Abstract
The present study attempted to clarify whether over-secretion of glucocorticoids in the serum caused by increased hypothalamus-pituitary-adrenal activity induces oxidative stress in the rat brain, and how the stress causes the emergence of cognitive deficits. When rats were subcutaneously injected with corticosterone, lipid hydroperoxides and protein carbonyls increased markedly in the hippocampus in association with a decrease in activity of antioxidative enzymes, such as superoxide dismutase, catalase and glutathione peroxidase. These results suggest that high-level corticosterone in the serum induces reactive oxygen species (ROS), leading to oxidative damage in the hippocampus. After administration of corticosterone to rats, glucose and superoxide levels in the serum increased markedly. Furthermore, pyramidal cell apoptosis was observed to accompany the loss of glucocorticoid receptors at the cornus ammonis 1 region of the hippocampus. Rats injected with corticosterone showed marked deficits in memory function. The present results imply that ROS generated from the glycation reaction of increased glucose levels caused by gluconeogenesis activation through glucocorticoid with proteins in the serum attack the hippocampus to induce neurodegeneration, resulting in cognitive deficits in rats.
Keywords: hypothalamus-pituitary-adrenal axis, reactive oxygen species, neurodegeneration, glucocorticoid, cognitive deficit
Introduction
Oxidative stress results from an imbalance between reactive oxygen species (ROS) generation and detoxification by antioxidants in living tissues, and neurodegeneration is considered to be accumulation of oxidative damage to the brain as a result of oxidative stress experienced over long periods of time during aging [1, 2]. Among organs in living tissues, neurons in the brain are considered to be more vulnerable to oxidative stress, leading to neuronal oxidative damage, and neurodegenerative disorders such as Alzheimer’s disease (AD), Parkinsonism and senile dementia [3]. Recent studies have demonstrated a decrease in polyunsaturated fatty acids, and increased levels of lipid peroxidation markers, protein oxidation, and DNA and RNA oxidation in AD [4].
Based on the oxidative stress theory of neurodegeneration, our previous work revealed that the levels of thiobarbituric acid reactive substances (TBARS), lipid hydroperoxides (LOOH), F2-isoprostane and conjugated dienes increase significantly in the rat brain through oxidative stress, and the activity of antioxidative enzymes and vitamin E content in the brain decrease markedly [5–7]. Furthermore, in accordance with these phenomena, young rats subjected to oxidative stress showed marked deficits in learning and memory functions. In addition to these phenomena, the delayed-type apoptosis of pyramidal cells and the accumulation of amyloid-β-like substances in the hippocampus of young rats were induced by oxidative stress [8, 9]. These abnormalities were also observed in both normal old rats and vitamin E-deficient young rats not subjected to oxidative stress. It is, therefore, reasonable to consider that impairment in cognitive function caused by oxidative stress during aging and in neurodegenerative disease results from oxidative neuronal cell death.
It has been confirmed that there are increased regional levels of oxidative stress in the AD brain, and hence, oxidative stress is known to be involved in several neurodegenerative disorders characterized by progressive cognitive deficits, and to induce dysfunction of the hypothalamus-pituitary-adrenal (H-P-A) axis in AD, resulting in increased corticoid levels in the serum and cerebrospinal fluid [10–12]. Glucocorticoids, which are steroid hormones secreted through the H-P-A axis, play a major role in glucose metabolism, anti-inflammation, growth processes, and neuroendocrine processes. The regulation of the H-P-A axis has been classified as a negative feedback action. Hence, an increase in the H-P-A activity is thought to be associated with a decline in glucocorticoid negative feedback inhibition over H-P-A activity [13]. This negative feedback is regulated by glucocorticoid receptors (GRs) in hippocampal pyramidal cells [14]. As hippocampal cell loss induces increased plasma corticoid levels in AD, which leads to further degenerative process [15], it is reasonable to suggest that the decrease in negative feedback action due to loss of GRs and H-P-A axis dysfunction arises from oxidative stress. However, the causes of these phenomena in AD have not been well understood.
