Growth factor receptor-bound protein 14: a new modulator of photoreceptor-specific cyclic-nucleotide-gated channel
The adapter protein Grb14 interacts with the rod photoreceptor-specific cyclic nucleotide gated channel alpha subunit (CNGA1) and decreases its affinity for cGMP. These data reveal a new role of Grb14 in the regulation of CNG channel in vivo.
Keywords: cyclic-nucleotide-gated channel, Grb14, photoreceptor cells, Ras-associating domain, tyrosine kinase signalling
Abstract
Growth factor receptor-bound protein 14 (Grb14) is an adaptor protein that is involved in receptor tyrosine kinase signalling. In this study, we report that Grb14 interacts with the rod photoreceptor-specific cyclic-nucleotide-gated channel alpha subunit (CNGA1) and decreases its affinity for cyclic guanosine monophosphate. Channel modulation is controlled by direct binding of the Grb14 Ras-associating domain with the carboxy-terminal region of CNGA1. We observed that the channel remains open in Grb14−/− mice that are exposed to light, suggesting that Grb14 is a normal physiological modulator of CNG channel function in vivo.
Introduction
Growth factor receptor-bound protein 14 (Grb14) is an adaptor protein that has an important role in receptor tyrosine kinase signalling pathways (Han et al, 2001; Depetris et al, 2005). The domain organization of Grb14 sequentially comprises a proline-rich region followed by a Ras-associating (RA) domain, a Pleckstrin-homology (PH) domain, a ‘Between the PH and SH2 (BPS) domain' and an Src-homology (SH2) domain (Daly et al, 1996). We previously identified Grb14 as an insulin receptor (IR)-interacting protein in the retina (Rajala & Chan, 2005). Recently, we demonstrated that Grb14 undergoes light-dependent intracellular redistribution on illumination (Rajala et al, 2009). The significance of this light-dependent translocation of Grb14 to rod outer segments (ROSs) is not known. Furthermore, it is not affected in rods in which the IR is deleted (Rajala et al, 2009). This suggests that there are other outer segment Grb14-binding partners in addition to the IR.
In this study, we found that Grb14 interacts with the rod photoreceptor cyclic-nucleotide-gated channel alpha subunit (CNGA1) and decreases its affinity for cyclic guanosine monophosphate (cGMP), and thereby inhibits the channel activity. The CNG channels have important roles in visual and olfactory transduction (Yau & Baylor, 1989; Finn et al, 1996; Biel et al, 1999; Kaupp & Seifert, 2002; Yau & Hardie, 2009). In the visual system, a cGMP-gated channel is found in the outer membrane of retinal photoreceptor cells. The cGMP-sensitive cation channel detects changes in cytoplasmic cGMP concentration brought about by light (Yau & Baylor, 1989). The RA domain of Grb10 and Grb14 was recently shown to bind to activated N-Ras, and it was proposed that this interaction might downregulate IR signalling (Depetris et al, 2009). The precise functional role of this domain has not been elucidated. We observed that the channel modulation is controlled by direct binding of the Grb14 RA domain to the carboxy-terminal region (CTR) in CNGA1. The functional consequence of this interaction is that Grb14 binds to CNGA1 in the light and facilitates the closure of the CNG channel. Our study identified an unanticipated and uncharacterized role of Grb14 as a CNG-interacting protein in light-promoting rod CNG channel closure. This is, to the best of our knowledge, the first molecular evidence that Grb14 is a normal physiological modulator of CNG channel function in vivo.
Results And Discussion
Grb14 interacts with the CNG channel subunits
Solubilized bovine ROSs were incubated with glutathione S-transferase (GST) and GST–Grb14 fusion proteins and subjected to a GST pull-down assay. A 240-kDa protein associating with GST–Grb14 was excised from Coomassie-stained sodium dodecyl sulphate gels and subjected to peptide mass fingerprinting (matrix-assisted laser desorption ionization time-of-flight mass spectrometry). The four peptide masses obtained (YMAFFEFNNR, AEDPGPGPWLLR, TANVVAHGFTNLFILDK and GGDIITDKK) were used to search GenBank (National Center for Biotechnology Information). This search identified the protein as the CNG channel beta subunit (CNGB1). The identifications of the 240-kDa CNGB1 and the 63-kDa CNGA1 subunits in GST–Grb14 pull-down analyses were confirmed by using immunoblot analysis with CNGA1 and CNGB1 subunit-specific antibodies, respectively (supplementary Fig S1A–D online).
