compared to that of cells treated with bezafibrate + MPA alone (P=0.017). The pro-apoptotic effect of bezafibrate + MPA + lycorine reached a plateau at doses of lycorine above 1.25 μM, with no significant increases in the level of apoptosis (P≤0.1 for all higher doses tested).
Figure 2.
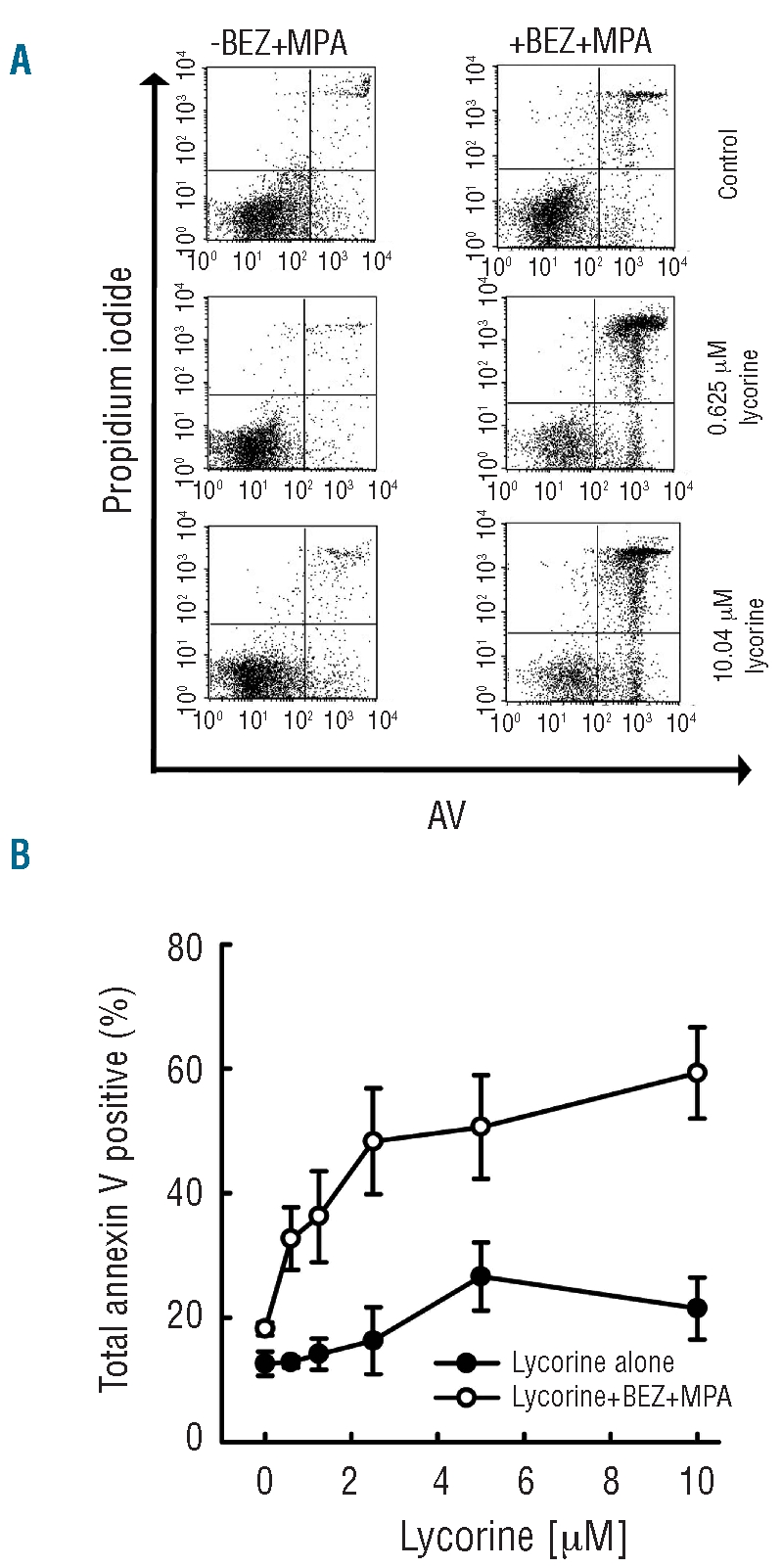
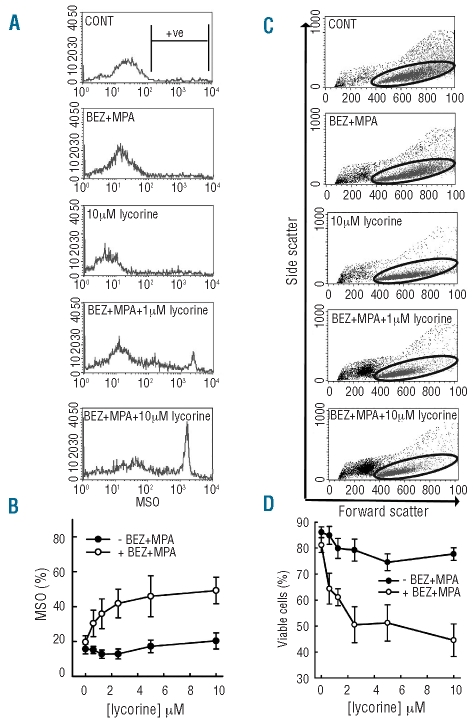
When CLL cells are cultured in the presence of CD40L, lycorine induces apoptosis in the presence of bezafibrate (BEZ)+MPA. CLL cells were cultured in a 10:1 ratio on stromal cells expressing CD40L and were treated with solvent control, or a dose titration of lycorine either with or without BEZ+MPA for 72 h prior to analysis of the binding of annexin V and exclusion of propidium iodide. (A) Representative plots (from six CLL samples) of solvent control, 0.625 μM and 10 μM lycorine with and without BEZ+MPA. The lower left (LL) quadrant shows viable cells, lower right (LR) indicates cells in early stages of apoptosis, upper right (UR) is indicative of late apoptosis. Total annexin V staining was calculated by adding the events in the LR with those in the UR. (B) Dose titration of lycorine (10 μM-0.625 μM) without (black circles) and with (open circles) BEZ+MPA. The plot shown represents total annexin V positivity of six CLL samples ±s.e.m.
Mitochondrial superoxide is produced in response to bezafibrate plus medroxyprogesterone plus lycorine
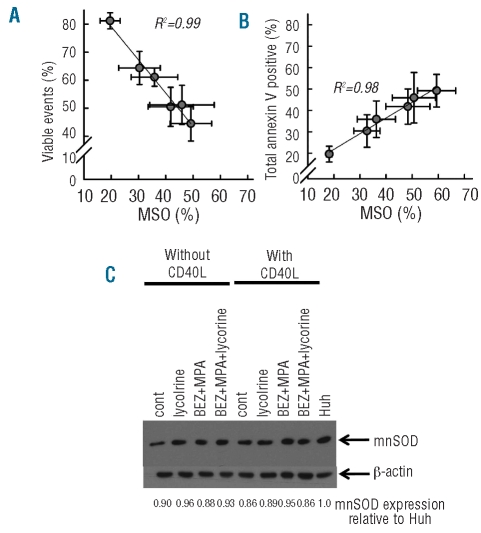
Our previous study showed that bezafibrate + MPA-induced apoptosis in the absence of CD40L was associated with the production of reactive oxygen species and mitochondrial superoxide. In contrast, the survival of bezafibrate + MPA-treated cells in the presence of CD40L was associated with the induction of reactive oxygen species but not mitochondrial