Our recent results also revealed that young rats subjected to hyperoxia showed hippocampal oxidative damage and pyramidal cell apoptosis accompanied by a marked decrease in GRs in hippocampal cornus ammonis 1 (CA1) region [16]. Furthermore, it was found that oxidative stress induces increase in secretion of corticotrophin-releasing hormone in the hypothalamus, and plasma levels of adrenocorticotrophic hormone and corticosterone [16]. Consequently, it implies that oxidative stress induces oxidative damage in the hippocampus and the H-P-A axis, resulting in cognitive deficits in rats, and that negative feedback inhibition of H-P-A activity was markedly dampened due to increased corticosterone levels caused by loss of GRs. However, the relevance of increased corticoid plasma levels to cognitive deficits remains unclear. The present study aimed to verify whether high level of glucocorticoids in the rat serum induces oxidative stress, leading to cognitive deficits due to the oxidative brain damages, in connection with the phenomena in AD.
Materials and Methods
Animals
All animal experiments were performed with the approval of the Animal Protection and Ethics Committee of the Shibaura Institute of Technology. Male Wistar rats (age, 3 months; Japan SLC Co., Hamamatsu, Japan) were fed ad libitum using standard diet and water. For biochemical analysis and the behavior test, the same rats (n = 9 for each group) were used.
Chemicals
Corticosterone, glucose analyzing kits (Glucose II Test Wako, Osaka, Japan) and apoptosis detection kits were purchased from Wako Pure Chemical Industries (Osaka, Japan). Lucigenin for analysis of superoxide, isoluminol and microperoxidase MP-11 were obtained from Sigma-Aldrich Co. (St. Louis, MO). Rat GR antibody was purchased from Thermo Fisher Scientific (Rockford, IL), and Elite ABC kit-PK6101 were obtained from Vector Laboratories Inc. (Burlingame, CA). All other chemicals were of the highest grade available.
Corticosterone administration to rats
In order to reduce the influence of circadian changes in rat physiological parameters, injection of corticosterone was performed daily at 6 p.m. After learning trials were conducted for 14 days, a suspension of corticosterone in olive oil was subcutaneously injected in rats (0.5 mg/kg body mass/day) for 14 days. Control rats were injected with the same volume of olive oil alone for 14 days. During the 14-day injection period, trials continued to be conducted.
Analyses of oxidized components in hippocampus
After corticosterone treatment for 14 days, rats were sacrificed, and brains were quickly removed, followed by dissection of the hippocampus on ice. For analysis of LOOH, tissues were extracted with a mixture of chloroform and methanol (2:1, v/v). After evaporation of the extract, residues were dissolved in 200 µl of methanol, and 80 µl of this solution was mixed with a chemiluminescent solution (mixture of 0.18 mg/ml isoluminol and 1 mg/ml microperoxidase in 70% methanol, 100:1, v/v). Chemiluminescence of the solution was then analyzed using a Luminescencer PSN apparatus (Atto Co., Tokyo, Japan), as described previously [17]. Protein carbonyls, an index of protein oxidation, were also analyzed. Homogenates of the hippocampus (100 µl) were incubated with a solution of 10 mM 2,4-dinitrophenyl hydrazine in 2 N HCl (400 µl) at 37°C for 60 min. This was mixed with a solution of 20% trichloro acetic acid (500 µl), followed by incubation in an ice bath for 10 min. After centrifugation at 3,000 rpm for 10 min at 4°C, the precipitate obtained was extracted with a mixture of ethanol and ethyl acetate (1:1) to remove excess 2,4-dinitrophenyl hydrazine. The precipitate was dissolved in 6 M guanidine solution (1 ml), and the optical density of the clear solution obtained was measured at 365 nm.
Measurement of antioxidative enzyme activity
The activity of superoxide dismutase (SOD) in the supernatant from aliquots of hippocampal homogenates was analyzed based on the reduction of nitroblue tetrazolium using the SOD analysis kit (Wako Pure Chemical Industries, Osaka, Japan). The activities of catalase and glutathione peroxidase were assessed as described previously [18, 19].
Detection of plasma levels of glucose and superoxide
Measurement of plasma glucose was performed by the Mutarotase-Glucose oxidase method using a glucose kit, as reported previously [20]. After 14 days of corticosterone injections, serum (20 µl) was mixed well with 3 ml of a solution from the kit, and incubated at 37°C for 5 min. Samples were measured for absorbance at 505 nm. For analysis of superoxide levels in the serum, 2.4 mM lucigenin solution in 10 mM Tris-HCl buffer (500 µl) was added, and the solution was diluted with 10 mM Tris-HCl buffer or 4 µM Fe(NO3)3 containing 10 mM Tris-HCl buffer (250 µl each). After this mixture was placed in a chemiluminescence detector, serum was added with stirring. Chemiluminescence was then measured for 30 min at room temperature.