An NH2-terminal-tagged Myc–CNGA1 and X-press-tagged Grb14 were co-expressed in HEK293T cells. Immunoprecipitation analysis revealed that the two proteins interacted with and reciprocally co-immunoprecipitated each other (supplementary Fig S1E–H online). The CNGB1 was also effectively co-immunoprecipitated with Myc–Grb14 when these two proteins were co-expressed (supplementary Fig S1I,J online). The interaction between CNGA1 and Grb14 was confirmed by using split-ubiquitin-based membrane yeast two-hybrid assays (supplementary Fig S1K,L online), which also identified this interaction. Together, the results from the cell culture and membrane yeast two-hybrid assays suggest that an interaction exists between CNGA1 and Grb14.
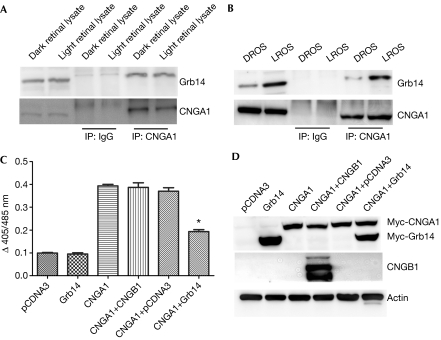
To investigate the in vivo interaction between CNGA1 and Grb14, either rat retinal lysates or ROSs from dark- and light-adapted animals were subjected to immunoprecipitation with CNGA1 antibody and examined for the association between CNGA1 and Grb14. Grb14 was observed to interact with CNGA1 in whole rat retinas exposed to light and complete darkness (Fig 1A). We recently reported that Grb14 undergoes light-dependent translocation from inner to outer segments in rod photoreceptor cells (Rajala et al, 2009). We observed increased association of Grb14 with CNGA1 in light-adapted compared with dark-adapted ROSs (Fig 1B). The presence of Grb14 in dark-adapted ROSs (Fig 1B, lanes 1 and 5) could be due to a slight contamination of outer segments with inner segments during ROS preparation, or a small portion of Grb14 could already be present in outer segments. These experiments suggest that Grb14 moves to ROS membranes in light and binds to its interacting partner CNGA1 there. This indicates that the interaction is mediated by spatial redistribution and not by light-induced exposure of binding sites in CNGA1, which might be unavailable in the dark.
Figure 1.
Growth factor receptor-bound protein 14 interacts with cyclic-nucleotide-gated channel alpha subunit in vivo and modulates its function. (A,B) Retinal lysates or solubilized ROS from dark- and light-adapted rats were immunoprecipitated with CNGA1 antibody and immunoblotted with Grb14 and CNGA1 antibodies. (C) CNGA1 was co-expressed with Grb14 in the HEK293T cells and channel activity in each case was measured as a function of Ca2+ influx in response to cGMP stimulation. The data are mean±s.d., n=3 independent transfections. Significance between CNGA1 and CNGA1+Grb14, *P<0.001. (D) Protein expression in cell lysates was determined by immunoblotting with Myc, CNGB1 and actin antibodies. cGMP, cyclic guanosine monophosphate; CNGA1, cyclic-nucleotide-gated channel alpha subunit; CNGB1, cyclic-nucleotide-gated channel beta subunit; Grb14, growth factor receptor-bound protein 14; IP, immunoprecipitated; ROS, rod outer segment.