superoxide. We argued that CD40L protects against bezafibrate + MPA-induced apoptosis by protecting against or countering mitochondrial superoxide production. We tested whether the capacity of lycorine to potentiate the ability of bezafibrate + MPA to induce apoptosis in cells exposed to CD40L was associated with the restoration of the mitochondrial superoxide response at 48 h. Consistent with the lack of cell death induced by lycorine alone, by itself this agent did not induce mitochondrial superoxide production (P≤0.1 for all) (Figure 3A and B). A small increase in mitochondrial superoxide was observed in response to 10 μM lycorine; however, even at this dose, the amount of mitochondrial superoxide produced was no more than that produced by bezafibrate + MPA alone (Figure 3A and B). In contrast, the combination of bezafibrate + MPA + lycorine induced substantial, lycorine-dose-dependent increases in mitochondrial superoxide, with bezafibrate + MPA + 1.25 μM lycorine producing the highest level of mitochondrial superoxide (P=0.004) (Figure 3B). Importantly, the lycorine-mitochondrial superoxide dose-response mirrored the induction of apoptosis as measured by annexin V staining and cell viability (Figures 2B, 3C and 3D). Direct comparison of total mitochondrial superoxide against viable events (Figure 4A) and annexin V staining against mitochondrial superoxide (Figure 4B) further demonstrated that an increase in mitochondrial superoxide was directly associated with the loss of viability and apoptosis. These data on two measures of apoptosis compared with mitochondrial superoxide production indicate that the apoptosis caused by the combination of bezafibrate + MPA + lycorine is likely to be due to restored accumulation of mitochondrial superoxide within the treated cells.
Figure 3.
