Abstract
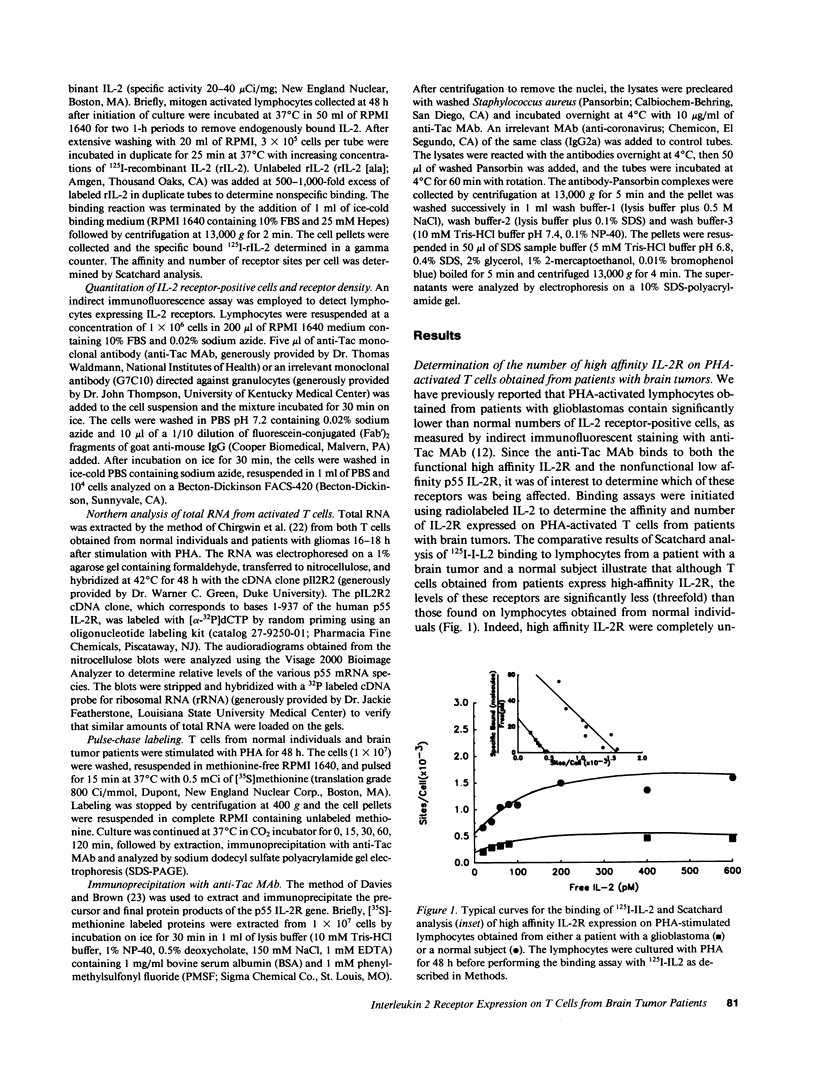
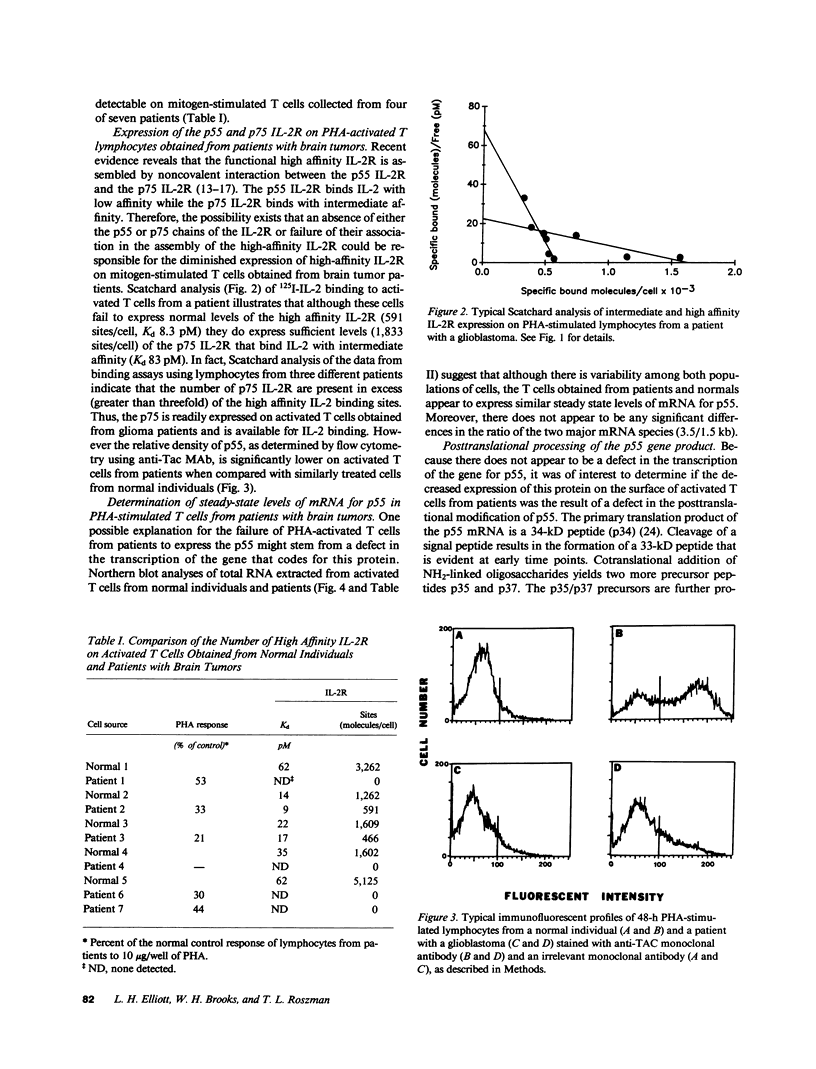
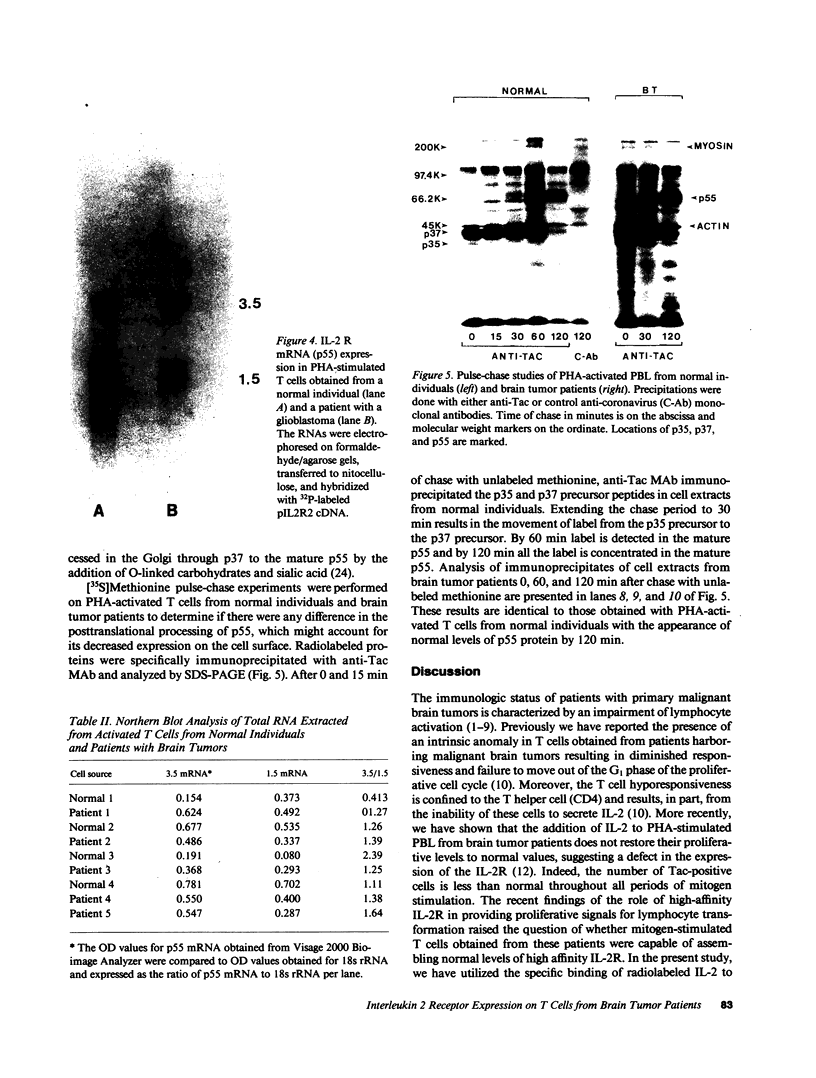
Patients with primary malignant brain tumors manifest a variety of abnormalities in cell-mediated and humoral immunity. Diminished T cell reactivity has been shown in these patients to be linked to deficiencies in interleukin 2 (IL-2) production that cannot be overcome by exogenous IL-2. In this study, specific binding of radiolabeled IL-2 to PHA-stimulated lymphocytes from brain tumor patients demonstrates that the number of high affinity interleukin 2 receptors (IL-2R) is greatly reduced. FACS analysis indicates that the relative density of the p55 protein (Tac protein) is lower on the mitogen-activated lymphocytes obtained from patients than on comparably treated lymphocytes from normal individuals. These data indicate that mitogen-stimulated lymphocytes obtained from patients have fewer functional high affinity IL-2R principally because of the failure to express sufficient levels of the p55 protein for association with the p75 protein. Northern analysis of total RNA isolated from mitogen-stimulated T cells from patients demonstrates normal levels of steady state mRNA, which codes for the p55 protein. Moreover, there is no defect in the postranslational processing of the primary translation product of this mRNA suggesting that normal levels of the p55 protein are produced in activated T cells from patients.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bich-Thuy L. T., Dukovich M., Peffer N. J., Fauci A. S., Kehrl J. H., Greene W. C. Direct activation of human resting T cells by IL 2: the role of an IL 2 receptor distinct from the Tac protein. J Immunol. 1987 Sep 1;139(5):1550–1556. [PubMed] [Google Scholar]
- Braun D. P., Penn R. D., Flannery A. M., Harris J. E. Immunoregulatory cell function in peripheral blood leukocytes of patients with intracranial gliomas. Neurosurgery. 1982 Feb;10(2):203–209. [PubMed] [Google Scholar]