Grb14 modulates CNG channel function
To test whether Grb14 could modulate CNGA1 function, we measured the 8-para-chloro phenyl thio (pCPT)-cGMP-induced increase in intracellular Ca2+ concentration ([Ca2+]i) in HEK293T cells overexpressing CNGA1 alone or in combination with Grb14. We measured this with the calcium-sensitive fluorescent dye indo-1/AM, as described in the supplementary information online. Fig 1C shows that overexpression of CNGA1 alone in HEK293T cells led to a fourfold increase in [Ca2+] in response to 8-pCPT-cGMP stimulation, compared with mock-transfected cells. Overexpression of Grb14 suppressed the 8-pCPT-cGMP-induced increase in [Ca2+]i seen in cells transfected with CNGA1 alone, by approximately 50%. Consistent with previous studies (Zheng et al, 2002; Zhong et al, 2003), the heterologous expression of CNGA1 forms functional cGMP-sensitive channels similar to those formed on co-expression of both CNGA1 and CNGB1 subunits, as measured by similar increases in [Ca2+]i in the transfected cells (Fig 1C). To exclude the possibility that Grb14 suppressed CNGA1 function by reducing the expression of CNGA1, transfected proteins were immunoblotted with Myc (for CNGA1 and Grb14) and CNGB1 antibodies. These experiments suggest that Grb14 modulates CNG channel function.
Effect of Grb14 on CNG channel function in vivo
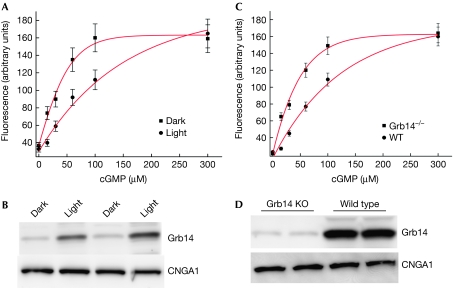
As Grb14 only associates with CNGA1 in ROS prepared from light-exposed animals (Fig 1B), the CNG channel activity was measured in ROS membrane vesicles prepared from dark- and light-adapted rats using Fluo-3, as described in the Methods section and in Fig 2A. We observed that the increase in Ca2+ release in response to varying concentrations of cGMP was greater in dark-adapted ROS than in light-adapted ROS (Fig 2A). Immunoblot analysis using the Grb14 antibody confirmed greater binding of Grb14 in light-adapted than in dark-adapted ROS (Fig 2B). To ensure equal amounts of CNGA1 in dark- and light-adapted ROS, the Grb14 blot was stripped and reprobed with CNGA1 antibody and the results indicate equal amounts of CNGA1 in both conditions (Fig 2B). These data suggest that Grb14 might be a physiological modulator of CNG channel activity in vivo.
Figure 2.
Effect of growth factor receptor-bound protein 14 on cyclic guanosine monophosphate-mediated Ca2+ influx. (A) ROS (5 mg/ml) vesicles were prepared from dark- and light-adapted rats and the Fluo-3 dye was trapped inside them. Ca2+ influx was induced by the addition of cGMP (0–300 μM). (B) ROS membranes prepared from dark- and light-adapted animals were immunoblotted with Grb14 and CNGA1 antibodies. (C) ROS micelles prepared from Grb14−/− and wild-type mice were stimulated with cGMP (0–300 μM) and the increase in fluorescence triggered by Ca2+ influx into micelles was monitored. (D) ROS membranes from wild-type and Grb14−/− mice were subjected to immunoblot analysis with Grb14 and CNGA1 antibodies. The data are represented as mean±s.d., n=3 independent ROS micelles. cGMP, cyclic guanosine monophosphate; CNGA1, cyclic-nucleotide-gated channel alpha subunit; Grb14, growth factor receptor-bound protein 14; KO, knockout; ROS, rod outer segment.