Tissue preparation for analysis of cell apoptosis and GR levels in hippocampus
Removed brains were immediately immersed in Mizuhira’s solution (3% paraformaldehyde in 0.1 M sodium cacodylate buffer containing 0.1% tannic acid, 2 mM CaCl2 and 1 mM MgCl2; pH 7.2–7.4) [21]. A microwave irradiation technique was used for the rapid penetration of fixative solution. Brains immersed in Mizuhira’s solution were exposed to microwaves in a water bath for 30 s at 25°C. After irradiation, samples were left to stand in fixative solution at room temperature overnight. Frontal sections were then serially sliced at 50 µm thickness using a microslicer (Vibratome, Lancer, Bannockburn, IL).
Cell apoptosis and GR analyses
Cell damage and loss of hippocampal area were assessed microscopically by hematoxylin-eosin staining. To visualize DNA cleavage, we performed terminal deoxynucleotidyl transferase (TdT)-mediated deoxyuridine triphosphate nick-end labeling (TUNEL) using the apoptosis detection kit (Wako Pure Chemical Industries, Ltd., Osaka, Japan), with slight modification [22]. Briefly, to inactivate endogenous peroxidase, sections were quenched with 2% H2O2 for 30 min at room temperature, and were then washed three times for 5 min each with phosphate-buffered saline (PBS), followed by incubation in terminal deoxynucleotidyl transferase buffer for 1 h at 37°C. Sections were washed again in PBS and incubated in buffer with peroxidase-conjugated antibodies for 30 min at room temperature. After washing with PBS, they were developed with diaminobenzidine and counterstained with methyl green. Changes in GRs in the hippocampal area were assessed microscopically by immunochemical staining using rat GR antibodies and Elite ABC kit-PK6101. Positive antigen-antibody reactions were visualized by incubating the slide in 250 µl of 0.3% 3,3'-diaminobenzidine-terahydrochloride in 50 mM Tris-HCl (pH 7.6) containing 3% H2O2. Negative staining was performed using the same method, except that the GR antibody was not used. Quantitative analysis of GRs was performed in order to evaluate the intensity of stained GRs using an imaging analyzer (LAS-3000; Fuji Film Co., Tokyo, Japan). The intensity was analyzed by a deduction of the intensity of background from that of the stained GRs.
Behavioral testing
Rats were tested for memory using a Morris water maze apparatus (140 cm in diameter and 45 cm in height) [23]. The bottom of the pool was divided into quadrants using white lines, and the transparent platform was submerged 2 cm below the surface of the water at the center of one of the quadrants; the water was maintained at 21 ± 1°C. For pre-training, the rats were allowed to swim freely in the pool for 60 s without the platform. Daily training consisted of one trial in which the rats swam from the start point to a fixed goal; this was conducted for 14 consecutive days. After learning the task, rats were injected with corticosterone. During the 14-day injection period, trials continued to be conducted. Thereafter, they were kept in an ordinary atmosphere at 21 ± 1°C for 48 h without training to assess their memory retention. Then the platform of the apparatus was removed, and the rats were placed opposite the quadrant where the platform had been located. The percentage of time spent in the quadrant where the platform had been was measured. This conducted for further 10 consecutive days.
Statistical analysis
Results are presented as means ± SE. All data were analyzed by Student’s t test and one-way ANOVA, followed by a Dunnett’s t test. Differences in experimental data were considered to be statistically significant when p values were less than 0.05.