- Brooks W. H., Netsky M. G., Normansell D. E., Horwitz D. A. Depressed cell-mediated immunity in patients with primary intracranial tumors. Characterization of a humoral immunosuppressive factor. J Exp Med. 1972 Dec 1;136(6):1631–1647. doi: 10.1084/jem.136.6.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks W. H., Roszman T. L., Mahaley M. S., Woosley R. E. Immunobiology of primary intracranial tumours. II. Analysis of lymphocyte subpopulations in patients with primary brain tumours. Clin Exp Immunol. 1977 Jul;29(1):61–66. [PMC free article] [PubMed] [Google Scholar]
- Brooks W. H., Roszman T. L., Rogers A. S. Impairment of rosette-forming T lymphocytes in patients with primary intracranial tumors. Cancer. 1976 Apr;37(4):1869–1873. doi: 10.1002/1097-0142(197604)37:4<1869::aid-cncr2820370435>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dukovich M., Wano Y., Le thi Bich Thuy, Katz P., Cullen B. R., Kehrl J. H., Greene W. C. A second human interleukin-2 binding protein that may be a component of high-affinity interleukin-2 receptors. Nature. 1987 Jun 11;327(6122):518–522. doi: 10.1038/327518a0. [DOI] [PubMed] [Google Scholar]
- Elliott L. H., Brooks W. H., Roszman T. L. Cytokinetic basis for the impaired activation of lymphocytes from patients with primary intracranial tumors. J Immunol. 1984 Mar;132(3):1208–1215. [PubMed] [Google Scholar]
- Elliott L., Brooks W., Roszman T. Role of interleukin-2 (IL-2) and IL-2 receptor expression in the proliferative defect observed in mitogen-stimulated lymphocytes from patients with gliomas. J Natl Cancer Inst. 1987 May;78(5):919–922. [PubMed] [Google Scholar]
- Fontana A., Hengartner H., de Tribolet N., Weber E. Glioblastoma cells release interleukin 1 and factors inhibiting interleukin 2-mediated effects. J Immunol. 1984 Apr;132(4):1837–1844. [PubMed] [Google Scholar]
- Jacobs S. K., Wilson D. J., Kornblith P. L., Grimm E. A. In vitro killing of human glioblastoma by interleukin-2-activated autologous lymphocytes. J Neurosurg. 1986 Jan;64(1):114–117. doi: 10.3171/jns.1986.64.1.0114. [DOI] [PubMed] [Google Scholar]
- Jacobs S. K., Wilson D. J., Kornblith P. L., Grimm E. A. Interleukin-2 and autologous lymphokine-activated killer cells in the treatment of malignant glioma. Preliminary report. J Neurosurg. 1986 May;64(5):743–749. doi: 10.3171/jns.1986.64.5.0743. [DOI] [PubMed] [Google Scholar]
- Kuppner M. C., Hamou M. F., Sawamura Y., Bodmer S., de Tribolet N. Inhibition of lymphocyte function by glioblastoma-derived transforming growth factor beta 2. J Neurosurg. 1989 Aug;71(2):211–217. doi: 10.3171/jns.1989.71.2.0211. [DOI] [PubMed] [Google Scholar]