To determine whether Grb14 is a normal physiological modulator of photoreceptor-specific CNG channel, we measured the CNG channel activity on cGMP stimulation in ROS vesicles prepared from light-adapted wild-type and Grb14−/− mice (Fig 2C). An increase in the free Ca2+ concentration was observed in the ROS vesicles prepared from the Grb14−/− mice, but this was suppressed in the ROS vesicles prepared from wild-type mice (Fig 2C). To confirm that Grb14 was not produced in the Grb14−/− mice, ROS membranes from wild-type and Grb14−/− mice were subjected to immunoblot analysis with Grb14 antibody (Fig 2D). The residual band in Grb14−/− mice could be nonspecific because we have previously observed that detection of this band cannot be prevented when the Grb14 antibody is pre-adsorbed with peptide antigen (Rajala et al, 2009). To ensure equal amounts of CNGA1, we reprobed the blot with CNGA1 antibody (Fig 2D). Our data suggest that Ca2+ uptake is greater in the dark than in the light and in Grb14−/− compared with wild-type ROS micelles. However, if the dye concentration had been different in the dark and light experiments or Grb14−/− and wild-type ROS micelles, the maximal fluorescence would be different, but not reflect the rate of Ca2+ uptake. To ensure that the difference we observed is not due to the difference in dye uptake of ROS micelles, we measured the channel activity at a high concentration of cGMP (300 μM). At 300 μM cGMP, the peak fluorescence in the two groups is the same (Fig 2A,C), suggesting that the observed channel activities are not due to differences in the dye uptake of ROS micelles and suggesting the possibility of competition between Grb14 and cGMP binding. These data further support the idea that Grb14 is a physiological modulator of CNG channel activity.
RA domain of Grb14 modulates CNG channel function
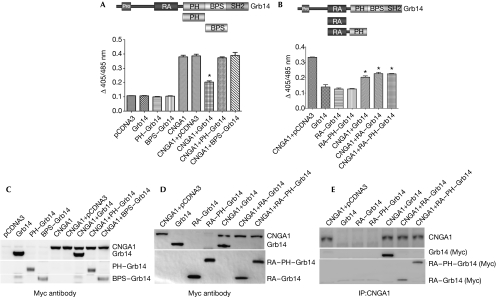
To determine which domain in Grb14 suppresses CNGA1 function, HEK293T cells were transiently co-transfected with Myc–CNGA1 and either full-length Grb14 (residues 1–540) or the specific Grb14 domains: PH (residues 234–341), BPS (residues 342–438), RA–PH (residues 105–355) and RA (residues 105–195). Protein expression was determined by immunoblotting and channel activity was determined by ratiometric measurement of [Ca2+]i in response to cGMP stimulation by means of the calcium-sensitive fluorescent dye indo-1/AM (Fig 3). No suppression of CNGA1 function was observed when the channel was co-expressed with either the PH or BPS domain of Grb14 (Fig 3A). However, a reduction in CNGA1 activity, similar to that seen with full-length Grb14 was observed when cells were transfected with the RA or RA–PH domains (Fig 3B). The lack of suppression of intracellular Ca2+ through CNGA1 in the presence of PH or BPS domains was not due to the lack of protein expression, as both of these domains were expressed at a level comparable with Grb14 (Fig 3C). The expression levels of RA or RA–PH domains in transfected cells were also comparable with full-length Grb14 (Fig 3D). These data point towards the RA domain as being responsible for the modulation of CNGA1 activity by Grb14. The RA and RA–PH domains of Grb14 were able to co-immunoprecipitate along with CNGA1, as was the case with full-length Grb14 (Fig 3E). CNGA1, Grb14, RA–Grb14 and RA–PH–Grb14 expressed alone were used as controls.
Figure 3.