When combined, lycorine+bezafibrate(BEZ)+MPA induce the production of mitochondrial superoxide and reduce cell viability. CLL cells were cultured in a 10:1 ratio on stromal cells expressing CD40L and were treated with solvent control or BEZ+MPA with (open circles) or without (black circles) a two-fold dose titration of lycorine. At 48 h CLL cells were removed from stroma and analyzed for the generation of mitochondrial superoxide (MSO). (A) Representative histograms for control, BEZ+MPA, 10 μM lycorine, BEZ+MPA+1.25 μM lycorine and BEZ+MPA+ 10 μM lycorine. Positivity was determined to be events captured above 102, indicated by the M-gate shown in the control plot and total positivity plotted in (B) for six CLL samples ± s.e.m. (C) At 72 h cells were removed from stroma and analyzed for cell viability by forward and side scatter using flow cytometry; representative dot plots shown for control, BEZ+MPA, 10 μM lycorine, BEZ+MPA + 1 μM lycorine and BEZ+MPA + 10 μM lycorine, with viable events shown in the oval gate. Total percentages in the viable gate plotted in (D) for six CLL samples ± s.e.m.
Figure 4.
Cell viability and total annexin V positivity correlates with mitochondrial superoxide (MSO) production but CD40L does not upregulate mnSOD. CLL cells were cultured in a 10:1 ratio on stromal cells expressing CD40L and were treated with bezafibrate(BEZ)+MPA alone and together with a two-fold dose titration of lycorine prior to analysis of generation of MSO at 48 h and binding of annexin V positivity as well as cell viability by forward scatter and side scatter at 72 h. (A) Total MSO plotted against viable events. (B) Total % MSO is plotted against total annexin V positivity, for each lycorine concentration. Plots are for six CLL samples ± s.e.m. CLL cells were treated with solvent control, 5 μM lycorine, BEZ+MPA or the combination on non-CD40L-expressing stroma or CD40L-expressing stroma for 24 and 48 h respectively, prior to western blot for mnSOD and β-actin. Densitometry was performed and total mnSOD protein calculated relative to Huh as displayed. The image is representative of samples from three patients.
Previous studies demonstrated that transcription of the mitochondrial superoxide antioxidant manganese super-oxide dismutase is up-regulated in cells exposed to tumor necrosis factor-α.46 As CD40 and its ligand are members of the tumor necrosis factor family, we hypothesized that CLL cells exposed to CD40L may also up-regulate manganese superoxide dismutase and, thereby, overcome the pro-apoptotic effects exerted by bezafibrate + MPA. Alternatively, bezafibrate + MPA may induce apoptosis in the absence of CD40L by down-regulating manganese superoxide dismutase and this action may be counteracted by CD40L; according to this hypothesis, lycorine, in the presence of CD40L, would reverse this down-regulation. However western blot analysis of protein expression revealed that CD40L did not up-regulate manganese superoxide dismutase (Figure 4C). Further, neither in the absence nor in the presence of CD40L was bezafibrate + MPA, lycorine or the combination able to induce down-regulation of this protein (Figure 4C).
Lycorine only modestly potentiates bezafibrate-plus medroxyprogesterone acetate-induced apoptosis in the absence of CD40-ligand
We next investigated the effects of lycorine on CLL cells in the absence of CD40L. Lycorine alone induced a modest dose-dependant increase in apoptosis (Figure 5). Even at the highest dose tested lycorine was no better at inducing apoptosis than bezafibrate + MPA (P=0.83). When combined with bezafibrate + MPA, lycorine did increase the amount of apoptosis observed; however, the effect was less striking than that detected in the presence of CD40L.
Figure 5.

Lycorine potentiates the level of apoptosis induced by bezafibrate(BEZ)+MPA in the absence of CD40L. CLL cells were cultured in a 10:1 ratio on stromal cells in the absence of CD40L and were treated with two-fold dose titration of lycorine (10 μM-0.625 μM) or solvent control with (open circles) or without (black circles) BEZ+MPA for 72 h. CLL cells were removed from stroma and analyzed for the binding of annexin V and exclusion of propidium iodide. The plot shown represents total annexin V positivity for four samples ± s.e.m.