Results
Changes in LOOH and oxidized protein, and activity of antioxidative enzymes in hippocampus after corticosterone injection
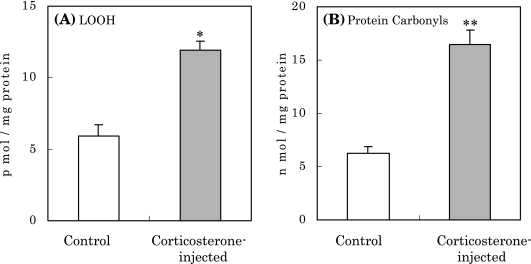
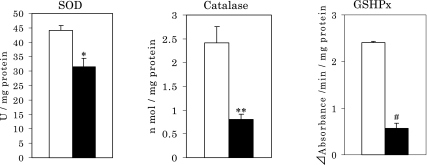
After injection of corticosterone, rats showed marked increases in levels of LOOH and oxidized protein by approximately 2 and 3 times compared to controls, respectively (Fig. 1(A) and (B)). These increases in the oxidized components in hippocampus suggest that corticosterone injection induces ROS, and hence, the hippocampus may be damaged oxidatively by the ROS attack to increase such oxidative components. To confirm this notion, the influence of corticosterone on the activity of antioxidative enzymes in the hippocampus was investigated. As shown in Fig. 2, the activities of SOD, catalase and glutathione peroxidase (GSHPx) in the hippocampus decreased markedly after injection. Based on these results, it is considered that high levels of corticosterone induce oxidative stress to induce oxidative damage in the hippocampus caused by ROS.
Fig. 1.
Increase in LOOH (A) and protein carbonyls (B) in the rat hippocampus caused by corticosterone injection. *p<0.01, **p<0.005 vs normal control; means ± SE, n = 9 for each group of rats.
Fig. 2.
Changes in SOD, catalase and glutathione peroxidase activities in the rat hippocampus caused by corticosterone injection. Open columns, normal control; Closed columns, rats injected with corticosterone. *p<0.05, **p<0.001, #p<0.002 vs normal control; means ± SE, n = 9 for each group of rats.
Effect of corticosterone on the plasma glucose level
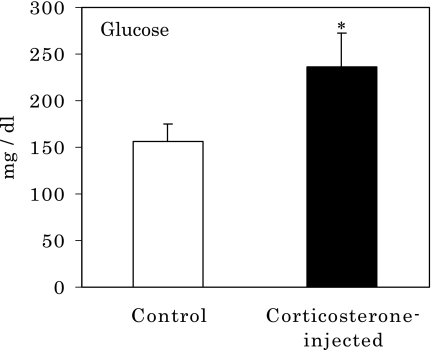
As corticoids are known to have anti-inflammatory effects and to induce increases in blood sugar levels, the effects of corticosterone on plasma glucose levels were investigated. When rats were subcutaneously administered corticosterone, they showed marked increases in plasma glucose levels at 14 days after injection (Fig. 3). Based on these results, it is evident that corticosterone induced high glucose levels in the serum through activated gluconeogenesis by corticosterone.
Fig. 3.
Increase in serum glucose in rats after corticosterone injection. *p<0.05 vs normal control; means ± SE, n = 9 for each group of rats.
Corticosterone-induced generation of superoxide in serum
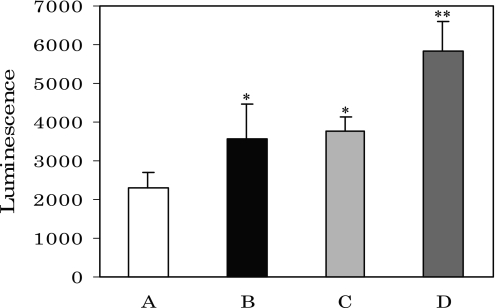
As it has been reported that human serum albumin and/or polypeptides are glycated by glucose nonenzymatically in vitro, followed by superoxide generation, and that generation of superoxide was accelerated by the presence of ferric ions [24], the increased serum glucose levels in this study most likely produced superoxide via glycation reactions with serum protein of rats administered corticosterone. As shown in Fig. 4, when rats were injected with corticosterone, levels of superoxide in the serum increased markedly compared to controls (Fig. 4, column B). Control sera mixed with ferric ions showed high levels of superoxide (Fig. 4, column C), but following addition of ferric ions to sera from rats injected with corticosterone, superoxide levels were more markedly increased (Fig. 4, column D). These results indicate that increased glucose in the serum after corticosterone administration reacted nonenzymatically with serum proteins via a glycation reaction to induce excess levels of superoxide.
Fig. 4.
