Maternal phosphatidylinositol 3-kinase signalling is crucial for embryonic genome activation and preimplantation embryogenesis
Maternal effect factors derived from oocytes are important for sustaining early embryonic development. This report shows that PI3K/PTEN-PDK1-AKT signaling in oocytes, as a novel maternal effect factor, is crucial for embryonic genome activation and preimplantation embryogenesis in mice.
Abstract
Maternal effect factors derived from oocytes are important for sustaining early embryonic development before the major wave of embryonic genome activation (EGA). In this study, we report a two-cell-stage arrest of embryos lacking maternal 3-phosphoinositide-dependent protein kinase 1 as a result of suppressed EGA. Concurrent deletion of maternal Pten completely rescued the suppressed EGA and embryonic progression through restored AKT signalling, which fully restored the fertility of double-mutant females. Our study identifies maternal phosphatidylinositol 3-kinase signalling as a new maternal effect factor that regulates EGA and preimplantation embryogenesis in mice.
Introduction
After fertilization, the mouse embryo undergoes three mitotic cell divisions before compacting at the eight-cell stage to form individually polarized cells (Rossant & Tam, 2004; Ohsugi et al, 2008). The first major wave of embryonic genome activation (EGA) in mouse embryos occurs at the two-cell stage, after the first mitotic division. A fully grown oocyte contains large amounts of maternal messenger RNAs and proteins, and embryonic development before EGA as well as EGA itself are believed to be dependent on these maternal effect factors (Schultz, 1993; Li et al, 2010). Once EGA occurs, the developmental programme of the embryo is shifted from maternal control to embryonic control. Such reprogramming is coupled with degradation of maternal transcripts and expression of the embryonic genome (for reviews, see Schultz, 2002; Schier, 2007; Li et al, 2010).
Phosphatidylinositol 3-kinase (PI3K) signalling is a fundamental pathway for the regulation of cell proliferation, survival, migration and metabolism in a variety of physiological and pathological processes. PI3Ks are lipid kinases that phosphorylate the 3′-OH group on the inositol ring of inositol phospholipids, whereas phosphatase and tensin homologue deleted on chromosome 10 (PTEN), a lipid phosphatase, reverses this process and thus functions as a negative regulator of PI3K action (Cantley, 2002; Engelman et al, 2006). In cultured mouse embryos, phosphatidylinositol 3,4,5-trisphosphate (PIP3) was constitutively produced as a PI3K product from the one-cell stage to the blastocyst stage, and treatment with the PI3K-specific inhibitor LY294002 arrested the embryonic progression during the two-to-four-cell transition (Halet et al, 2008). These studies indicate that PI3K signalling in embryos might be important for preimplantation embryogenesis in mice.
A considerable proportion of PI3K signalling converges at 3-phosphoinositide-dependent protein kinase 1 (PDK1). PDK1 activates AKT by co-binding to PIP3 (Mora et al, 2004; Engelman et al, 2006); PDK1 also functions as a master kinase to activate other protein kinases of the AGC family (cAMP-dependent, cGMP-dependent and protein kinase C), such as p70 S6 kinase 1 (S6K1) and p90 ribosomal S6 kinase (Mora et al, 2004).
In this study, to determine the functions of maternally derived PI3K signalling in preimplantation embryogenesis, we deleted the Pdk1 gene (also known as Pdpk1 or Pkb kinase) or both the Pdk1 and Pten genes from oocytes and studied the development of these embryos. In this study, we provide experimental evidence to show that maternal PI3K signalling is an indispensable maternal effect factor that regulates EGA and preimplantation embryogenesis in mice.
Results
Developmental arrest of Pdk1 maternally mutant embryos
We deleted the Pdk1 gene in oocytes by crossing Pdk1loxP/loxP mice (Hashimoto et al, 2006) with transgenic mice expressing a Zona pellucida 3 (Zp3) promoter-mediated Cre recombinase (de Vries et al, 2000). By using western blot analysis, we confirmed the absence of PDK1 expression in growing and ovulated oocytes (supplementary Fig S1A,B online). The mutant (Pdk1loxP/loxP;Zp3-Cre) females were observed to be sterile when housed with wild-type males during a testing period from 10–30 weeks of age (supplementary Fig S2B online). However, their follicular development and oocyte maturation were normal (supplementary Fig S1C,D online).