The bezafibrate plus medroxyprogesterone acetate plus lycorine combination, but not dasatinib plus fludarabine, is selective for chronic lymphocytic leukemia cells
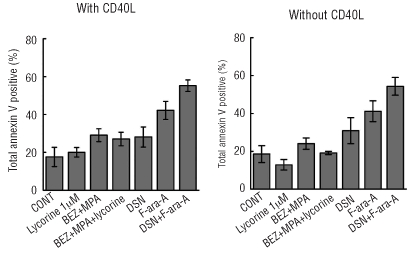
In our previous study,15 we demonstrated that bezafibrate + MPA had minimal effects on normal B cells, indicating tumor cell selectivity. Here we tested the effect of dasatinib + fludarabine on normal peripheral blood mononuclear cell apoptosis and compared the response with that of bezafibrate + MPA with and without lycorine. Neither bezafibrate + MPA, 1.25 μM lycorine, or the triple combination significantly increased apoptosis of peripheral blood mononuclear cells either in the presence or the absence of CD40L (P≤0.1 for all) (Figure 6). The combination of bezafibrate + MPA +10 μM lycorine induced some apoptosis (data not shown); however, since the most significant anti-CLL activity of lycorine + bezafibrate + MPA was observed at a dose of 1.25 μM lycorine we suggest that a dose of 10 μM would not be used as CLL therapy. Dasatinib induced a small amount of peripheral blood mononuclear cell apoptosis (P=0.048) (Figure 6). In contrast, fludarabine increased apoptosis of peripheral blood mononuclear cells cultured both with and without CD40L. Furthermore, there was a trend to significantly increased apoptosis for the combination of dasatinib + fludarabine compared to control (P=0.001) (Figure 6).
Figure 6.
The combination of lycorine+bezafibrate(BEZ)+MPA induces significantly less apoptosis in normal peripheral blood cells than does dasatinib(DSN)+fludarabine(F-ara-A). Normal peripheral blood mononuclear cells were cultured in a 10:1 ratio on CD40L expressing stromal cells or non- CD40L-expressing stromal cells and were treated with solvent control, 1 μM lycorine, BEZ+MPA, BEZ+MPA+1 μM lycorine, 30 μM DSN, 5 μM F-ara-A, or DSN+F-ara-A for 72 h. The cells were removed from the stroma and immediately analyzed for binding of annexin V and exclusion of propidium iodide. The plots represent total annexin V positivity for four sample ± s.e.m.
Discussion
Despite many developments in the treatment of CLL and improved response rates, the disease remains incurable and overcoming chemoresistance without high levels of toxicity is a priority. Migration of circulating CLL cells to lymph nodes provides the cells with survival signals from CD40L, causing the development of pro-survival niches that are widely believed to be the source of therapy resistance and relapse.17,47 Chemoresistance has been reported in CLL cells that have been exposed to CD40L13,14 and Vogler et al. recently demonstrated that CLL cells exposed to CD40L have 1000-fold increased resistance to the novel therapeutic ABT-737.16 It appears, therefore, that CD40L can protect CLL cells against a variety of potential treatments. Thus it could be speculated that CLL cells effectively ‘seek cover’ from anti-neoplastic therapy by exploiting CD40L within the lymph nodes. We have recently shown that bezafibrate + MPA can induce apoptosis of circulating CLL cells, while significantly reducing the proliferative capabilities of CLL cells when engaged with CD40L.15 However, the lack of apoptosis within an environment mimicking the lymph node led to our search for a third agent to combine with bezafibrate + MPA with the aim of exerting apoptosis both in the absence and presence of CD40L. The recent study by Hallaert et al. identified the possibility of using dasatinib as an agent to sensitize CLL cells to other therapies.17 However, the experiments herein have demonstrated that the ability of dasatinib to promote apoptosis requires that the cells are no longer perceiving protective signals from CD40L. Thus, the implications of the study of Hallaert et al. are not dissimilar to those of Veldurthy et al.48 and Amrein et al.49 who demonstrated dasatinib potentiation of fludarabine-induced apoptosis in CLL cells not provided with CD40L. Furthermore, in this scenario, the actions of combined dasatinib + fludarabine were no greater than those of bezafibrate + MPA.
However, lycorine was able to sensitize CLL cells to bezafibrate + MPA-induced apoptosis in the continuous presence of CD40L. Furthermore, the ability to do so was associated with restored accumulation of mitochondrial superoxide in response to bezafibrate + MPA. This would indicate that either lycorine reinstates the capacity of bezafibrate + MPA to induce mitochondrial superoxide in CD40L-stimulated cells or interferes with the CD40L-mediated clearance of mitochondrial superoxide. This is the first study to show a correlation between the generation of mitochondrial superoxide and cell death in CLL.