Superoxide generation in rat serum caused by corticosterone injection. A, control; B, rats injected by corticosterone; C, Fe(NO3)3-added A; D, Fe(NO3)3-added B. *p<0.05 vs A, **p<0.01 vs B; means ± SE, n = 9 for each group of rats.
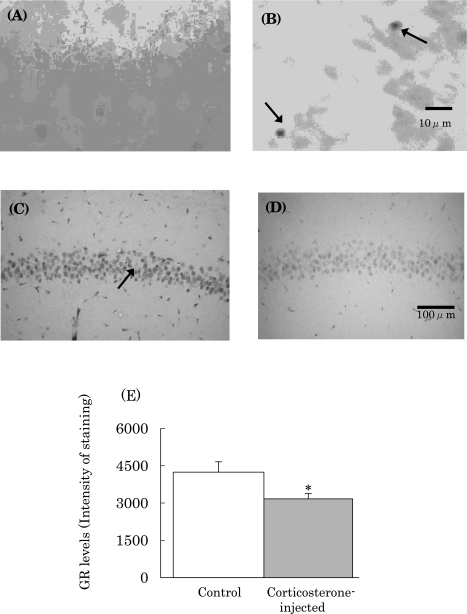
Appearance of apoptosis and changes in glucocorticoid receptor (GR) levels after corticosterone injection
The superoxide generated leads to production of various ROS, and may attack the brain to induce the oxidative damage. This contention is supported by the previous findings that oxidative stress induces ROS, followed by the apoptosis of pyramidal cells in the hippocampus and the loss of GRs [9, 16]. When rats were subcutaneously administered corticosterone, pyramidal cell apoptosis was seen in the CA1 region of the hippocampus (Fig. 5(B)). In addition to cell apoptosis, it was found that GR levels in the hippocampus decreased markedly (Fig. 5(D) and (E)).
Fig. 5.
Pyramidal cell apoptosis (B) and GR loss (D) and (E) in CA1 region in rat hippocampus. (A) and (C), normal controls; (B) and (D), rats injected with corticosterone; Arrowhead in (B) shows apoptotic cells in CA1 region, and that in (C) shows immunochemically stained GR in pyramidal cells. Magnification for each photograph is indicated by the bars in (B) and (D). *p<0.05 vs normal control.
Influence of corticosterone injection on memory function in rats
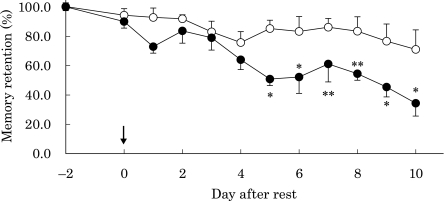
As pyramidal cell loss in the hippocampus, which modulates memory function, was observed after injection of corticosterone, we examined the influence of corticosterone on memory function in rats. As shown in Fig. 6, control rats mostly retained their memories of the platform location throughout the experimented period. However, when rats were injected with corticosterone, they retained their memories for 4 days after resting for 48 h. However, their memories suddenly declined beginning at 5 days after the rest period. In the final test after administration of corticosterone, memory function decreased by 50–60%.
Fig. 6.
Changes in memory retention in rats after corticosterone injection. Open circles, control rats; closed circles, rats injected with corticosterone. Arrowhead shows endpoint of rest without trial, and “−2 days” indicates the start point of resting. *p<0.01, **p<0.05 vs normal control; means ± SE, n = 9 for each group of rats.
Discussion
Oxidative stress is elevated in the brains of subjects with AD to induce pyramidal cell death in the hippocampus, leading to loss of GRs. AD is also associated with high serum levels of glucocorticoids and glucose [10–12]. Glucocorticoid levels are regulated by negative feedback in the H-P-A axis via the GR response, and GR loss in AD patients plays a role in activated H-P-A activity, resulting in over-secretion of glucocorticoids. However, the relationship between these abnormalities and cognitive impairment in AD is poorly understood. As AD patients treated with prednisone, which is a glucocorticoid used as an anti-inflammatory agent, show impaired cognition when compared with placebo-treated patients, glucocorticoids are thought to have a detrimental role in AD [12]. However, the role of glucocorticoids in cognitive impairment through neurodegeneration is unclear.
