Abstract
It is beneficial to seek scientific basis for the effects of functional foods. Natural pigments derived from plants are widely known as possible antioxidants. Black soybean contains a larger amount of anthocyanins than regular soybean. Here we studied the antioxidative effect of a beverage obtained via citric acid fermentation of black soybean (BBS), using a rat model of renal oxidative injury induced by a renal carcinogen, ferric nitrilotriacetate. BBS (10 ml/kg) was orally administered 30 min before ferric nitrilotriacetate treatment. Renal lipid peroxidation was significantly suppressed in the BBS-pretreated animals concomitant with decrease in 4-hydroxy-2-nonenal-modified proteins and 8-hydroxy-2'-deoxyguanosine. Maintenance of renal activities of antioxidative enzymes including catalase, glutathione peroxidase, glutathione reductase, glutathione S-transferase, glucose-6-phosphate dehydrogenase and quinone reductase was significantly better in the BBS-pretreated rats. Elevation of serum creatinine and urea nitrogen was significantly suppressed in the BBS-pretreated rats. These data suggest that dietary intake of BBS is useful for the prevention of renal tubular oxidative damage mediate by iron, and warrant further investigation.
Keywords: black soybean, anthocyanin, ferric nitrilotriacetate, oxidative stress, lipid peroxidation
Introduction
Life style-related pathologic conditions, such as obesity, diabetes mellitus, hypertension and hyperlipidemia, are causatively associated with oxidative stress [1]. Thus, consuming antioxidative food in the daily life may decrease the burden of oxidative stress and may be beneficial for the promotion of health [2]. Recently, many kinds of functional foods claiming their supportive role are already in the market. However, only small fraction of those has been evaluated with established methods. It would be useful to provide evidence that antioxidative food is really functional with a scientific approach.
Among the functional foods, black soybeans are one of the most popular beans in Japan. Black soybean contains higher levels of anthocyanins than regular soybean [3]. Anthocyanins are water-soluble pigments that are found in many plants, including black soybean. Dietary intake of anthocyanins is estimated to be 180 to 225 mg/day in the United States [4], and has an antioxidant effect and prescribed as medicine in many countries [5]. The major anthocyanin in black soybean is cyanidin-3-O-β-glucoside (C3G). C3G shows well-known red color below pH 4, when it forms most stable flavylium cation [6]. C3G is reactive towards reactive oxygen species (ROS) since its structure is able to donate electrons or transfer hydrogen atoms from hydroxyl moieties to free radicals [7].
Traditional fermented foods have been recognized as healthy food, and attracted much attention because they are related to longevity in Japan [8]. During fermentation, ingredients may be converted to well-balanced forms, which are beneficial for animals [9]. Thus, we may expect synergistic effect of antioxidative potential in the combination of anthocyanins and fermentation. We focused on citric acid fermentation beverage of black soybean (BBS) and investigated its antioxidative effect. BBS also contains other antioxidants that are yet to be elucidated.
To study the antioxidative effect of BBS in vivo, we used an animal model of oxidative renal tubular damage induced by ferric nitrilotriacetate (Fe-NTA) [10]. Nitrilotriacetate (NTA) is an aminotricarboxylic acid that efficiently forms water-soluble chelate complexes with many metal cations at neutral pH [11]. This iron chelate catalyzes the generation of ROS and accelerates lipid peroxidation in the kidney through the intraperitoneal (i.p.) administration in rats and mice [12, 13]. Repeated i.p. administration of Fe-NTA produces acute and sub-acute renal proximal tubular oxidative damage and remodeling of tubular structure that finally lead to a high incidence of renal cell carcinoma [14–16]. At the acute phase, oxidatively modified molecules are increased in the kidney after Fe-NTA treatment. For example, an increase in malondialdehyde [17], thiobarbituric acid-reactive substances (TBARS), oxidized glutathione [18], 4-hydroxy-2-nonenal (HNE)-modified proteins [19–21], 8-hydroxy-2'-deoxyguanosine (8-OHdG) [22, 23] and prostaglandin F2α [24] was shown. Regarding genetic alternations of this carcinogenetic model, homogygous deletion of p16INK4A tumor suppressor gene that is an inhibitor of cyclin-dependent kinase was a frequent observation [25] with its monoallelic loss occurring as early as 3 weeks after the start of the protocol [26]. The other target genes in carcinogenesis and progression include annexin2 [27], ptprz1 [28] and aminoacylase1 [29]. Although the potent action of hepatic tumor promotion was also reported [30], Fe-NTA administration alone does not induce primary hepatic tumor.
Well-known antioxidants such as Vitamin E [31], curcumin [32], probucol [33], lycopene [34], nordihydroguairetic acid, garlic oil [35], propolis (artepillin C) [36] and colored rice [37] have been reported to show protective effect in this carcinogenesis model. Based on these studies, this experimental model is useful for the evaluation of antioxidants in vivo. In the present study, we show for the first time that beverage containing fermented black soybean has advantageous effects on oxidative stress-induced renal injury in vivo. Possible mechanisms and its implication will be discussed.
Materials and Methods
Chemicals
Iron nitrate enneahydrate, nitrilotriacetic acid, oxidized and reduced glutathione, glutathione reductase, hydrogen peroxide were purchased from Wako (Osaka, Japan). Nicotinamide adenine dinucleotide phosphate reduced, glucose-6-phosphate, 1-chloro-2,4-dinitrobenzene (CDNB), 2,6-dichloroindophenol sodium salt hydrate (DCIP), 5,5'-dithio-bis-2-nitrobenzoic acid (DTNB), bovine serum albumin, trichloroacetic acid, tween 20 were from Sigma (St. Louis, MO). 2-Thiobarbituric acid was from Merck (Darmstadt, Germany). BCA assay kit was from Pierce (Rockford, IL). Citric acid fermentation beverage of black soybean (BBS; Gokoku-maroyaka-su) was a kind gift from Kimise Shoyu (Okayama, Japan). Normal goat serum was from Vector Laboratories (Burlingame, CA) and Histofine Simple Stain rat Max-PO (multi) was from Nichirei (Tokyo, Japan). Liquid 3-3'-diaminobenzidine (DAB) was from DAKO Cytomation (Kyoto, Japan). Two monoclonal antibodies against 8-OHdG (N45.1) [23] and HNE-modified proteins (HNE-J2) [38] were from Japan Institute for the Control of Aging (Shizuoka, Japan). All the other chemicals were of the highest quality available from Wako (Osaka, Japan).