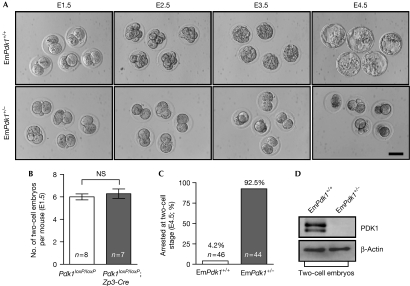
To determine the cause of sterility, mutant and control (Pdk1loxP/loxP) females were housed with wild-type males, and two-cell embryos were recovered from their oviducts on embryonic day 1.5 (E1.5). The morphologies and numbers of two-cell embryos from mutant females were comparable with those from control females (Fig 1A,B), indicating that fertilization of mutant oocytes was normal and that the first cleavage was normal. Subsequently, the two-cell embryos from the mutant females (referred to as EmPdk1+/− embryos) and from the control females (referred to as EmPdk1+/+ embryos) were cultured in vitro. After a 3-day culture period, 92.5% of the EmPdk1+/− embryos were observed to be arrested at the two-cell stage, compared with only 4.2% of EmPdk1+/+ embryos (Fig 1A,C). The EmPdk1+/− embryos eventually died. These results indicate that the sterility of mutant females is caused by the cleavage-stage arrest of their embryos.
Figure 1.
Cleavage-stage arrest of 3-phosphoinositide-dependent protein kinase 1 maternally mutant embryos. (A) Developmental arrest at the two-cell stage of EmPdk1+/− embryos. EmPdk1+/+ and EmPdk1+/− embryos were collected on E1.5 and were cultured for 3 days. Scale bar, 50 μm. (B) The average numbers of two-cell embryos per Pdk1loxP/loxP and Pdk1loxP/loxP;Zp3-Cre female after mating. Numbers of mice used (n) are shown. (C) Percentages of EmPdk1+/+ and EmPdk1+/− embryos that were arrested at the two-cell stage. Numbers of embryos used (n) are shown. (D) Western blots showing the absence of PDK1 protein in two-cell EmPdk1+/− embryos. β-Actin was used as an internal control. NS, not significant; PDK1, 3-phosphoinositide-dependent protein kinase 1.
Notably, the two-cell EmPdk1+/− embryos did not express PDK1 protein (Fig 1D), although they were heterozygous for the Pdk1 gene. This showed that PDK1 protein had not yet been synthesized from the paternal allele in two-cell EmPdk1+/− embryos.
Defective EGA and second cell cycle in mutant embryos
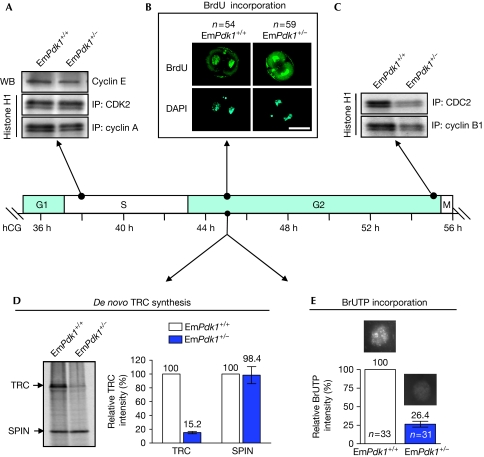
We then investigated the various phases of the second mitotic cell cycle (G1, S, G2 and M) in two-cell EmPdk1+/− embryos. The corresponding hours after human chorionic gonadotropin (hCG) injection are listed for each phase of the cell cycle on the basis of a well-established timeline shown in Fig 2 (Artus & Cohen-Tannoudji, 2008). We observed that in EmPdk1+/− embryos, the kinase profiles for S phase (38 h after hCG injection)—including the expression level of cyclin E and kinase activities of CDK2–cyclin A complex—were not altered (Fig 2A). Furthermore, bromodeoxyuridine was incorporated normally into the nuclei of two-cell EmPdk1+/− embryos after S phase, suggesting that DNA synthesis was unaffected (Fig 2B). We therefore concluded that the G1/S phases of the second cell cycle are normal in EmPdk1+/− embryos.
Figure 2.