In order to clarify whether over-secretion of glucocorticoids caused by increased H-P-A activity induces oxidative stress to injure the brain, and how this may be involved in the emergence of cognitive deficits, we investigated the influence of glucocorticoids on oxidative brain damage using young normal rats injected subcutaneously with corticosterone. As shown in Fig. 1(A) and (B), when rats were administered corticosterone, LOOH and oxidized protein increased markedly in the hippocampus when compared to control rats. As both compounds are known to be oxidation products of lipids and proteins, it was evident that glucocorticoid induced oxidative stress to generate ROS in the rat brain. Based on these results, changes in the activity of antioxidant enzymes, such as SOD, catalase and GSHPx, were analyzed in the rat hippocampus. Although mRNA levels of these antioxidant enzymes were not analyzed in this study, it is considered that the activities of these enzymes might be markedly decreased for scavenging of ROS without further production of these enzymes (Fig. 2).
These results suggest that glucocorticoids may damage the hippocampus by generating ROS. To confirm this notion, immunochemical analysis of the hippocampus was performed by TUNEL. As shown in Fig. 5(B), pyramidal cell apoptosis was found in the CA1 region of the rat hippocampus. Consequently, it appears that the ROS generated by corticosterone attacked the hippocampus to damage the pyramidal cells, resulting in neuronal cell apoptosis.
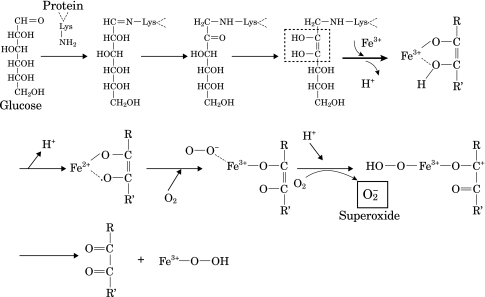
It is widely recognized that glucocorticoids have a role in both anti-inflammation and gluconeogenesis activation. Hence, the high glucocorticoid levels in this study would be expected to produce elevated glucose levels in the serum based on the effects of gluconeogenesis activation. As shown in Fig. 3, when rats were administered corticosterone, serum glucose levels increased markedly. It is known that lysine residues in serum proteins are glycated by glucose to induce superoxide in the presence of ferric ions (Fig. 7) [24]. The link between glucocorticoid and ROS generation remains poorly understood, and hence, the probability of superoxide generation due to high glucose levels after corticosterone administration was investigated. Superoxide generation increased markedly following the injection of corticosterone (Fig. 4, column B). When ferric ions were added to the serum of rats injected with corticosterone, superoxide generation was further increased significantly (Fig. 4, column D). When ferric ions were added to control serum, superoxide generation was also increased (Fig. 4, column C).
Fig. 7.
Glycation reaction of serum protein in the presence of Fe(NO3)3 and superoxide generation [24].
The fact obtained here that higher superoxide levels were observed in the serum added by ferric ions may show the probability of superoxide generation due to the reaction of glycated proteins with ferric ions. However, it is unclear at the present day whether glycated proteins in the rat serum were actually produced, and reacted with ferric ions to induce superoxide. In order to verify this notion, further investigations are necessarily needed.
Thus, it can imply that ROS arising from superoxide generated by glucocorticoids attack the hippocampus to induce oxidative damage, resulting in the pyramidal cell apoptosis.
As negative feedback via H-P-A activity is regulated by GRs in hippocampal pyramidal cells [14], the hippocampal cell loss observed here (Fig. 5(B)) may induce decreases in GR levels in the hippocampus, leading to over-secretion of corticosterone. To confirm this notion, changes in GRs in the hippocampal area were assessed microscopically by immunochemical staining using rat GR antibodies. As shown in Fig. 5(D) and (E), GR levels in the CA1 region in the hippocampus decreased markedly. Therefore, ROS generated by serum protein glycation must have attacked pyramidal cells in the hippocampus to induce GR loss, leading to an increase in H-P-A activity and, ultimately, over-secretion of glucocorticoids.
The results obtained in this study prompted us to assess the memory function of rats injected with corticosterone. After rats learned the location of the platform in the pool, the influence of corticosterone on memory function was assessed. Control rats retained their memory function throughout the experimental period. When rats were subcutaneously injected with corticosterone, they retained their memories within four days after resting. However, their memories began to decrease five days after resting (Fig. 6). Thus, it is evident that the oxidative pyramidal cell loss caused by increased serum glucocorticoid induces impairment of memory function in rats.