Growth factor receptor-bound protein 14 modulates cyclic nucleotide-gated channel alpha subunit function through its Ras-associating domain. The domain organization of Grb14 is shown. CNGA1 was co-expressed (A) with the PH and BPS domains of Grb14 in HEK293T cells or (B) with the RA or RA–PH domains of Grb14 and examined for their effect on Ca2+ influx in response to cGMP. The data are represented as mean±s.d., n=3 independent transfections. Significance between CNGA1 and CNGA1 plus Grb14 or CNGA1 plus RA–Grb14 or RA–PH–Grb14, *P<0.001. Protein expression in cellular lysates was determined by immunoblotting with Myc antibody for (C) CNGA1, Grb14, PH–Grb14, BPS–Grb14 or (D) CNGA1, Grb14, RA–Grb14 or RA–PH–Grb14, respectively. (E) The physical interaction of the domains shown in (D) with CNGA1 was analysed by standard co-immunoprecipitation procedures using CNGA1 antibody. The immunoprecipitates were immunoblotted with Myc antibody and reprobed with CNGA1 antibody. BPS, between the PH and SH2 domain; cGMP, cyclic guanosine monophosphate; CNGA1, cyclic-nucleotide-gated channel alpha subunit; Grb14, growth factor receptor-bound protein 14; PH, Pleckstrin homology; RA, Ras-associating.
Kinetics of the CNGA1 and Grb14 interaction
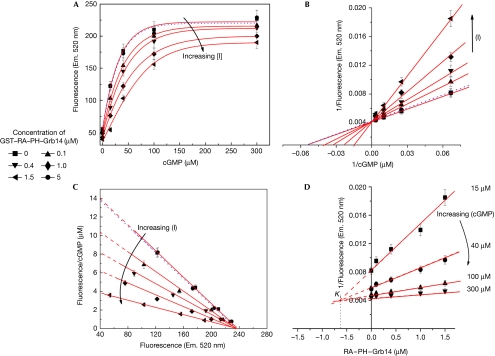
The direct effect of Grb14 on CNGA1 channel activity was investigated using an in vitro fluorometric Ca2+ influx assay, as described in Fig 2. The ROS membrane vesicles were prepared and loaded with the fluorescent calcium indicator Fluo-3. The release of Ca2+ in the vesicles was measured in response to varying concentrations of cGMP, in the presence or absence of exogenously added recombinant GST–RA–PH or GST alone (control; Fig 4). To assess the effect of Grb14 on CNG channel activity, kinetic parameters Vmax, Km and inhibitor constant (Ki) were calculated from initial velocity (Fig 4A), Eadie–Hofstee (Fig 4C) and Dixon plots (Fig 4D). The apparent Michaelis–Menten constant (Km) for cGMP increased from 17±5 μM to 23±5, 30±5, 35±5 and 60±10 μM at GST–RA–PH–Grb14 concentrations of 0, 0.1, 0.4, 1.0 and 1.5 μM, respectively (Fig 4B). Our data indicate that Grb14 interacts with CNGA1 in a competitive manner and increases the Km value of cGMP for CNGA1. A Ki value of 0.65±0.2 μM was calculated for RA–PH domain of Grb14 towards CNG channel activity as determined by Dixon plots (Fig 4D). This finding suggests that Grb14 might be competing for the cGMP binding site, and its inhibitory effect can be greatly alleviated by increasing the concentration of cGMP (Fig 4). It is interesting to note that the purified RA domain (residues 105–195) of Grb14 failed to modulate channel function—as determined by Ca2+ release from ROS vesicles—in response to cGMP stimulation (supplementary Fig S2 online). However, expression of the RA domain (residues 105–195) with CNGA1 in HEK293T cells resulted in suppression of the effect of CNGA1 on [Ca2+]i in response to cGMP. This discrepancy could be due to the improper folding and/or stability of the RA domain of Grb14 in the bacteria. This kinetic data along with the co-expression of Grb14 with CNG channel experiments show that Grb14 did not affect the level of CNGA1 expression, indicating that Grb14 modulates CNG channel function.
Figure 4.