Determination of nutrients, trace metals and C3G in BBS
Diet composition and trace metals were analyzed according to the standard procedure. Anthocyanidin was measured using HPLC by the method of Miyazawa et al. [39].
Preparation of Fe-NTA solution
The Fe-NTA solution was prepared by the method of Awai et al. [40]. In brief, nitrilotriacetic acid (NTA) and iron nitrate enneahydrate were dissolved in distilled water. The pH was adjusted to 7.0 with sodium bicarbonate. The molar ratio Fe to NTA was 1:4.
Animal experiments
The Animal Care Committee of Okayama University Graduate School of Medicine and Dentistry approved this experiment. Care and handling of the animals were in accordance with National Institutes of Health Guidelines. Male Wistar rats (7 week-old) were purchased from Japan SLC (Hamamatsu, Japan). They were housed in a temperature-controlled (25°C with alternating 12 h light/12 h dark cycles), and were allowed free access to distilled water and standard chow diet (MF; Oriental Yeast, Tokyo, Japan) during experiment. They were used for experiments after passing one week of acclimatization.
A total of 42 rats (190–210 g) were used for the following experiments. Each animal received 10 ml/kg of BBS using gastric tube, 30 min prior to the i.p. injection of 9.0 mg iron/kg body weight Fe-NTA. In a preliminary study, we prepared condensed BBS and performed a dose-dependence study, which determined the administration of BBS containing 130 µg/kg body weight of cyanidin (refer to Table 1) as the most effective dose for Fe-NTA-induced renal tubular damage. After sacrifice, the kidneys were immediately removed for enzyme assays and histological examination.
Table 1.
Constituents of citric acid fermentation beverage of black soybean
| Anthocyanidins | |
| Delphinidin | n.d. |
| Cyanidin | 6.5 µg/ml |
| Petunidin | n.d. |
| Pelargonidin | n.d. |
| Peonidin | n.d. |
| Malvidin | n.d. |
| Vitamins | |
| Ascorbic acid | n.d. |
| α-tocopherol | n.d. |
| Minerals and metals | |
| Phosphorus | 384 µg/ml |
| Iron | 1.2 µg/ml |
| Calcium | 30 µg/ml |
| Magnesium | 105 µg/ml |
| Copper | 0.2 µg/ml |
| Zinc | 0.05 µg/ml |
| Manganese | 0.04 µg/ml |
n.d.: not detected. Detection limit; ascorbic acid, 10 µg/ml; α-tocopherol, 1 µg/ml; anthocyanidins, 0.1 µg/ml.
Determination of TBA-reactive substances
Lipid peroxidation was measured via production of TBARS by the method of Hamazaki et al. [10] with slight modification described by Iqbal et al. [41].
Determination of creatinine and blood urea nitrogen (BUN)
Blood urea nitrogen and creatinine in sera were measured by auto-analyzer (Hitachi 7600-110S).
Determination of renal antioxidant enzyme activities
These enzymatic activities were assayed by post-mitochondrial supernatant of renal homogenate. Glutathione peroxidase activity was measured by the method of nicotinamide adenine dinucleotide phosphate, reduced (NADPH) oxidation in a coupled system [42]. Glutathione reductase activity was measured by the method of NADPH oxidation [43]. Glutathione S-transferase activity toward CDNB as a substrate was measured by the method of Habig et al. [44]. Glucose-6-phosphate dehydrogenase activity was measured by the method of NADPH formation [45]. Catalase activity was measured by the method of H2O2 degradation [46]. Quinone reductase activity was measured by the method of Benson et al. [47]. Reduced glutathione toward DTNB as a substrate was measured by the method of Mohandas et al. [42].
Determination of NADPH
NADPH was measured by the method of Zhang et al. [48].
Hematoxylin and eosin (HE) staining
Kidneys were transversely cut including renal pelvis at 5 mm thickness, and immediately fixed with 10% phosphate-buffered formalin. The samples were fixed overnight, and subjected to paraffin embedding. The paraffin-embedded tissues were cut at 4 µm and mounted on glass slides. These slides were used for hematoxylin and eosin staining and immunohistochemical analyses.
Immunohistochemical analysis
Immunohistochemical analyses were performed as previously described [23, 49]. Immunostainings were quantified using NIH image 1.63 as described [23].
Statistical analysis
Statistical analyses were performed with one-way analysis of variance (ANOVA) and an unpaired t test. The difference was considered significance when p<0.05. In animal studies, data are presented as means ± standard deviation (N = 6–7) unless otherwise specified.
Results
Nutrients, trace metals and anthocyanidins in BBS
Nutrients and levels of trace metals and anthocyanidins in the BBS are summarized in Table 1. Only cyanidin was detectable among 6 forms of anthocyanidins. The levels of ascorbic acid and α-tocopherol were below the detection limit.