In conclusion, when rats are subjected to oxidative stress, serious damage to pyramidal cells induce an increase in H-P-A activity, leading to over-secretion of corticosterone [16]. Increased serum corticosterone may induce ROS through glycation of serum protein, and the hippocampus is further damaged oxidatively. After H-P-A function is damaged by the oxidative stress, this process may be exaggerated through increased glucocorticoid production, and cognitive functions are ultimately impaired. Thus, it implies that over-secretion of glucocorticoids caused by oxidative stress further induce oxidative stress to damage hippocampal cells. It has been reported that glucocorticoid enhances cell death in cultured hippocampal cells after amyloid β protein and glutamate challenge in vitro [25]. Our in vivo data may explain the cause of these findings, and suggest a link between activated H-P-A activity and cognitive impairment in AD.
Acknowledgment
This study has been supported in part, by MEXT HAITEKU (2007), and a Grant-in-Aid from Eisai Company Ltd.
Abbreviations
- AD
Alzheimer’s disease
- CA1
cornus ammonis
- GRs
glucocorticoid receptors
- GSHPx
glutathione peroxidase
- H-P-A
hypothalamus-pituitary-adrenal
- LOOH
lipid hydroperoxides
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- TBARS
thiobarbituric acid reactive substances
References
- 1.Harman D. Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 2.Harman D. Free radical theory of aging: the free radical diseases. Age. 1984;7:111–131. [Google Scholar]
- 3.Halliwell B. Oxidants and the central nervous system: some fundamental questions. Acta Neurol. Scand. 1989;126:23–33. doi: 10.1111/j.1600-0404.1989.tb01779.x. [DOI] [PubMed] [Google Scholar]
- 4.Davis K.L., Davis B.M., Greenwald B.S., Mohs R.C., Mathé A.A., Johns C.A., Horvath T.B. Cortisol and Alzheimer’s disease. I: Basal studies. Am. J. Psychiatry. 1986;143:300–305. doi: 10.1176/ajp.143.3.300. [DOI] [PubMed] [Google Scholar]
- 5.Onodera K., Omoi N., Fukui K., Hayasaka T., Shinkai T., Suzuki S., Abe K., Urano S. Oxidative damage of rat cerebral cortex and hippocampus, and changes in antioxidative defense systems caused by hyperoxia. Free Radic. Res. 2003;37:367–372. doi: 10.1080/1071576031000090019. [DOI] [PubMed] [Google Scholar]
- 6.Nishio T., Miyadera R., Sakai R., Abe K., Kanazawa H., Fukui K., Urano S. Increased F2-isoprostane levels in the rat brain and plasma caused by oxidative stress and aging, and inhibitory effect of vitamin E. J. Clin. Biochem. Nutr. 2006;38:161–166. [Google Scholar]
- 7.Urano S., Asai Y., Makabe S., Matsuo M., Izumiyama N., Ohtsubo K., Endo T. Oxidative injury of synapse and alteration of antioxidative defense systems in rats, and its prevention by vitamin E. Eur. J. Biochem. 1997;245:64–70. doi: 10.1111/j.1432-1033.1997.00064.x. [DOI] [PubMed] [Google Scholar]
- 8.Fukui K., Omoi N., Hayasaka T., Shinkai T., Suzuki S., Abe K., Urano S. Cognitive impairment of rats caused by oxidative stress and aging, and its prevention by vitamin E. Ann. N.Y. Acad. Sci. 2002;959:275–284. doi: 10.1111/j.1749-6632.2002.tb02099.x. [DOI] [PubMed] [Google Scholar]
- 9.Fukui K., Takatsu H., Shinkai T., Suzuki S., Abe K., Urano S. Appearance of amyloid β-like substances and delayed-type apoptosis in rat hippocampus CA1 region through aging and oxidative stress. J. Alzheimer’s Dis. 2005;8:299–309. doi: 10.3233/jad-2005-8309. [DOI] [PubMed] [Google Scholar]
- 10.Masugi F., Ogihara T., Sakaguchi K., Otsuka A., Tsuchiya Y., Morimoto S., Kumahara Y., Saeki Y., Nishide M. High plasma levels of cortisol in patients with senile dementia of the Alzheimer’s type. Methods Find. Exp. Clin. Pharmacol. 1989;11:707–710. [PubMed] [Google Scholar]