Growth factor receptor-bound protein 14 competitively inhibits Ca2+ influx across the membrane through its Ras-associating domain. (A) Fluo-3 dye was trapped inside the vesicles prepared from bovine ROS and these vesicles were incubated with various concentrations of GST–RA–PH–Grb14 (see key) and followed for the effect of cGMP (0–300 μM)-induced enhancement of fluorescence as a function of Ca2+ influx into the vesicles as indicated. GST (5 μM) is shown with a dotted line. The experiment was performed in triplicate and data were plotted as mean±s.d. (B) Double reciprocal plot of the data shown in (A). (C) Eadie–Hofstee plot of the data further indicating the competitive nature of inhibition. (D) Dixon plot of various concentrations of GST–RA–PH–Grb14 against the reciprocal of fluorescence intensity. cGMP, cyclic guanosine monophosphate; CNGA1, cyclic-nucleotide-gated channel alpha subunit; Em, emission; Grb14, growth factor receptor-bound protein 14; PH, Pleckstrin homology; RA, Ras-associating; ROS, rod outer segment.
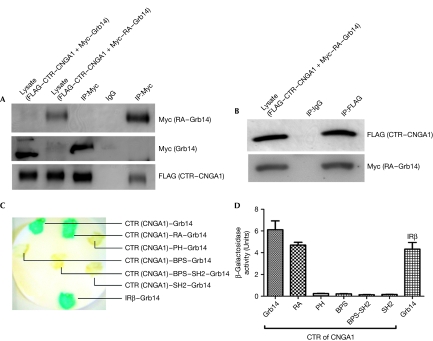
RA domain interacts with the C-terminus of CNGA1
Our kinetic data indicate that Grb14 is a competitive inhibitor of the CNG channel and that the cGMP-binding site is located in the C-terminus of the channel. We expressed the C-terminal region (CTR, residues 483–690) of CNGA1 as a FLAG-tagged protein. The FLAG-tagged CTR–CNGA1 was co-expressed in HEK293T cells with either full-length Myc–Grb14 or Myc–RA–Grb14. FLAG CTR–CNGA1 co-immunoprecipitated with full-length Myc–Grb14 and Myc–RA Grb14 (Fig 5A). Similarly, FLAG CTR–CNGA1 was able to reciprocally co-immunoprecipitate Myc–RA–Grb14 (Fig 5B). The interactions between CTR–CNGA1 and full-length Grb14 and RA–Grb14 were also evaluated by yeast two-hybrid assays. CTR–CNGA1 interacts with both full-length Grb14 and the RA domain of Grb14 (Fig 5C,D). No such interactions were observed when CTR–CNGA1 was co-expressed with the PH, BPS, BPS-SH2 or SH2 domains of Grb14 (Fig 5C,D). These experiments indicate that the RA domain of Grb14 is necessary and sufficient to be able to interact with the CTR region of CNGA1 and inhibit its activity by competing with the cGMP-binding site. The RA domain of Grb14 has recently been shown to bind to activated N-Ras (Depetris et al, 2009). The Ras proteins are small GTPases (Symons & Takai, 2001) whereas CNGA1 is not. However, both proteins are nucleotide-binding proteins. The binding interaction between Grb14 and CNG channel suggests that Grb14 might regulate other ion channels, perhaps through nucleotide-binding sites present in their cytosolic domains. Grb14 functions as a negative regulator of two different proteins: the IR tyrosine kinase (Depetris et al, 2005) and cGMP-gated channel. Both the IR and CNG channels are localized in the plasma membrane of retinal photoreceptor outer segments and Grb14 can interact with these proteins and regulate their activities.
Figure 5.
Carboxy-terminal region of cyclic nucleotide-gated channel alpha subunit interacts with the Ras-associating domain of growth factor receptor-bound protein 14. (A) RA domain of Grb14 or full-length Grb14 were co-expressed with CTR–CNGA1 in HEK293T cells. Protein extracts expressing Myc–RA–Grb14 or Myc–Grb14 were co-immunoprecipitated with Myc antibody. The presence of CTR–CNGA1 in the immunoprecipitate was tested by immunoblot analysis using FLAG antibody and the blot was reprobed with Myc antibody for RA–Grb14 and Grb14. (B) Protein extracts expressing CTR–CNGA1 and RA–Grb14 were subjected to reciprocal co-immunoprecipitation with FLAG antibody. The presence of RA–Grb14 in the immunoprecipitate was tested by immunoblot analysis using Myc antibody and the blot was reprobed with FLAG antibody for CTR–CNGA1. (C) The L40 yeast strain was transformed with pLexA–CTR–CNGA1 and Grb14 or various individual domains of Grb14 and analysed by yeast two-hybrid assay. Positive control included the co-expression of IRβ and Grb14. Transformants were assayed for β-galactosidase by colony colour by filter lift assay and (D) the β-galactosidase activity was quantitatively determined by the solution assay. cGMP, cyclic guanosine monophosphate; CNGA1, cyclic nucleotide-gated channel alpha subunit; CTR, C-terminal region; Grb14, growth factor receptor-bound protein 14; IR, insulin receptor β-subunit; PH, Pleckstrin homology; RA, Ras-associating.