TBARS
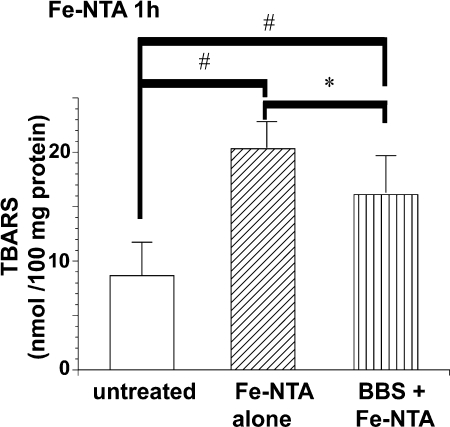
TBARS was elevated 1 h after Fe-NTA treatment in comparison with untreated animals. This elevation was significantly suppressed by BBS pretreatment (Fig. 1).
Fig. 1.
Renal TBA-reactive substances 1 h after intraperitoneal injection of ferric nitrilotriacetate (Fe-NTA). Prior intake of beverage containing fermented black soybean (BBS) decreased TBA-reactive substances. Refer to text for details (ANOVA, p = 0.0001; #p<0.05 vs untreated; *p<0.05 vs Fe-NTA alone).
Serum creatinine and BUN
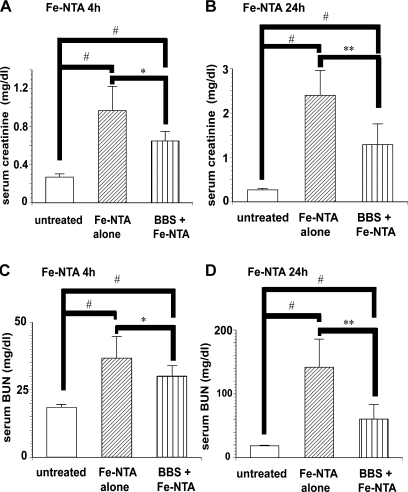
Four and 24 h after Fe-NTA administration, an elevation of creatinine and BUN in sera was evident. Pretreatment with BBS suppressed the elevations of these parameters at both time points. Essentially the same effect was observed by the data of creatinine and BUN (Fig. 2 A–D).
Fig. 2.
Serum markers for renal dysfunction 4 h and 24 h after Fe-NTA administration. (A) Serum creatinine: Fe-NTA 4 h, (B) serum creatinine: Fe-NTA 24 h, (C) serum BUN: Fe-NTA 4 h, (D) serum BUN: Fe-NTA 24 h. Protective effect of BBS was observed (ANOVA, p<0.0001 for A–D; #p<0.05 vs untreated; *p<0.05 and **p<0.01 vs Fe-NTA alone).
Antioxidative enzyme activity
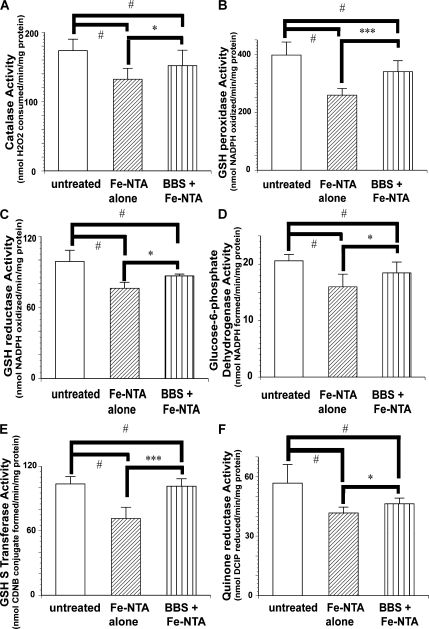
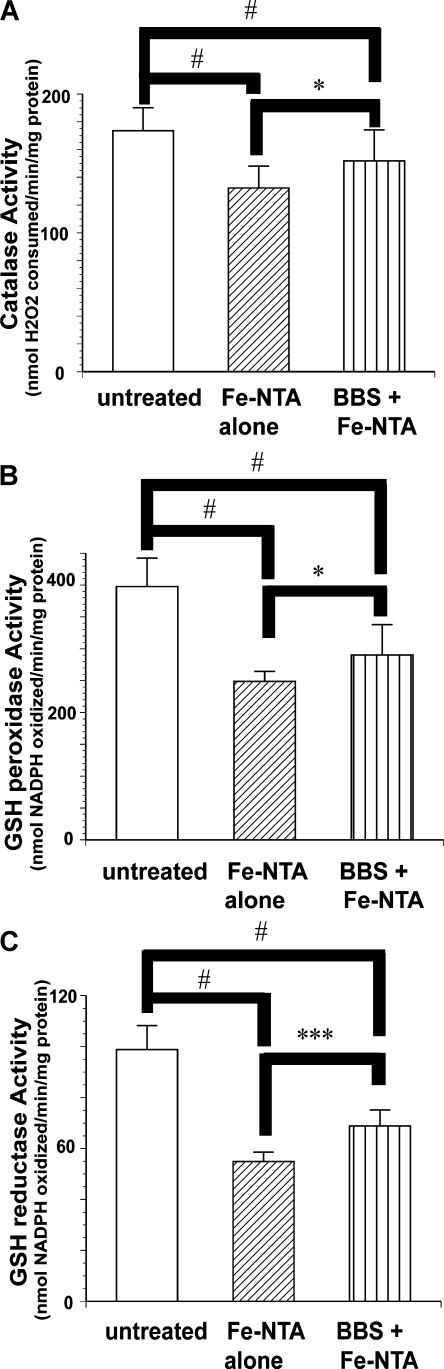
Activities of all the antioxidative enzymes were decreased 4 h after Fe-NTA treatment, and BBS pretreatment prevented this decrease (Fig. 3). The same tendency was observed in many of the representative enzymes 24 h after Fe-NTA treatment (Fig. 4).
Fig. 3.
Activities of renal antioxidative enzymes 4 h after Fe-NTA administration. (A) Catalase, (B) GSH peroxidase, (C) GSH reductase, (D) Glucose 6-phosphate dehydorgenase, (E) GSH S-transferase, (F) quinone reductase. Protective effects of BBS pretreatment on Fe-NTA-induced renal oxidative damage were observed in all the parameters examined (ANOVA, p = 0.0004, p<0.0001, p = 0.0012, p<0.0014, p<0.0001 and p = 0.0009 for A–F, respectively; #p<0.05 vs untreated; *p<0.05 and ***p<0.001 vs Fe-NTA alone).