We postulated that a possible mechanism of action of lycorine and/or bezafibrate + MPA could be disrupted regulation of the mitochondrial superoxide manganese super-oxide dismutase. However, we found that CD40L does not induce up-reglaution of this superoxide dismutase, and that the agents studied were not interfering with this pathway. Nonetheless, the possibility that lycorine exerts its effects via mitochondrial mechanisms is supported by studies in Saccharomyces cerevisiae, which have demonstrated that cells lacking mitochondrial DNA are resistant to lycorine.50
Elderly CLL patients are commonly excluded from clinical trials because of their susceptibility to the development of cytopenia and infections and in younger patients there is an inevitable development of chemoresistance to conventional agents. Thus more targeted therapies are urgently required. Studies on mononuclear cells isolated from normal donors demonstrated that the combination of dasatinib + fludarabine caused significant apoptosis, whereas the combination of bezafibrate + MPA + lycorine left the normal cells relatively unaffected, further highlighting the potential of our approach.
Currently, many plant compounds are being investigated with the intention of gaining FDA approval for their therapeutic use. Recently, the prescription drug Reminyl was approved for the treatment of Alzheimer’s disease. The active ingredient of this compound is the alkaloid galanthamine isolated from another member of the Amaryllidaceae family.42,51 Therefore a precedent has been set for the use of this group of agents in the treatment of diseases. Lycorine has been identified as a novel lead for anticancer drug design24,39 and Jones et al. have published the first successful study of the synthesis of the framework of one of lycorine’s sister compounds.40 Thus, the concept of lycorine ultimately being synthesized successfully and gaining FDA approval for therapeutic use is a real possibility. Nonetheless the time scale and cost of developing new drugs are limiting and in addition the in vivo toxicity of lycorine is as yet unknown and will have to be determined prior to possible trials in combination with bezafibrate + MPA.
In conclusion, our studies continue to indicate bezafibrate + MPA as potential therapies for CLL. Importantly this study provides the proof of principle that strategies using bezafibrate + MPA can be developed with the potential to target cells both in the periphery and in the protective lymph node environment and demonstrates that the generation of mitochondrial superoxide is critical for apoptosis. It remains possible that other already available drugs could also target the cells within the lymph node as effectively as lycorine. Unfortunately, these do not appear to include dasatinib but screens of other agents could yield promising results. Our data would suggest that agents targeting mitochondria may be strong candidates. We, therefore, propose that rational drug screening for a third agent should be based on the generation of mitochondrial super-oxide as measured by MitoSox. If a suitable agent could be found this would be the most expedient way to develop the regime as a clinically viable treatment.
Footnotes
Funding: this work was supported by grants from Leukaemia and Lymphoma Research (LLR, UK).
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Ghia P, Ferreri AM, Caligaris-Cappio F. Chronic lymphocytic leukemia. Crit Rev Oncol Hematol. 2007;64(3):234–46. doi: 10.1016/j.critrevonc.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 2.Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. N Engl J Med. 2005;352(8):804–15. doi: 10.1056/NEJMra041720. [DOI] [PubMed] [Google Scholar]
- 3.Rozman C, Montserrat E. Chronic lymphocytic leukemia. N Engl J Med. 1995;333(16):1052–7. doi: 10.1056/NEJM199510193331606. [DOI] [PubMed] [Google Scholar]
- 4.Ghia P, Ferreri AM, Galigaris-Cappio F. Chronic lymphocytic leukemia. Crit Rev Oncol Hematol. 2007;64(3):234–46. doi: 10.1016/j.critrevonc.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 5.Eichhorst B, Goede V, Hallek M. Treatment of elderly patients with chronic lymphocytic leukemia. Leuk Lymphoma. 2009;50(2):171–8. doi: 10.1080/10428190802688517. [DOI] [PubMed] [Google Scholar]