Suppressed embryonic genome activation and defective G2/M phases in two-cell EmPdk1+/− embryos. Embryos were cultured for different lengths of time after hCG injection, corresponding to different stages of the second mitotic cell cycle. The arrows indicate time points when embryos were collected for different assays. (A) Levels of cyclin E and kinase activities of CDK2/cyclin A, showing the regular G1-to-S transition in two-cell EmPdk1+/− embryos. (B) Incorporation of BrdU into the nuclei, showing the completion of DNA synthesis in the indicated numbers (n) of EmPdk1+/+ and EmPdk1+/− embryos. Scale bar, 50 μm. (C) Kinase activities of CDC2/cyclin B1, showing the defective G2-to-M transition in EmPdk1+/− embryos. (D) Representative autoradiograph (left) and a histogram (right) showing the restrained TRC synthesis in EmPdk1+/− embryos at G2 phase. SPIN was the internal control. (E) Incorporation of BrUTP into the nuclei, showing the repression of global RNA synthesis in EmPdk1+/– embryos at G2 phase. Representative nuclei are shown above bars in the histogram. The number of nuclei analysed is denoted by n. BrdU, 5-bromo-2′deoxyuridine; BrUTP, 5-bromouridine-5′-triphosphate; DAPI, 4′,6-diamidino-2-phenylindole; hCG, human chorionic gonadotropin; IP, immunoprecipitation; SPIN, spindlin; TRC, transcription-requiring complex; WB, western blot.
In mice, the first major wave of EGA takes place at the two-cell stage (Schultz, 2002). Transcription-requiring complex (TRC), which is synthesized at the G2 phase of the second mitotic cell cycle (42–45 h after hCG injection), is a hallmark of EGA in two-cell embryos (Schultz, 1993; Zeng & Schultz, 2005). By metabolically labelling embryos with 35S-methionine, we observed that the de novo synthesis of TRC in EmPdk1+/− embryos was only 15.2% of that in EmPdk1+/+ embryos (Fig 2D). As an internal control, the expression of spindlin—a protein derived from maternal messenger RNA in early embryos (Oh et al, 1997)—was monitored and observed to be similar in mutant and control embryos, suggesting that the suppressed synthesis of TRC in EmPdk1+/− embryos was not due to global repression.
We further evaluated the overall transcriptional activity in mutant and control two-cell embryos by using 5-bromouridine-5'-triphosphate incorporation assay (Aoki et al, 1997), and observed that de novo RNA synthesis in two-cell EmPdk1+/− embryos was only 26.4% of that in EmPdk1+/+ embryos (Fig 2E). Thus, the loss of maternal Pdk1 led to a suppressed EGA in two-cell EmPdk1+/− embryos.
In addition, for entry into mitosis from the G2 phase, maturation-promoting factor activity, which is a heterodimeric complex of CDC2 (also called CDK1) and cyclin B, is required (Hunter, 1995). In late two-cell EmPdk1+/− embryos (55 h after hCG injection), the kinase activities of CDC2 and cyclin B1 were significantly reduced compared with those in EmPdk1+/+ embryos (Fig 2C), suggesting that the two-cell arrest in EmPdk1+/− embryos was a consequence of restricted maturation-promoting factor activities during G2-to-M transition.
In summary, the above data show that the two-cell arrest of EmPdk1+/− embryos correlated with suppressed EGA and defective G2-to-M transition of the second mitotic cell cycle. This finding is consistent with previous studies suggesting that EGA is required for embryonic progression beyond the two-cell stage (Schultz, 1993).