Fig. 4.
Activities of renal antioxidative enzymes 24 h after Fe-NTA administration. Representative results of antioxidative enzymes are shown. (A) Catalase, (B) GSH Peroxidase, (C) GSH Reductase. Protective effects of BBS pretreatment on Fe-NTA-induced renal oxidative damage were observed (ANOVA, p<0.0001 for A–C; #p<0.05 vs untreated; *p<0.05 and ***p<0.001 vs Fe-NTA alone).
Determination of Reduced Glutathione (GSH) and NADPH
Renal reduced GSH showed remarkable depletion 1 h after Fe-NTA administration with or without BBS pretreatment (Untreated 6.90 ± 0.71; Fe-NTA-1h, ###1.99 ± 0.20; BBS + Fe-NTA-1h, ###2.02 ± 0.37; mmol/g tissue, N = 6–7, means ± SEM; ANOVA, p<0.0001, ###p<0.001 vs untreated). At 4 h, BBS pretreatment did not show protective effects against oxidative stress. However at 24 h, BBS pretreatment restored GSH level faster than Fe-NTA alone group (Fe-NTA-4h, ##3.69 ± 0.27, BBS + Fe-NTA-4h, ##3.63 ± 1.25; Fe-NTA-24h, ##3.99 ± 0.12; BBS + Fe-NTA-24h, *5.33 ± 0.20; mmol/g tissue, N = 6–7, means ± SEM; ANOVA, p = 0.017 and p = 0.0005 for 4 h and 24 h, respectively; *p<0.05 vs FeNTA alone, ##p<0.01 vs untreated). NADPH showed the same tendency as GSH (Untreated 10.24 ± 0.71; Fe-NTA-1h, ##4.54 ± 0.37; BBS + Fe-NTA-1h, ##4.95 ± 0.55; Fe-NTA-4h, ##5.03 ± 0.56; BBS + Fe-NTA-4h, ##5.12 ± 0.51, Fe-NTA-24h, ###3.75 ± 0.43; BBS + Fe-NTA-24h, ##4.82 ± 0.62; µmol/g tissue, N = 6–7, means ± SEM; ANOVA, p<0.0001, p<0.0001 and p<0.0001 for 1 h, 4 h and 24 h, respectively; ##p<0.01 and ###p<0.001 vs untreated).
Histology
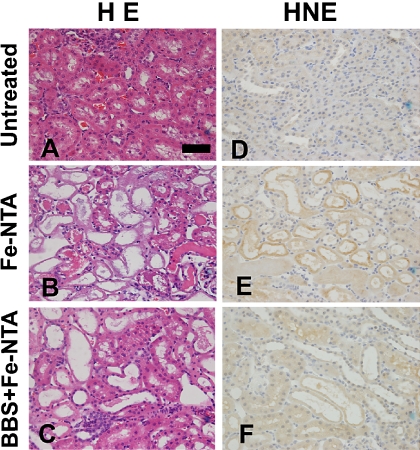
Representative renal histology of Fe-NTA-treated rats 4 h after Fe-NTA treatment is shown (Fig. 5B). Acute tubular necrosis was apparent in the proximal tubules. Pretreated rats with BBS 30 min before Fe-NTA administration were highly protected from tubular injury (Fig. 5C).
Fig. 5.
Histological and immunohistochemical analyses of kidney 4 h after Fe-NTA administration. Hematoxylin and eosin staining (HE). (A) untreated, (B) Fe-NTA, (C) BBS pretreatment 30 min before Fe-NTA (BBS + Fe-NTA). Representative images are shown. Scattered necrotic tubules are seen in (B). In (C), only a few degenerative tubules were observed. Immunohistichemical staining of 4-hydroxy-2-nonenal-modified proteins (HNE). (D) untreated, (E) Fe-NTA, (F) BBS + Fe-NTA. HNE immunostaining revealed accumulation of oxidatively modified proteins. Whereas no positive tubule in the HNE immunostaining was observed in (D), many tubules revealed positivity in (E). Immunopositivities were significantly decreased in (F) (bar, 50 µm).
In the cases of Fe-NTA-treated rats, degenerative proximal tubular cells revealing HNE-modified proteins were observed. However, the number of HNE-positive tubules was significantly decreased in the BBS-treated group. No positive tubules with apparent HNE-modified proteins were observed in the untreated group (Fig. 5 D–F).
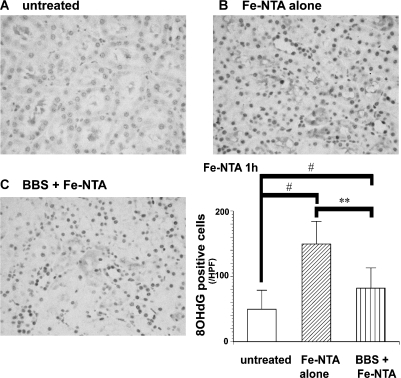
Increased 8-OHdG positive cells were observed in Fe-NTA-treated groups. However, BBS-pretreated group revealed suppression in the number of 8-OHdG positive renal tubular cells in each rat. This protective effect was statistically significant (Fig. 6).
Fig. 6.
Immunohistochemical evaluation of 8-hydroxy-2'-deoxyguanosine (8-OHdG) in the kidney 1 h after Fe-NTA administration. Representative images in each group are shown; (A) untreated, (B) Fe-NTA, (C) BBS + Fe-NTA. Fe-NTA treatment induced intense nuclear staining in the proximal tubular cells. 8-OHdG immunostainings were quantified with NIH image 1.63. Significant decrease in the number of positive cells was observed in BBS-pretreated rats (ANOVA, p = 0.0001; #p<0.05 vs untreated; **p<0.01 and vs Fe-NTA alone).