- 6.Delgado J, Briones J, Sierra J. Emerging therapies for patients with advanced chronic lymphocytic leukaemia. Blood Rev. 2009;23(5):217–24. doi: 10.1016/j.blre.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Kay NE, Rai KR, O’Brien S. Chronic lymphocytic leukemia: current and emerging treatment approaches. Clin Adv Hematol Oncol. 2006;4(11 Suppl 22):1–12. [PubMed] [Google Scholar]
- 8.Coiffier B, Lepretre S, Pedersen LM, Gadeberg O, Fredriksen H, van Oers MH, et al. Safety and efficacy of ofatumumab, a fully human monoclonal anti-CD20 antibody, in patients with relapsed or refractory B-cell chronic lymphocytic leukemia: a phase 1–2 study. Blood. 2008;111(3):1094–100. doi: 10.1182/blood-2007-09-111781. [DOI] [PubMed] [Google Scholar]
- 9.Byrd JC, Lin TS, Dalton JT, Wu D, Phelps MA, Fischer B, et al. Flavopiridol administered using a pharmacologically derived schedule is associated with marked clinical efficacy in refractory, genetically high-risk chronic lymphocytic leukemia. Blood. 2007;109(2):399–404. doi: 10.1182/blood-2006-05-020735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munk Pedersen I, Reed J. Microenvironmental interactions and survival of CLL B-cells. Leuk Lymphoma. 2004;45(12):2365–72. doi: 10.1080/10428190412331272703. [DOI] [PubMed] [Google Scholar]
- 11.Luqman M, Klabunde S, Lin K, Georgakis GV, Cherukuri A, Holash J, et al. The antileukemia activity of a human anti-CD40 antagonist antibody, HCD122, on human chronic lymphocytic leukemia cells. Blood. 2008;112(3):711–20. doi: 10.1182/blood-2007-04-084756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ranheim EA, Kipps TJ. Activated T cells induce expression of B7/BB1 on normal or leukemic B cells through a CD40-dependent signal. J Exp Med. 1993;177(4):925–35. doi: 10.1084/jem.177.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kitada S, Zapata JM, Andreeff M, Reed JC. Bryostatin and CD40-ligand enhance apoptosis resistance and induce expression of cell survival genes in B-cell chronic lymphocytic leukaemia. Br J Haematol. 1999;106(4):995–1004. doi: 10.1046/j.1365-2141.1999.01642.x. [DOI] [PubMed] [Google Scholar]
- 14.Kater AP, Evers LM, Remmerswaal EB, Jaspers A, Oosterwijk MF, van Lier RA, et al. CD40 stimulation of B-cell chronic lymphocytic leukaemia cells enhances the anti-apoptotic profile, but also Bid expression and cells remain susceptible to autologous cytotoxic T-lymphocyte attack. Br J Haematol. 2004;127(4):404–15. doi: 10.1111/j.1365-2141.2004.05225.x. [DOI] [PubMed] [Google Scholar]
- 15.Hayden RE, Pratt G, Davies NJ, Khanim FL, Birtwistle J, Delgado J, et al. Treatment of primary CLL cells with bezafibrate and medroxyprogesterone acetate induces apoptosis and represses the pro-proliferative signal of CD40-ligand, in part through increased 15dDelta12,14,PGJ2. Leukemia. 2009;23(2):292–304. doi: 10.1038/leu.2008.283. [DOI] [PubMed] [Google Scholar]
- 16.Vogler M, Butterworth M, Majid A, Walewska RJ, Sun XM, Dyer MJ, et al. Concurrent up-regulation of BCL-XL and BCL2A1 induces approximately 1000-fold resistance to ABT-737 in chronic lymphocytic leukemia. Blood. 2009;113(18):4403–13. doi: 10.1182/blood-2008-08-173310. [DOI] [PubMed] [Google Scholar]
- 17.Hallaert DY, Jaspers A, van Noesel CJ, van Oers MH, Kater AP, Eldering E. c-Abl kinase inhibitors overcome CD40-mediated drug resistance in CLL: implications for therapeutic targeting of chemoresistant niches. Blood. 2008;112(13):5141–9. doi: 10.1182/blood-2008-03-146704. [DOI] [PubMed] [Google Scholar]
- 18.Morabito F, Messina G, Oliva B, Ramirez F, Callea V, Brugiatelli M, et al. In vitro chemosensitivity of chronic lymphocytic leukemia B-cells to multidrug regimen (CEOP) compounds using the MTT colorimetric assay. Haematologica. 1993;78(4):213–8. [PubMed] [Google Scholar]
- 19.Gandhi V, Kemena A, Keating MJ, Plunkett W. Cellular pharmacology of fludarabine triphosphate in chronic lymphocytic leukemia cells during fludarabine therapy. Leuk Lymphoma. 1993;10(1–2):49–56. doi: 10.3109/10428199309147356. [DOI] [PubMed] [Google Scholar]