Co-deletion of maternal Pten rescued the two-cell arrest
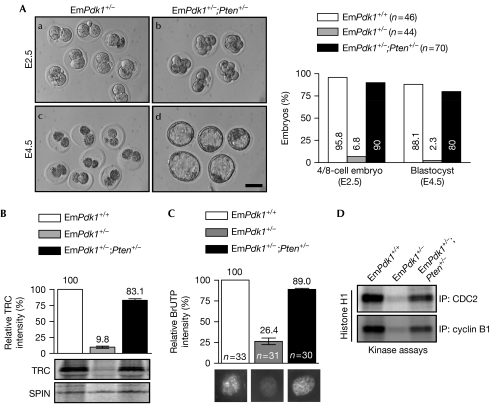
To determine the functional relationship between the maternal PI3K–PDK1 signalling and PTEN in regulating EGA and preimplantation embryogenesis, we generated double-mutant female mice lacking both Pten and Pdk1 in oocytes (referred to as Pdk1loxP/loxP;PtenloxP/loxP;Zp3-Cre mice), and studied the preimplantation development of embryos derived from them. We observed that during the period from 10–30 weeks of age, the fertility of double-mutant females was completely restored when housed with wild-type males (supplementary Fig S2A,B online). It is thus not surprising that embryos derived from such double-mutant females and wild-type males (referred to as EmPdk1+/−;Pten+/− embryos) showed normal preimplantation development. During a 3-day in vitro culture period from E1.5 to E4.5, compared with the two-cell arrest and subsequent demise of EmPdk1+/− embryos (Fig 3Aa,c), most of the EmPdk1+/−;Pten+/− embryos reached the four-to-eight-cell stage (Fig 3Ab) and then the blastocyst stage (Fig 3Ad). Their proportions were comparable with those of EmPdk1+/+ embryos (Fig 3A, histogram). Thus, the concurrent loss of maternal Pten rescued the two-cell arrest of EmPdk1+/− embryos and completely restored the fertility of double-mutant females.
Figure 3.
Concurrent loss of maternal phosphatase and tensin homologue deleted on chromosome 10 restored suppressed embryonic development through recovery of embryonic genome activation and G2-to-M transition in two-cell embryos. (A) EmPdk1+/− and EmPdk1+/−;Pten+/− embryos were collected and cultured as described in Fig 1. Representative images (left) and a histogram (right) indicate the rescued developmental progression of EmPdk1+/−;Pten+/− embryos. The number of embryos is denoted by n. Scale bar, 50 μm. (B) Representative autoradiograph and a histogram showing the rescued TRC synthesis in two-cell EmPdk1+/−;Pten+/− embryos. SPIN was the internal control. (C) Incorporation of BrUTP into the nuclei, showing the rescued global RNA synthesis in two-cell EmPdk1+/−;Pten+/− embryos. Representative nuclei are shown below bars in the histogram. The number of nuclei analysed is denoted by n. (D) Kinase activities of CDC2/cyclin B1 showing the rescued G2-to-M transition in two-cell EmPdk1+/−;Pten+/− embryos. BrUTP, 5-bromouridine-5′-triphosphate; SPIN, Spindlin; TRC, transcription-requiring complex.
Rescued EGA and second cell cycle in DKO embryos
We observed that in EmPdk1+/−;Pten+/− (DKO) embryos, the concurrent loss of Pten had largely rescued the suppressed EGA, as shown by the elevated TRC synthesis (Fig 3B) and global RNA transcription (Fig 3C) in these embryos. Such rescue effects were observed to be largely inhibited by the PI3K-specific inhibitor LY294002 (supplementary Fig S3 online), indicating that the concurrent loss of maternal Pten rescued the development of EmPdk1+/− embryos through elevated PIP3 levels. Furthermore, in vitro kinase assays showed recovered CDC2 and cyclin B1 activities in late two-cell EmPdk1+/−;Pten+/− embryos (Fig 3D), indicating a normal G2-to-M transition. These data suggest that the maternal PI3K/PTEN signalling directly controls early embryogenesis through regulation of EGA. The restored EGA in EmPdk1+/−;Pten+/− embryos enabled the continuous development of EmPdk1+/−;Pten+/− embryos.