Discussion
BBS aimed to make the best of antioxidant effect of anthocyanins. The analysis of digested ingredients demonstrated that cyanidin was the major source among 6 forms of anthocyanidins, and ascorbic acid and α-tocopherol were under detection limit. These results suggest that major antioxidants in BBS were cyanidin and its glycosylated derivatives. The distribution of C3G in kidney is reported to be the maximum 30 to 45 min after oral administration in animals [39, 50, 51]. We adopted this protocol after preliminary experiments.
Prior oral BBS administration to animals that were subjected to Fe-NTA treatment significantly reduced the formation of TBARS and 8-OHdG in the kidney. Serum markers for renal dysfunction showed a marked decrease with BBS pretreatment. Activities of renal antioxidative enzymes were well preserved after Fe-NTA treatment in the BBS pretreated rats. Thus, BBS played a role in scavenging radicals and protected tissue from oxidative damage. Our study for the first time demonstrated that extracts containing fermentated black soybean have a protective effect on iron-catalyzed oxidative tissue injury.
There are four possible explanations for the antioxidative effects of anthocyanins; namely, direct and indirect antioxidative effects, effects on iron absorption/transportation, and nutritional factor. In our preliminary experiments, the antioxidative effect of BBS was not proportionally dose-dependent. We found that most effective dosage of BBS as oral administration was 10 ml/kg containing 130 µg/kg of cyanidin. This may be due to saturation effect under pharmacokinetic characteristics of cyanidin and other components. We speculate that the present effect of BBS was synergistic with other BBS ingredients because the dosage of cyanidin was very low compared with the dosage previously reported [37]. This synergistic antioxidative effect to modulate the above mentioned four possible mechanisms may have been partially induced by fermentation as reported for black soybean [52] and green tea [9]. Fermentation of black soybean enhanced not only the contents of aglycon and vitamin K2 but also the superoxide dismutase-like activity [52], thus altering the ingredients to well-balanced functional forms for animals.
Our results demonstrated the functional role of BBS in vivo. However, we have to be careful about the general use of BBS since the present study was focused on the iron-catalyzed oxidative renal tubular injury in rats. To investigate the functional role in humans, we also performed epidemiological studies with the approval of Human Investigation Ethics Committee of Okayama University Graduate School of Medicine and Dentistry. We measured antioxidant parameters such as coenzyme Q10 [53], uric acid, polyunsaturated fatty acid, total free fatty acid [54] and urinary excretion of 8-OHdG [55, 56]. Only uric acid and monoenoic acid, which are thought as innate antioxidative molecules, showed a trend for increase but within the normal reference value after feeding of 50 ml BBS (Gokoku-maroyaka-su) for 4 weeks by the use of 45 volunteers. BBS may protect its consumption of uric acid and unsaturated fatty acid. There were no significant effects on coenzyme Q10, cholesterol, triacylglycerol, HDL cholesterol, and body weight. During the period of this trial, 6 persons dropped off. Most frequent reason was mild diarrhea. One person complained of allergic dermatitis. After quitting the drink, her redness resolved immediately. There were no persistent complaints and symptoms in all the volunteers. The details will be published elsewhere. Further studies would be necessary to identify which component is important for the antioxidative effect and to clarify the beneficial effects to prevent life style-related diseases.
In conclusion, we for the first time observed that citric acid fermentation beverage of black soybean had a protective effect against Fenton reaction-based renal tubular injury model in rats. Further study is warranted to find human pathologic conditions or genotypes this kind of chemoprevention is most useful.
Acknowledgments
This work was supported by the grants from Bioactive Okayama.
Abbreviations
- ANOVA
analysis of variance
- BBS
citric acid fermentation beverage of black soybean
- BUN
blood urea nitrogen
- CDNB
1-chloro-2,4-dinitrobenzene
- C3G
cyanidin-3-O-β-glucoside
- DAB
3-3'-diaminobenzidine
- DCIP
2,6-dichloroindophenol
- DTNB
5,5'-dithio-bis-2-nitrobenzoic acid
- Fe-NTA
ferric nitilotriacetate
- GSH
glutathione, reduced form
- HNE
4-hydroxy-2-nonenal
- NADPH
nicotinamide adenine dinucleotide phosphate, reduced
- 8-OHdG
8-hydroxy-2'-deoxyguanosine
- TBARS
thiobarbituric acid-reactive substances
References
- 1.Arai S., Osawa T., Ohigashi H., Yoshikawa M., Kaminogawa S., Watanabe M., Ogawa T., Okubo K., Watanabe S., Nishino H., Shinohara K., Esashi T., Hirahara T. A mainstay of functional food science in Japan--history, present status, and future outlook. Biosci. Biotechnol. Biochem. 2001;65:1–13. doi: 10.1271/bbb.65.1. [DOI] [PubMed] [Google Scholar]