The function of Grb14 interaction with the CNG channel in rod cell physiology is not known. However, we observed that the channel opens at a lower concentration of cGMP in Grb14−/− mice. To maintain the channels in an off state, it is necessary not only to have a precise balance of the cGMP levels, but also to have a control on the cGMP sensitivity of the CNG channels itself. In this study, we show that the sensitivity of the channel to cGMP might be controlled by certain physiological agents, such as Grb14 that is localized in the rod inner segment and translocated to outer segments by bright light stimulus (Rajala et al, 2009). Receptor tyrosine kinase signalling has been shown to modulate the CNG channel function and regulate the sensitivity of rods to light (Molokanova et al, 1997); such a role for Grb14 cannot be ruled out. Grb14 has an important role in IR signalling, which is neuroprotective in the retina (Rajala et al, 2008, 2010). It is tempting to speculate that Grb14–CNG channel interaction might have a role in photoreceptor neuroprotection. Another possible function of Grb14 in rods might be to reduce the membrane noise and make them responsive to slight changes in light conditions. The next question we aim to address is whether light intensity is sufficiently dim for modulation to occur within the physiological range. The binding might instead have some role in protection from light damage. Studies are underway in our laboratory to investigate these questions.
Methods
Cell lines and culture condition. Human embryonic kidney 293T cells were maintained in DMEM medium containing 10% (v/v) fetal bovine serum at 37°C. Approximately 2.5 × 105 cells were seeded in each culture dish 12–18 h before transfection. Calcium phosphate-mediated DNA transfection was performed and cells were collected for experiments approximately 48 h after transfection.
Assessment of the channel activity in ROS vesicles. The effect of Grb14 on CNG channel activity was investigated using a fluorometric Ca2+ assay. Bovine ROS was prepared on a continuous sucrose gradient (Hsu & Molday, 1993), whereas ROS from rat and mouse was prepared on a discontinuous sucrose density gradient centrifugation (Rajala et al, 2009). The ROS membranes (19 mg/ml) were suspended in buffer containing 10 μM Fluo-3, a fluorescent Ca2+indicator and sonicated. The sonicated ROS membranes were then extruded three times through a lipid extruder containing two layers of nucleopore polycarbonate membranes with pore sizes of 400 and 200 nm. The vesicles were dialysed extensively at 4°C for 6 h to exclude untrapped dye. The whole experiment was carried out in the dark to minimize the light bleaching effect on Fluo-3. The sample was diluted and incubated with various concentrations of exogenously added GST–RA–PH–Grb14, GST–RA–Grb14 or GST (5 μM) as indicated. Stock CaCl2 was then added to achieve a final concentration of 100 μM. At 1 min after the addition of Ca2+, the Ca2+ release assay was initiated by the addition of different cGMP concentrations. Free Ca2+ concentration was measured by emission at 520 nm using a fluorometer.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
We thank R.S. Molday (University of British Columbia, Canada) for providing channel antibodies and mammalian expression constructs of CNG channel, L. Tsiokas and M. Elliott (University of Oklahoma) for critical comments on the paper and D. Allen for technical help. This study was supported by grants from the National Institutes of Health (EY016507; EY00871; EY12190).
Footnotes
The authors declare that they have no conflict of interest.