- Kuppner M. C., Hamou M. F., de Tribolet N. Immunohistological and functional analyses of lymphoid infiltrates in human glioblastomas. Cancer Res. 1988 Dec 1;48(23):6926–6932. [PubMed] [Google Scholar]
- Leonard W. J., Depper J. M., Krönke M., Robb R. J., Waldmann T. A., Greene W. C. The human receptor for T-cell growth factor. Evidence for variable post-translational processing, phosphorylation, sulfation, and the ability of precursor forms of the receptor to bind T-cell growth factor. J Biol Chem. 1985 Feb 10;260(3):1872–1880. [PubMed] [Google Scholar]
- Leonard W. J., Krönke M., Peffer N. J., Depper J. M., Greene W. C. Interleukin 2 receptor gene expression in normal human T lymphocytes. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6281–6285. doi: 10.1073/pnas.82.18.6281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy N. L. Cell-mediated cytotoxicity and serum-mediated blocking: evidence that their associated determinants on human tumor cells are different. J Immunol. 1978 Sep;121(3):916–922. [PubMed] [Google Scholar]
- Mahaley M. S., Jr, Brooks W. H., Roszman T. L., Bigner D. D., Dudka L., Richardson S. Immunobiology of primary intracranial tumors. Part 1: studies of the cellular and humoral general immune competence of brain-tumor patients. J Neurosurg. 1977 Apr;46(4):467–476. doi: 10.3171/jns.1977.46.4.0467. [DOI] [PubMed] [Google Scholar]
- Merchant R. E., Grant A. J., Merchant L. H., Young H. F. Adoptive immunotherapy for recurrent glioblastoma multiforme using lymphokine activated killer cells and recombinant interleukin-2. Cancer. 1988 Aug 15;62(4):665–671. doi: 10.1002/1097-0142(19880815)62:4<665::aid-cncr2820620403>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Miescher S., Whiteside T. L., de Tribolet N., von Fliedner V. In situ characterization, clonogenic potential, and antitumor cytolytic activity of T lymphocytes infiltrating human brain cancers. J Neurosurg. 1988 Mar;68(3):438–448. doi: 10.3171/jns.1988.68.3.0438. [DOI] [PubMed] [Google Scholar]