- 2.Diplock A.T., Charleux J.L., Crozier-Willi G., Kok F.J., Rice-Evans C., Roberfroid M., Stahl W., Vina-Ribes J. Functional food science and defence against reactive oxidative species. Br. J. Nutr. 1998;80 Suppl 1:S77–112. doi: 10.1079/bjn19980106. [DOI] [PubMed] [Google Scholar]
- 3.Choung M.G., Baek I.Y., Kang S.T., Han W.Y., Shin D.C., Moon H.P., Kang K.H. Isolation and determination of anthocyanins in seed coats of black soybean (Glycine max (L.) Merr.) J. Agric. Food Chem. 2001;49:5848–5851. doi: 10.1021/jf010550w. [DOI] [PubMed] [Google Scholar]
- 4.Kuhnau J. The flavonoids. A class of semi-essential food components: their role in human nutrition. World Rev. Nutr. Diet. 1976;24:117–191. [PubMed] [Google Scholar]
- 5.Galvano F., La Fauci L., Lazzarino G., Fogliano V., Ritieni A., Ciappellano S., Battistini N.C., Tavazzi B., Galvano G. Cyanidins: metabolism and biological properties. J. Nutr. Biochem. 2004;15:2–11. doi: 10.1016/j.jnutbio.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen I.L., Haren G.R., Magnussen E.L., Dragsted L.O., Rasmussen S.E. Quantification of anthocyanins in commercial black currant juices by simple high-performance liquid chromatography. Investigation of their pH stability and antioxidative potency. J. Agric. Food Chem. 2003;51:5861–5866. doi: 10.1021/jf034004+. [DOI] [PubMed] [Google Scholar]
- 7.Tsuda T., Horio F., Osawa T. Dietary cyanidin 3-O-beta-D-glucoside increases ex vivo oxidation resistance of serum in rats. Lipids. 1998;33:583–588. doi: 10.1007/s11745-998-0243-5. [DOI] [PubMed] [Google Scholar]
- 8.Murooka Y., Yamshita M. Traditional healthful fermented products of Japan. J. Ind. Microbiol. Biotechnol. 2008;35:791–798. doi: 10.1007/s10295-008-0362-5. [DOI] [PubMed] [Google Scholar]
- 9.Nakamoto K., Takayama F., Mankura M., Hidaka Y., Egashira T., Ogino T., Kawasaki H., Mori A. Beneficial effects of fermented green tea extract in a rat model of non-alcoholic steatohepatitis. J. Clin. Biochem. Nutr. 2009;44:239–246. doi: 10.3164/jcbn.08-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamazaki S., Okada S., Ebina Y., Midorikawa O. Acute renal failure and glucosuria induced by ferric nitrilotriacetate in rats. Toxicol. Appl. Pharmacol. 1985;77:267–274. doi: 10.1016/0041-008x(85)90326-6. [DOI] [PubMed] [Google Scholar]
- 11.Anderson R.L., Bishop W.E., Campbell R.L. A review of the environmental and mammalian toxicology of nitrilotriacetic acid. Crit. Rev. Toxicol. 1985;15:1–102. doi: 10.3109/10408448509023766. [DOI] [PubMed] [Google Scholar]
- 12.Hamazaki S., Okada S., Ebina Y., Li J.L., Midorikawa O. Effect of dietary vitamin E on ferric nitrilotriacetate-induced nephrotoxicity in rats. Toxicol. Appl. Pharmacol. 1988;92:500–506. doi: 10.1016/0041-008x(88)90190-1. [DOI] [PubMed] [Google Scholar]
- 13.Toyokuni S., Okada S., Hamazaki S., Minamiyama Y., Yamada Y., Liang P., Fukunaga Y., Midorikawa O. Combined histochemical and biochemical analysis of sex hormone dependence of ferric nitrilotriacetate-induced renal lipid peroxidation in ddY mice. Cancer Res. 1990;50:5574–5580. [PubMed] [Google Scholar]
- 14.Okada S., Midorikawa O. Induction of rat renal adenocarcinoma by Fe-nitrilotriacetate (Fe-NTA) Jpn. Arch. Intern. Med. 1982;29:485–491. [Google Scholar]
- 15.Ebina Y., Okada S., Hamazaki S., Ogino F., Li J.L., Midorikawa O. Nephrotoxicity and renal cell carcinoma after use of iron- and aluminum-nitrilotriacetate complexes in rats. J. Natl. Cancer Inst. 1986;76:107–113. [PubMed] [Google Scholar]
- 16.Li J.L., Okada S., Hamazaki S., Ebina Y., Midorikawa O. Subacute nephrotoxicity and induction of renal cell carcinoma in mice treated with ferric nitrilotriacetate. Cancer Res. 1987;47:1867–1869. [PubMed] [Google Scholar]
- 17.Uchida K., Fukuda A., Kawakishi S., Hiai H., Toyokuni S. A renal carcinogen ferric nitrilotriacetate mediates a temporary accumulation of aldehyde-modified proteins within cytosolic compartment of rat kidney. Arch. Biochem. Biophys. 1995;317:405–411. doi: 10.1006/abbi.1995.1181. [DOI] [PubMed] [Google Scholar]
- 18.Okada S. Iron-induced tissue damage and cancer: the role of reactive oxygen free radicals. Pathol. Int. 1996;46:311–332. doi: 10.1111/j.1440-1827.1996.tb03617.x. [DOI] [PubMed] [Google Scholar]
- 19.Toyokuni S., Uchida K., Okamoto K., Hattori-Nakakuki Y., Hiai H., Stadtman E.R. Formation of 4-hydroxy-2-nonenal-modified proteins in the renal proximal tubules of rats treated with a renal carcinogen, ferric nitrilotriacetate. Proc. Natl. Acad. Sci. U.S.A. 1994;91:2616–2620. doi: 10.1073/pnas.91.7.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toyokuni S., Luo X.P., Tanaka T., Uchida K., Hiai H., Lehotay D.C. Induction of a wide range of C2-12 aldehydes and C7-12 acyloins in the kidney of Wistar rats after treatment with a renal carcinogen, ferric nitrilotriacetate. Free Radic. Biol. Med. 1997;22:1019–1027. doi: 10.1016/s0891-5849(96)00489-3. [DOI] [PubMed] [Google Scholar]