References
- Biel M, Zong X, Ludwig A, Sautter A, Hofmann F (1999) Structure and function of cyclic nucleotide-gated channels. Rev Physiol Biochem Pharmacol 135: 151–171 [DOI] [PubMed] [Google Scholar]
- Daly RJ, Sanderson GM, Janes PW, Sutherland RL (1996) Cloning and characterization of GRB14, a novel member of the GRB7 gene family. J Biol Chem 271: 12502–12510 [DOI] [PubMed] [Google Scholar]
- Depetris RS, Hu J, Gimpelevich I, Holt LJ, Daly RJ, Hubbard SR (2005) Structural basis for inhibition of the insulin receptor by the adaptor protein Grb14. Mol Cell 20: 325–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depetris RS, Wu J, Hubbard SR (2009) Structural and functional studies of the Ras-associating and Pleckstrin-homology domains of Grb10 and Grb14. Nat Struct Mol Biol 16: 833–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn JT, Grunwald ME, Yau KW (1996) Cyclic nucleotide-gated ion channels: an extended family with diverse functions. Annu Rev Physiol 58: 395–426 [DOI] [PubMed] [Google Scholar]
- Han DC, Shen TL, Guan JL (2001) The Grb7 family proteins: structure, interactions with other signaling molecules and potential cellular functions. Oncogene 20: 6315–6321 [DOI] [PubMed] [Google Scholar]
- Hsu YT, Molday RS (1993) Modulation of the cGMP-gated channel of rod photoreceptor cells by calmodulin. Nature 361: 76–79 [DOI] [PubMed] [Google Scholar]
- Kaupp UB, Seifert R (2002) Cyclic nucleotide-gated ion channels. Physiol Rev 82: 769–824 [DOI] [PubMed] [Google Scholar]
- Molokanova E, Trivedi B, Savchenko A, Kramer RH (1997) Modulation of rod photoreceptor cyclic nucleotide-gated channels by tyrosine phosphorylation. J Neurosci 17: 9068–9076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajala A, Daly RJ, Tanito M, Allen DT, Holt LJ, Lobanova ES, Arshavsky VY, Rajala RV (2009) Growth factor receptor-bound protein 14 undergoes light-dependent intracellular translocation in rod photoreceptors: functional role in retinal insulin receptor activation. Biochemistry 48: 5563–5572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajala A, Tanito M, Le YZ, Kahn CR, Rajala RV (2008) Loss of neuroprotective survival signal in mice lacking insulin receptor gene in rod photoreceptor cells. J Biol Chem 283: 19781–19792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajala RV, Chan MD (2005) Identification of a NPXY Motif in growth factor receptor-bound protein 14 (Grb14) and its interaction with the phosphotyrosine-binding (PTB) domain of IRS-1. Biochemistry 44: 7929–7935 [DOI] [PubMed] [Google Scholar]
- Rajala RV, Tanito M, Neel BG, Rajala A (2010) Enhanced retinal insulin receptor-activated neuroprotective survival signal in mice lacking the protein-tyrosine phosphatase-1B gene. J Biol Chem 285: 8894–8904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons M, Takai Y (2001) Ras GTPases: singing in tune. Sci STKE 2001: E1. [DOI] [PubMed] [Google Scholar]
- Yau KW, Baylor DA (1989) Cyclic GMP-activated conductance of retinal photoreceptor cells. Annu Rev Neurosci 12: 289–327 [DOI] [PubMed] [Google Scholar]
- Yau KW, Hardie RC (2009) Phototransduction motifs and variations. Cell 139: 246–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Trudeau MC, Zagotta WN (2002) Rod cyclic nucleotide-gated channels have a stoichiometry of three CNGA1 subunits and one CNGB1 subunit. Neuron 36: 891–896 [DOI] [PubMed] [Google Scholar]
- Zhong H, Lai J, Yau KW (2003) Selective heteromeric assembly of cyclic nucleotide-gated channels. Proc Natl Acad Sci USA 100: 5509–5513 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.