Restored AKT signalling in EmPdk1+/−;Pten+/− embryos
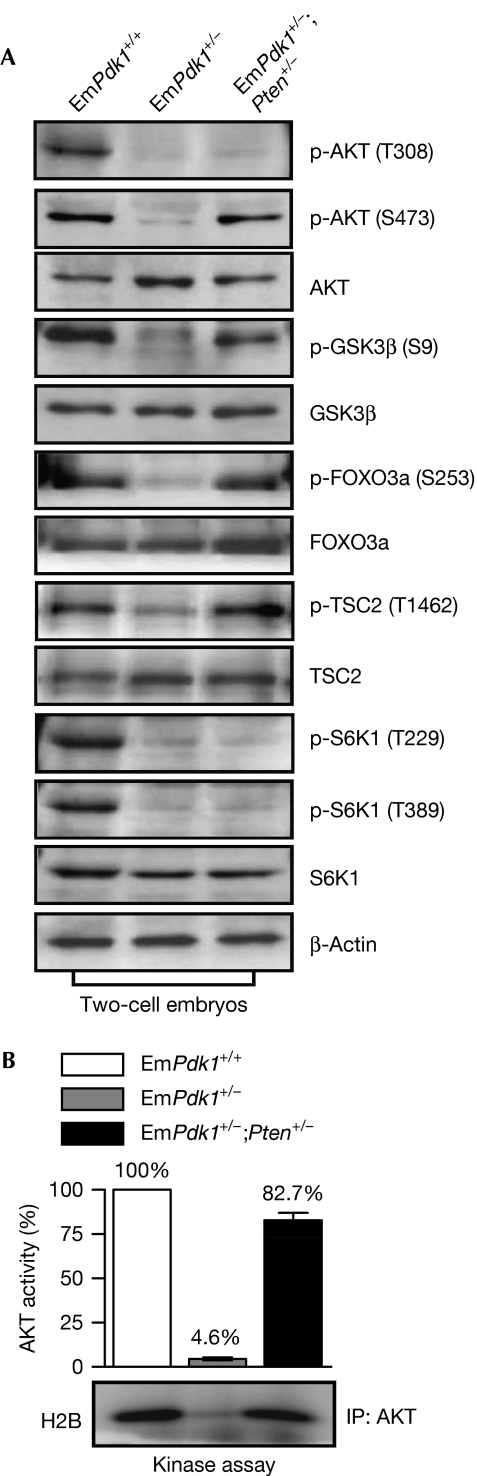
To determine how the concurrent loss of maternal Pten rescued EGA and embryonic development in EmPdk1+/−;Pten+/− embryos, we studied the activation of AKT that is downstream from PI3K and PTEN. In two-cell EmPdk1+/−;Pten+/− embryos, although the phosphorylation of AKT at Thr 308 that is performed by PDK1 (Mora et al, 2004) was still absent, the phosphorylation of AKT at Ser 473 that is achieved through mammalian target of rapamycin complex 2 (Sarbassov et al, 2005) was fully recovered, in contrast with the absence of phosphorylation of AKT at Ser 473 in two-cell EmPdk1+/− embryos (Fig 4A). The recovery of AKT phosphorylation at Ser 473 in EmPdk1+/−;Pten+/− embryos led to at least partly restored AKT kinase activity, as the phosphorylation of several known AKT substrates, including forkhead box O3, glycogen synthase kinase-3β and tuberous sclerosis 2 (Manning & Cantley, 2007) was restored in EmPdk1+/−;Pten+/− embryos (Fig 4A). The partial recovery of AKT activity in two-cell EmPdk1+/−;Pten+/− embryos was confirmed by in vitro AKT kinase assay (Fig 4B).
Figure 4.
Concurrent deletion of maternal Pten restored phosphatidylinositol 3-kinase–AKT signaling in two-cell EmPdk1+/−;Pten+/− embryos. (A) Levels of p-AKT (Thr 308), p-AKT (Ser 473), p-GSK3β (Ser 9), p-FOXO3a (Ser 253), p-TSC2 (Thr 1462), p-S6K1 (Thr 229), and p-S6K1 (Thr 389) were determined by using western blotting. Note that p-AKT (Ser 473) was recovered in EmPdk1+/−;Pten+/− embryos, restoring AKT activity as shown by the elevated levels of p-GSK3β (Ser 9), p-FOXO3a (Ser 253), and p-TSC2 (Thr 1462). Levels of p-S6K1 (Thr 229) and p-S6K1 (Thr 389) were not restored. (B) In vitro AKT kinase assay showing the restoration of AKT activity in two-cell EmPdk1+/−;Pten+/− embryos. FOXO3a, forkhead box O3; GSK3β, glycogen synthase kinase 3 beta; H2B, recombinant histone H2B; IP, immunoprecipitation; PI3K, phosphatidylinositol 3-kinase; S6K1, ribosomal protein S6 kinase, polypeptide 1; TSC2, tuberous sclerosis 2.
With elevated AKT activity, the loss of maternal Pten probably allows the EmPdk1+/−;Pten+/− embryos to initiate EGA and to develop beyond the two-cell stage, the subsequent expression of PDK1 from the embryonic genome then sustains later development of EmPdk1+/−;Pten+/− embryos and pups.