- 21.Iqbal M., Giri U., Giri D.K., Alam M.S., Athar M. Age-dependent renal accumulation of 4-hydroxy-2-nonenal (HNE)-modified proteins following parenteral administration of ferric nitrilotriacetate commensurate with its differential toxicity: implications for the involvement of HNE-protein adducts in oxidative stress and carcinogenesis. Arch. Biochem. Biophys. 1999;365:101–112. doi: 10.1006/abbi.1999.1135. [DOI] [PubMed] [Google Scholar]
- 22.Toyokuni S., Mori T., Dizdaroglu M. DNA base modifications in renal chromatin of Wistar rats treated with a renal carcinogen, ferric nitrilotriacetate. Int. J. Cancer. 1994;57:123–128. doi: 10.1002/ijc.2910570122. [DOI] [PubMed] [Google Scholar]
- 23.Toyokuni S., Tanaka T., Hattori Y., Nishiyama Y., Ochi H., Hiai H., Uchida K., Osawa T. Quantitative immunohistochemical determination of 8-hydroxy-2'-deoxyguanosine by a monoclonal antibody N45.1: its application to ferric nitrilotriacetate-induced renal carcinogenesis model. Lab. Invest. 1997;76:365–374. [PubMed] [Google Scholar]
- 24.Iqbal M., Giri U., Giri D.K., Athar M. Evidence that Fe-NTA-induced renal prostaglandin F2α is responsible for hyperplastic response in kidney: implications for the role of cyclooxygenase-dependent arachidonic acid metabolism in renal tumor promotion. Biochem. Mol. Biol. Int. 1997;42:1115–1124. doi: 10.1080/15216549700203581. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka T., Iwasa Y., Kondo S., Hiai H., Toyokuni S. High incidence of allelic loss on chromosome 5 and inactivation of p15INK4B and p16INK4A tumor suppressor genes in oxystress-induced renal cell carcinoma of rats. Oncogene. 1999;18:3793–3797. doi: 10.1038/sj.onc.1202707. [DOI] [PubMed] [Google Scholar]
- 26.Hiroyasu M., Ozeki M., Kohda H., Echizenya M., Tanaka T., Hiai H., Toyokuni S. Specific allelic loss of p16INK4A tumor suppressor gene after weeks of iron-mediated oxidative damage during rat renal carcinogenesis. Am. J. Pathol. 2002;160:419–424. doi: 10.1016/S0002-9440(10)64860-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanaka T., Akatsuka S., Ozeki M., Shirase T., Hiai H., Toyokuni S. Redox regulation of annexin 2 and its implications for oxidative stess-induced renal carcinogenesis and metastasis. Oncogene. 2004;23:3980–3989. doi: 10.1038/sj.onc.1207555. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y.-T., Shang D.-G., Akatsuka S., Ohara H., Dutta K.K., Mizushima K., Naito Y., Yoshikawa T., Izumiya M., Abe K., Nakagama H., Noguchi N., Toyokuni S. Chronic oxidative stress causes amplification and overexpresson of ptprz1 protein tyrosine phosphatase to activate β-catenin pathway. Am. J. Pathol. 2007;171:1978–1988. doi: 10.2353/ajpath.2007.070741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhong Y., Onuki J., Yamasaki T., Ogawa O., Akatsuka S., Toyokuni S. Genome-wide analysis identifies a tumor suppressor role for aminoacylase 1 in iron-induced rat renal cell carcinoma. Carcinogenesis. 2009;30:158–164. doi: 10.1093/carcin/bgn255. [DOI] [PubMed] [Google Scholar]
- 30.Iqbal M., Giri U., Athar M. Ferric nitrilotriacetate (Fe-NTA) is a potent hepatic tumor promoter and acts through the generation of oxidative stress. Biochem. Biophys. Res. Commun. 1995;212:557–563. doi: 10.1006/bbrc.1995.2006. [DOI] [PubMed] [Google Scholar]
- 31.Zhang D., Okada S., Yu Y., Zheng P., Yamaguchi R., Kasai H. Vitamin E inhibits apoptosis, DNA modification, and cancer incidence induced by iron-mediated peroxidation in Wistar rat kidney. Cancer Res. 1997;57:2410–2414. [PubMed] [Google Scholar]
- 32.Okazaki Y., Iqbal M., Okada S. Suppressive effects of dietary curcumin on the increased activity of renal ornithine decarboxylase in mice treated with a renal carcinogen, ferric nitrilotriacetate. Biochim. Biophys. Acta. 2005;1740:357–366. doi: 10.1016/j.bbadis.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 33.Qin X., Zhang S., Zarkovic M., Yamazaki Y., Oda H., Nakatsuru Y., Ishikawa T. Inhibitory effect of probucol on nephrotoxicity induced by ferric nitrilotriacetate (Fe-NTA) in rats. Carcinogenesis. 1995;16:2549–2552. doi: 10.1093/carcin/16.10.2549. [DOI] [PubMed] [Google Scholar]
- 34.Matos H.R., Capelozzi V.L., Gomes O.F., Mascio P.D., Medeiros M.H. Lycopene inhibits DNA damage and liver necrosis in rats treated with ferric nitrilotriacetate. Arch. Biochem. Biophys. 2001;396:171–177. doi: 10.1006/abbi.2001.2611. [DOI] [PubMed] [Google Scholar]
- 35.Iqbal M., Athar M. Attenuation of iron-nitrilotriacetate (Fe-NTA)-mediated renal oxidative stress, toxicity and hyperproliferative response by the prophylactic treatment of rats with garlic oil. Food Chem. Toxicol. 1998;36:485–495. doi: 10.1016/s0278-6915(98)00008-8. [DOI] [PubMed] [Google Scholar]
- 36.Kimoto T., Koya S., Hino K., Yamamoto Y., Nomura Y., Micallef M.J., Hanaya T., Arai S., Ikeda M., Kurimoto M. Renal carcinogenesis induced by ferric nitrilotriacetate in mice, and protection from it by Brazilian propolis and artepillin C. Pathol. Int. 2000;50:679–689. doi: 10.1046/j.1440-1827.2000.01097.x. [DOI] [PubMed] [Google Scholar]