- 20.Danhauser L, Plunkett W, Keating M, Cabanillas F. 9-beta-D-arabinofuranosyl-2-fluoroadenine 5′-monophosphate pharmacokinetics in plasma and tumor cells of patients with relapsed leukemia and lymphoma. Cancer Chemother Pharmacol. 1986;18(2):145–52. doi: 10.1007/BF00262285. [DOI] [PubMed] [Google Scholar]
- 21.Khanim FL, Hayden RE, Birtwistle J, Lodi A, Tiziani S, Davies NJ, et al. Combined bezafibrate and medroxyprogesterone acetate: potential novel therapy for acute myeloid leukaemia. PLoS One. 2009;4(12):e8147. doi: 10.1371/journal.pone.0008147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fenton SL, Luong QT, Sarafeim A, Mustard KJ, Pound J, Desmond JC, et al. Fibrates and medroxyprogesterone acetate induce apoptosis of primary Burkitt’s lymphoma cells and cell lines: potential for applying old drugs to a new disease. Leukemia. 2003;17(3):568–75. doi: 10.1038/sj.leu.2402843. [DOI] [PubMed] [Google Scholar]
- 23.Murray JA, Khanim FL, Hayden RE, Craddock CF, Holyoake TL, Jackson N, et al. Combined bezafibrate and medroxyprogesterone acetate have efficacy without haematological toxicity in elderly and relapsed acute myeloid leukaemia (AML) Br J Haematol. 2010;149(1):65–9. doi: 10.1111/j.1365-2141.2009.08055.x. [DOI] [PubMed] [Google Scholar]
- 24.Evidente A, Kireev AS, Jenkins AR, Romero AE, Steelant WF, Van Slambrouck S, et al. Biological evaluation of structurally diverse Amaryllidaceae alkaloids and their synthetic derivatives: discovery of novel leads for anticancer drug design. Planta Med. 2009;75(5):501–7. doi: 10.1055/s-0029-1185340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hampson P, Chahal H, Khanim F, Hayden R, Mulder A, Assi LK, et al. PEP005, a selective small-molecule activator of protein kinase C, has potent antileukemic activity mediated via the delta isoform of PKC. Blood. 2005;106(4):1362–8. doi: 10.1182/blood-2004-10-4117. [DOI] [PubMed] [Google Scholar]
- 26.Flescher E. Jasmonates – a new family of anti-cancer agents. Anticancer Drugs. 2005;16(9):911–6. doi: 10.1097/01.cad.0000176501.63680.80. [DOI] [PubMed] [Google Scholar]
- 27.Flescher E. Jasmonates in cancer therapy. Cancer Lett. 2007;245(1–2):1–10. doi: 10.1016/j.canlet.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 28.Heyfets A, Flescher E. Cooperative cytotoxicity of methyl jasmonate with anticancer drugs and 2-deoxy-D-glucose. Cancer Lett. 2007;250(2):300–10. doi: 10.1016/j.canlet.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 29.Davies NJ, Hayden RE, Simpson PJ, Birtwistle J, Mayer K, Ride JP, et al. AKR1C isoforms represent a novel cellular target for jasmonates alongside their mitochondrial-mediated effects. Cancer Res. 2009;69 (11):4769–75. doi: 10.1158/0008-5472.CAN-08-4533. [DOI] [PubMed] [Google Scholar]
- 30.Liu J, Hu WX, He LF, Ye M, Li Y. Effects of lycorine on HL-60 cells via arresting cell cycle and inducing apoptosis. FEBS Lett. 2004;578(3):245–50. doi: 10.1016/j.febslet.2004.10.095. [DOI] [PubMed] [Google Scholar]
- 31.Liu J, Li Y, Tang LJ, Zhang GP, Hu WX. Treatment of lycorine on SCID mice model with human APL cells. Biomed Pharmacother. 2007;61(4):229–34. doi: 10.1016/j.biopha.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu XS, Jiang J, Jiao XY, Wu YE, Lin JH, Cai YM. Lycorine induces apoptosis and down-regulation of Mcl-1 in human leukemia cells. Cancer Lett. 2009;274(1):16–24. doi: 10.1016/j.canlet.2008.08.029. [DOI] [PubMed] [Google Scholar]
- 33.Lin MS, Chen WC, Bai X, Wang YD. Activation of peroxisome proliferator-activated receptor γ inhibits cell growth via apoptosis and arrest of the cell cycle in human colorectal cancer. J Dig Dis. 2007;8(2):82–8. doi: 10.1111/j.1443-9573.2007.00290.x. [DOI] [PubMed] [Google Scholar]
- 34.Hwang YC, Chu JJ, Yang PL, Chen W, Yates MV. Rapid identification of inhibitors that interfere with poliovirus replication using a cell-based assay. Antiviral Res. 2008;77 (3):232–6. doi: 10.1016/j.antiviral.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deng L, Dai P, Ciro A, Smee DF, Djaballah H, Shuman S. Identification of novel antipoxviral agents: mitoxantrone inhibits vaccinia virus replication by blocking virion assembly. J Virol. 2007;81(24):13392–402. doi: 10.1128/JVI.00770-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li SY, Chen C, Zhang HQ, Guo HY, Wang H, Wang L, et al. Identification of natural compounds with antiviral activities against SARS-associated coronavirus. Antiviral Res. 2005;67(1):18–23. doi: 10.1016/j.antiviral.2005.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mackey ZB, Baca AM, Mallari JP, Apsel B, Shelat A, Hansell EJ, et al. Discovery of trypanocidal compounds by whole cell HTS of Trypanosoma brucei. Chem Biol Drug Des. 2006;67(5):355–63. doi: 10.1111/j.1747-0285.2006.00389.x. [DOI] [PubMed] [Google Scholar]