Given that the phosphorylation of AKT at Thr 308 by PDK1 has been considered to be a prerequisite for subsequent phosphorylation at Ser 473 (Mora et al, 2004), it would be interesting to study the molecular mechanisms underlying the partial recovery of Ser 473 phosphorylation and AKT activity in double-mutant embryos in the absence of a priming Thr 308 phosphorylation by PDK1.
In addition, the phosphorylation of S6K1 at Thr 229 and Thr 389 in two-cell EmPdk1+/− or EmPdk1+/−;Pten+/− embryos were at very low levels (Fig 4A), indicating that the mTORC1–S6K1 signalling was not restored in two-cell EmPdk1+/−;Pten+/− embryos, and was therefore irrelevant for the resumption of embryonic progression.
Discussion
During embryonic development before implantation in mice, maternal effect factors are believed to be important for sustaining embryonic development, at least up to the time when the embryonic genome is activated (Schultz, 1993; Li et al, 2010). In this study, we have shown that blockage of maternal PI3K signalling by deletion of Pdk1 from oocytes leads to the arrest of resultant embryos at the two-cell stage, which is most probably a consequence of suppressed EGA and a defective G2/M phase at the two-cell stage. Furthermore, concurrent loss of maternal Pten recovered the impaired AKT activation, rescued the suppressed EGA and two-cell arrest of embryos, and restored the fertility of double-mutant females. We therefore identified the maternal PI3K/PTEN–PDK1–AKT signalling cascade as an indispensable maternal effect factor in triggering EGA and sustaining preimplantation embryogenesis in mice.
Preimplantation embryos from mice and humans can survive and develop in vitro in a defined culture medium lacking exogenous growth factors or serum (Whitten & Biggers, 1968; Edwards et al, 1969), which suggests that early embryos contain intrinsic signals that promote their survival and development (O'Neill, 2008). Previous studies have indicated that certain paracrine or autocrine factors might activate intracellular signalling events that are needed for early embryonic development (Kane et al, 1997; O'Neill, 2008). In this study, we have presented several lines of in vivo evidence that maternal PI3K signalling provides intrinsic signals to the embryo for sustaining its autonomous preimplantation development.
In recent years, a growing number of maternal effect factors have been identified, such as Ago2, Npm2, Brg1, Hsf1, Bnc1, Stella, Zar1 and the subcortical maternal complex (for a review, see Li et al, 2010). It would therefore be of interest to investigate the relationships between maternal PI3K/PTEN–PDK1–AKT signalling and these maternal effect factors. Furthermore, it is important to identify the downstream effectors of maternal PI3K signalling during preimplantation embryogenesis. Studies of how maternal PI3K signalling might regulate preimplantation embryogenesis in humans would also be useful lines of investigation.
Methods
Most of the methods used are described in the supplementary information online.
Collection and culture of embryos. For studies of embryonic progression in vitro, five-week-old female mice were housed with wild-type males and vaginal plugs were checked every morning. The day on which a vaginal plug was observed was defined as E0.5. On E1.5, two-cell embryos were flushed out of the oviducts with M2 medium (Sigma-Aldrich) and cultured in potassium simplex optimization medium (KSOM) supplemented with amino acids (Chemicon) for 3 days in a 37°C incubator under 5% CO2. For western blot and kinase activity assay, superovulation was primed by intraperitoneal injection of 7.5 IU pregnant mare serum gonadotropin (Sigma-Aldrich) per female, followed by injection of 7.5 IU hCG (Sigma-Aldrich) 48 h later. The female mice were housed with wild-type males at the time of hCG injection. One-cell embryos were collected from the oviducts 20 h after hCG injection and were cultured for different lengths of time corresponding to different stages of the first two mitotic cell cycles, based on the well-established timeline of in vitro embryonic development (Artus & Cohen-Tannoudji, 2008).
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
We thank M. Kasuga for kindly providing the Pdk1loxP/loxP mice. This study was supported by grants to K.L. from the Swedish Research Council, the Swedish Cancer Foundation, the Young Researcher Award of Umeå University, Sweden, the Torsten and Ragnar Söderberg Foundation, Sweden, and the Novo Nordisk Foundation, Denmark. This work was also supported by the Cutting-Edge Research Grant from the County Council of Västerbotten, Sweden, to E.L.