- Nelson D. L., Rubin L. A., Kurman C. C., Fritz M. E., Boutin B. An analysis of the cellular requirements for the production of soluble interleukin-2 receptors in vitro. J Clin Immunol. 1986 Mar;6(2):114–120. doi: 10.1007/BF00918743. [DOI] [PubMed] [Google Scholar]
- Robb R. J., Greene W. C. Internalization of interleukin 2 is mediated by the beta chain of the high-affinity interleukin 2 receptor. J Exp Med. 1987 Apr 1;165(4):1201–1206. doi: 10.1084/jem.165.4.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb R. J., Greene W. C., Rusk C. M. Low and high affinity cellular receptors for interleukin 2. Implications for the level of Tac antigen. J Exp Med. 1984 Oct 1;160(4):1126–1146. doi: 10.1084/jem.160.4.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb R. J., Rusk C. M., Yodoi J., Greene W. C. Interleukin 2 binding molecule distinct from the Tac protein: analysis of its role in formation of high-affinity receptors. Proc Natl Acad Sci U S A. 1987 Apr;84(7):2002–2006. doi: 10.1073/pnas.84.7.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roszman T. L., Brooks W. H., Elliott L. H. Immunobiology of primary intracranial tumors. VI. Suppressor cell function and lectin-binding lymphocyte subpopulations in patients with cerebral tumors. Cancer. 1982 Oct 1;50(7):1273–1279. doi: 10.1002/1097-0142(19821001)50:7<1273::aid-cncr2820500709>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Roszman T. L., Brooks W. H., Elliott L. H. Inhibition of lymphocyte responsiveness by a glial tumor cell-derived suppressive factor. J Neurosurg. 1987 Dec;67(6):874–879. doi: 10.3171/jns.1987.67.6.0874. [DOI] [PubMed] [Google Scholar]
- Roszman T. L., Brooks W. H. Immunobiology of primary intracranial tumours. III. Demonstration of a qualitative lymphocyte abnormality in patients with primary brain tumours. Clin Exp Immunol. 1980 Feb;39(2):395–402. [PMC free article] [PubMed] [Google Scholar]
- Rubin L. A., Kurman C. C., Fritz M. E., Biddison W. E., Boutin B., Yarchoan R., Nelson D. L. Soluble interleukin 2 receptors are released from activated human lymphoid cells in vitro. J Immunol. 1985 Nov;135(5):3172–3177. [PubMed] [Google Scholar]
- Rutka J. T., Rosenblum M. L., Stern R., Ralston H. J., 3rd, Dougherty D., Giblin J., DeArmond S. Isolation and partial purification of growth factors with TGF-like activity from human malignant gliomas. J Neurosurg. 1989 Dec;71(6):875–883. doi: 10.3171/jns.1989.71.6.0875. [DOI] [PubMed] [Google Scholar]