- 38.Sener B, Orhan I, Satayavivad J. Antimalarial activity screening of some alkaloids and the plant extracts from Amaryllidaceae. Phytother Res. 2003;17(10):1220–3. doi: 10.1002/ptr.1346. [DOI] [PubMed] [Google Scholar]
- 39.Lamoral-Theys D, Decaestecker C, Mathieu V, Dubois J, Kornienko A, Kiss R, et al. Lycorine and its derivatives for anticancer drug design. Mini Rev Med Chem. 2010;10(1):41–50. doi: 10.2174/138955710791112604. [DOI] [PubMed] [Google Scholar]
- 40.Jones MT, Schwartz BD, Willis AC, Banwell MG. Rapid and enantioselective assembly of the lycorine framework using chemoenzymatic techniques. Org Lett. 2009;11(15):3506–9. doi: 10.1021/ol901364n. [DOI] [PubMed] [Google Scholar]
- 41.El Tahchy A, Boisbrun M, Ptak A, Dupire F, Chretien F, Henry M, et al. New method for the study of Amaryllidaceae alkaloid biosynthesis using biotransformation of deuterium-labeled precursor in tissue cultures. Acta Biochim Pol. 2010;57(1):75–82. [PubMed] [Google Scholar]
- 42.McNulty J, Nair JJ, Bastida J, Pandey S, Griffin C. Structure-activity studies on the lycorine pharmacophore: a potent inducer of apoptosis in human leukemia cells. Phytochemistry. 2009;70(7):913–9. doi: 10.1016/j.phytochem.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oscier D, Fegan C, Hillmen P, Illidge T, Johnson S, Maguire P, et al. Guidelines on the diagnosis and management of chronic lymphocytic leukaemia. Br J Haematol. 2004;125(3):294–317. doi: 10.1111/j.1365-2141.2004.04898.x. [DOI] [PubMed] [Google Scholar]
- 44.Gagro A, McCloskey N, Challa A, Holder M, Grafton G, Pound JD, et al. CD5-positive and CD5-negative human B cells con-verge to an indistinguishable population on signalling through B-cell receptors and CD40. Immunology. 2000;101(2):201–9. doi: 10.1046/j.1365-2567.2000.00098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li Y, Liu J, Tang LJ, Shi YW, Ren W, Hu WX. Apoptosis induced by lycorine in KM3 cells is associated with the G0/G1 cell cycle arrest. Oncol Rep. 2007;17(2):377–84. [PubMed] [Google Scholar]
- 46.Warner BB, Stuart L, Gebb S, Wispe JR. Redox regulation of manganese superoxide dismutase. Am J Physiol. 1996;271(1 Pt 1):L150–8. doi: 10.1152/ajplung.1996.271.1.L150. [DOI] [PubMed] [Google Scholar]
- 47.Hartmann TN, Grabovsky V, Wang W, Desch P, Rubenzer G, Wollner S, et al. Circulating B-cell chronic lymphocytic leukemia cells display impaired migration to lymph nodes and bone marrow. Cancer Res. 2009;69(7):3121–30. doi: 10.1158/0008-5472.CAN-08-4136. [DOI] [PubMed] [Google Scholar]
- 48.Veldurthy A, Patz M, Hagist S, Pallasch CP, Wendtner CM, Hallek M, et al. The kinase inhibitor dasatinib induces apoptosis in chronic lymphocytic leukemia cells in vitro with preference for a subgroup of patients with unmutated IgVH genes. Blood. 2008;112(4):1443–52. doi: 10.1182/blood-2007-11-123984. [DOI] [PubMed] [Google Scholar]
- 49.Amrein L, Hernandez TA, Ferrario C, Johnston J, Gibson SB, Panasci L, et al. Dasatinib sensitizes primary chronic lymphocytic leukaemia lymphocytes to chlorambucil and fludarabine in vitro. Br J Haematol. 2008;143(5):698–706. doi: 10.1111/j.1365-2141.2008.07418.x. [DOI] [PubMed] [Google Scholar]
- 50.Davey MW, Persiau G, De Bruyn A, Van Damme J, Bauw G, Van Montagu M. Purification of the alkaloid lycorine and simultaneous analysis of ascorbic acid and lycorine by micellar electrokinetic capillary chromatography. Anal Biochem. 1998;257(1):80–8. doi: 10.1006/abio.1997.2544. [DOI] [PubMed] [Google Scholar]
- 51.Houghton PJ, Ren Y, Howes MJ. Acetylcholinesterase inhibitors from plants and fungi. Nat Prod Rep. 2006;23(2):181–99. doi: 10.1039/b508966m. [DOI] [PubMed] [Google Scholar]