- 11.Swanwick G.R., Kirby M., Bruce I., Buggy F., Coen R.F., Coakley D., Lawlor B.A. Hypothalamic-pituitary-adrenal axis dysfunction in Alzheimer’s disease: lack of association between longitudinal and cross-sectional findings. Am. J. Psychiatry. 1998;155:286–289. doi: 10.1176/ajp.155.2.286. [DOI] [PubMed] [Google Scholar]
- 12.Aisen P.S., Davis K.L., Berg J.D., Schafer K., Campbell K., Thomas R.G., Weiner M.F., Farlow M.R., Sano M., Grundman M.R., Thal L.J. A randomized controlled trial of prednisone in Alzheimer’s disease. Alzheimer’s disease Cooperative Study. Neurology. 2000;54:588–593. doi: 10.1212/wnl.54.3.588. [DOI] [PubMed] [Google Scholar]
- 13.Sapolsky R.M., Krey L., McEwen B.S. The adrenocortical response in the aged mail rats: impairment of recovery from stress. Exp. Gerontol. 1983;18:55–64. doi: 10.1016/0531-5565(83)90051-7. [DOI] [PubMed] [Google Scholar]
- 14.Jacobson J., Sapolsky R.M. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr. Rev. 1991;12:118–134. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- 15.Sapolsky R.M., McEwen B.S. In: Stress, glucocorticoids and their role in degenerative changes in the aging hippocampus, in Treatment Development Strategies for Alzheimer’s Disease. Krook T., Bartus R., Ferris S., editors. Markpowley Associates; New Cannan CT, Connecticut, U.S.A.: 1986. pp. 151–172. [Google Scholar]
- 16.Kobayashi K., Machida T., Takahashi T., Takatsu H., Shinkai T., Abe K., Urano S. Elevation by oxidative stress and aging of hypothalamic-pituitary-adrenal activity in rats and its prevention by vitamin E. J. Clin. Biochem. Nutr. 2009;45:207–213. doi: 10.3164/jcbn.09-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Omoi N., Arai M., Saito M., Takatsu H., Shibata A., Fukuzawa K., Sato K., Abe K., Fukui K., Urano S. Influence of oxidative stress on fusion of pre-synaptic plasma membranes of the rat brain with phosphatidyl choline liposomes, and protective effect of vitamin E. J. Nutr. Sci. Vitaminol. 2006;52:248–255. doi: 10.3177/jnsv.52.248. [DOI] [PubMed] [Google Scholar]
- 18.Aebi H. Catalase in vivo. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 19.Flohé L., Gunzler W.A. Assays of glutathione peroxidase. Methods Enzymol. 1992;105:114–121. doi: 10.1016/s0076-6879(84)05015-1. [DOI] [PubMed] [Google Scholar]
- 20.Miwa I., Okuda J., Maeda K., Okuda G. Mutarotase effect on colorimetric determination of blood glucose with -D-glucose oxidase. Clin. Chim. Acta. 1972;37:538–540. doi: 10.1016/0009-8981(72)90483-4. [DOI] [PubMed] [Google Scholar]
- 21.Mizuhira V., Hasagawa H. Microwave fixation method for cytochemistry. For conventional electron microscopy, enzymoimmunocytochemistry, autoradiography elemental distribution studies and staining methods. Eur. J. Morphol. 1996;34:385–391. doi: 10.1076/ejom.34.5.385.13055. [DOI] [PubMed] [Google Scholar]
- 22.Gavrieli Y., Sherman Y., Ben-Sasson S.A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J. Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morris R. Development of water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 24.Sakurai T., Tsuchiya S. Superoxide production from nonenzymatically glycated protein. FEBS Lett. 1988;236:406–410. doi: 10.1016/0014-5793(88)80066-8. [DOI] [PubMed] [Google Scholar]
- 25.Behl C., Lezoual C.H.F., Trapp T., Widmann M., Skutella T., Holsboer F. Glucocorticoids enhance oxidative stress-induced cell death in hippocampal neurons in vitro. Endocrinology. 1997;138:101–106. doi: 10.1210/endo.138.1.4835. [DOI] [PubMed] [Google Scholar]