- 37.Toyokuni S., Itani T., Morimitsu Y., Okada K., Ozeki M., Kondo S., Uchida K., Osawa T., Hiai H., Tashiro T. Protective effect of colored rice over white rice on Fenton reaction-based renal lipid peroxidation in rats. Free Radic. Res. 2002;36:583–592. doi: 10.1080/10715760290025960. [DOI] [PubMed] [Google Scholar]
- 38.Toyokuni S., Miyake N., Hiai H., Hagiwara M., Kawakishi S., Osawa T., Uchida K. The monoclonal antibody specific for the 4-hydroxy-2-nonenal histidine adduct. FEBS Lett. 1995;359:189–191. doi: 10.1016/0014-5793(95)00033-6. [DOI] [PubMed] [Google Scholar]
- 39.Miyazawa T., Nakagawa K., Kudo M., Muraishi K., Someya K. Direct intestinal absorption of red fruit anthocyanins, cyanidin-3-glucoside and cyanidin-3,5-diglucoside, into rats and humans. J. Agric. Food Chem. 1999;47:1083–1091. doi: 10.1021/jf9809582. [DOI] [PubMed] [Google Scholar]
- 40.Awai M., Narasaki M., Yamanoi Y., Seno S. Induction of diabetes in animals by parenteral administration of ferric nitrilotriacetate. A model of experimental hemochromatosis. Am. J. Pathol. 1979;95:663–673. [PMC free article] [PubMed] [Google Scholar]
- 41.Iqbal M., Okazaki Y., Sharma S.D., Okada S. Nitroglycerin, a nitric oxide generator attenuates ferric nitrilotriacetate-induced renal oxidative stress, hyperproliferative response and necrosis in ddY mice. Biochim. Biophys. Acta. 2003;1623:98–108. doi: 10.1016/j.bbagen.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 42.Mohandas J., Marshall J.J., Duggin G.G., Horvath J.S., Tiller D.J. Low activities of glutathione-related enzymes as factors in the genesis of urinary bladder cancer. Cancer Res. 1984;44:5086–5091. [PubMed] [Google Scholar]
- 43.Carlberg I., Mannervik B. Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J. Biol. Chem. 1975;250:5475–5480. [PubMed] [Google Scholar]
- 44.Habig W.H., Pabst M.J., Jakoby W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol.Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 45.Kletsova V.L., Kiriukhin Y.M., Chistoserdov Y.A., Tsygankov D.Y. Glucose 6-phosphate dehyderogenase and 6-phosphogluconate dehydrogenase from Methylobacillus flagellatum. Methods Enzymol. 1990;188:335–345. [Google Scholar]
- 46.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 47.Benson A.M., Hunkeler M.J., Talaley P. Increase of NAD(P)H:quinone reductase by dietary antioxidants: possible role in protection against carcinogenesis and toxicity. Proc. Natl. Acad. Sci. U.S.A. 1980;77:5216–5220. doi: 10.1073/pnas.77.9.5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Z., Yu J., Stanton R.C. A method for determination of pyridine nucleotides using a single extract. Anal. Biochem. 2000;285:163–167. doi: 10.1006/abio.2000.4701. [DOI] [PubMed] [Google Scholar]
- 49.Tanaka T., Nishiyama Y., Okada K., Hirota K., Matsui M., Yodoi J., Hiai H., Toyokuni S. Induction and nuclear translocation of thioredoxin by oxidative damage in the mouse kidney: independence of tubular necrosis and sulfhydryl depletion. Lab. Invest. 1997;77:145–155. [PubMed] [Google Scholar]
- 50.Tsuda T., Horio F., Osawa T. Absorption and metabolism of cyanidin 3-O-beta-D-glucoside in rats. FEBS Lett. 1999;449:179–182. doi: 10.1016/s0014-5793(99)00407-x. [DOI] [PubMed] [Google Scholar]
- 51.Matsumoto H., Inaba H., Kishi M., Tominaga S., Hirayama M., Tsuda T. Orally administered delphinidin 3-rutinoside and cyanidin 3-rutinoside are directly absorbed in rats and humans and appear in the blood as the intact forms. J. Agric. Food Chem. 2001;49:1546–1551. doi: 10.1021/jf001246q. [DOI] [PubMed] [Google Scholar]
- 52.Wu C.H., Chou C.C. Enhancement of aglycone, vitamin K2 and superoxide dismutase activity of black soybean through fermentation with Bacillus subtilis BCRC 14715 at different temperatures. J. Agric. Food Chem. 2009;57:10695–10700. doi: 10.1021/jf902752t. [DOI] [PubMed] [Google Scholar]
- 53.Yamashita S., Yamamoto Y. Simultaneous detection of ubiquinol and ubiquinone in human plasma as a marker of oxidative stress. Anal. Biochem. 1997;250:66–73. doi: 10.1006/abio.1997.2187. [DOI] [PubMed] [Google Scholar]
- 54.Hara K., Yamashita S., Fujisawa A., Ishiwa S., Ogawa T., Yamamoto Y. Oxidative stress in newborn infants with and without asphyxia as measured by plasma antioxidants and free fatty acids. Biochem. Biophys. Res. Commun. 1999;257:244–248. doi: 10.1006/bbrc.1999.0436. [DOI] [PubMed] [Google Scholar]
- 55.Lunec J., Holloway K.A., Cooke M.S., Faux S., Griffiths H.R., Evans M.D. Urinary 8-oxo-2'-deoxyguanosine: redox regulation of DNA repair in vivo? Free Radic. Biol. Med. 2002;33:875–885. doi: 10.1016/s0891-5849(02)00882-1. [DOI] [PubMed] [Google Scholar]
- 56.Cooke M., Henderson P.T., Evans M.D. Sources of extracellular, oxidatively-modified DNA lesions: implications for their measurement in urine. J. Clin. Biochem. Nutr. 2009;45:255–270. doi: 10.3164/jcbn.SR09-41. [DOI] [PMC free article] [PubMed] [Google Scholar]