- Saragovi H., Malek T. R. The murine interleukin 2 receptor. Irreversible cross-linking of radiolabeled interleukin 2 to high affinity interleukin 2 receptors reveals a noncovalently associated subunit. J Immunol. 1987 Sep 15;139(6):1918–1926. [PubMed] [Google Scholar]
- Sawamura Y., Abe H., Aida T., Hosokawa M., Kobayashi H. Isolation and in vitro growth of glioma-infiltrating lymphocytes, and an analysis of their surface phenotypes. J Neurosurg. 1988 Nov;69(5):745–750. doi: 10.3171/jns.1988.69.5.0745. [DOI] [PubMed] [Google Scholar]
- Sharon M., Klausner R. D., Cullen B. R., Chizzonite R., Leonard W. J. Novel interleukin-2 receptor subunit detected by cross-linking under high-affinity conditions. Science. 1986 Nov 14;234(4778):859–863. doi: 10.1126/science.3095922. [DOI] [PubMed] [Google Scholar]
- Smith K. A. The interleukin 2 receptor. Adv Immunol. 1988;42:165–179. doi: 10.1016/s0065-2776(08)60844-5. [DOI] [PubMed] [Google Scholar]
- Teshigawara K., Wang H. M., Kato K., Smith K. A. Interleukin 2 high-affinity receptor expression requires two distinct binding proteins. J Exp Med. 1987 Jan 1;165(1):223–238. doi: 10.1084/jem.165.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsudo M., Kozak R. W., Goldman C. K., Waldmann T. A. Demonstration of a non-Tac peptide that binds interleukin 2: a potential participant in a multichain interleukin 2 receptor complex. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9694–9698. doi: 10.1073/pnas.83.24.9694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside T. L., Wang Y. L., Selker R. G., Herberman R. B. In vitro generation and antitumor activity of adherent lymphokine-activated killer cells from the blood of patients with brain tumors. Cancer Res. 1988 Nov 1;48(21):6069–6075. [PubMed] [Google Scholar]
- Wood G. W., Morantz R. A. In vitro reversal of depressed T-lymphocyte function in the peripheral blood of brain tumor patients. J Natl Cancer Inst. 1982 Jan;68(1):27–33. [PubMed] [Google Scholar]
- Young H. F., Sakalas R., Kaplan A. M. Inhibition of cell-mediated immunity in patients with brain tumors. Surg Neurol. 1976 Jan;5(1):19–23. [PubMed] [Google Scholar]