Footnotes
The authors declare that they have no conflict of interest.
References
- Aoki F, Worrad DM, Schultz RM (1997) Regulation of transcriptional activity during the first and second cell cycles in the preimplantation mouse embryo. Dev Biol 181: 296–307 [DOI] [PubMed] [Google Scholar]
- Artus J, Cohen-Tannoudji M (2008) Cell cycle regulation during early mouse embryogenesis. Mol Cell Endocrinol 282: 78–86 [DOI] [PubMed] [Google Scholar]
- Cantley LC (2002) The phosphoinositide 3-kinase pathway. Science 296: 1655–1657 [DOI] [PubMed] [Google Scholar]
- de Vries WN, Binns LT, Fancher KS, Dean J, Moore R, Kemler R, Knowles BB (2000) Expression of Cre recombinase in mouse oocytes: a means to study maternal effect genes. Genesis 26: 110–112 [PubMed] [Google Scholar]
- Edwards RG, Bavister BD, Steptoe PC (1969) Early stages of fertilization in vitro of human oocytes matured in vitro. Nature 221: 632–635 [DOI] [PubMed] [Google Scholar]
- Engelman JA, Luo J, Cantley LC (2006) The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet 7: 606–619 [DOI] [PubMed] [Google Scholar]
- Halet G, Viard P, Carroll J (2008) Constitutive PtdIns(3,4,5)P3 synthesis promotes the development and survival of early mammalian embryos. Development 135: 425–429 [DOI] [PubMed] [Google Scholar]
- Hashimoto N et al. (2006) Ablation of PDK1 in pancreatic beta cells induces diabetes as a result of loss of beta cell mass. Nat Genet 38: 589–593 [DOI] [PubMed] [Google Scholar]
- Hunter T (1995) Protein kinases and phosphatases: the yin and yang of protein phosphorylation and signaling. Cell 80: 225–236 [DOI] [PubMed] [Google Scholar]
- Kane MT, Morgan PM, Coonan C (1997) Peptide growth factors and preimplantation development. Hum Reprod Update 3: 137–157 [DOI] [PubMed] [Google Scholar]
- Li L, Zheng P, Dean J (2010) Maternal control of early mouse development. Development 137: 859–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning BD, Cantley LC (2007) AKT/PKB signaling: navigating downstream. Cell 129: 1261–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora A, Komander D, van Aalten DM, Alessi DR (2004) PDK1, the master regulator of AGC kinase signal transduction. Semin Cell Dev Biol 15: 161–170 [DOI] [PubMed] [Google Scholar]
- O'Neill C (2008) The potential roles for embryotrophic ligands in preimplantation embryo development. Hum Reprod Update 14: 275–288 [DOI] [PubMed] [Google Scholar]
- Oh B, Hwang SY, Solter D, Knowles BB (1997) Spindlin, a major maternal transcript expressed in the mouse during the transition from oocyte to embryo. Development 124: 493–503 [DOI] [PubMed] [Google Scholar]
- Ohsugi M, Zheng P, Baibakov B, Li L, Dean J (2008) Maternally derived FILIA–MATER complex localizes asymmetrically in cleavage-stage mouse embryos. Development 135: 259–269 [DOI] [PubMed] [Google Scholar]
- Rossant J, Tam PP (2004) Emerging asymmetry and embryonic patterning in early mouse development. Dev Cell 7: 155–164 [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM (2005) Phosphorylation and regulation of Akt/PKB by the rictor–mTOR complex. Science 307: 1098–1101 [DOI] [PubMed] [Google Scholar]
- Schier AF (2007) The maternal–zygotic transition: death and birth of RNAs. Science 316: 406–407 [DOI] [PubMed] [Google Scholar]
- Schultz RM (1993) Regulation of zygotic gene activation in the mouse. Bioessays 15: 531–538 [DOI] [PubMed] [Google Scholar]
- Schultz RM (2002) The molecular foundations of the maternal to zygotic transition in the preimplantation embryo. Hum Reprod Update 8: 323–331 [DOI] [PubMed] [Google Scholar]
- Whitten WK, Biggers JD (1968) Complete development in vitro of the pre-implantation stages of the mouse in a simple chemically defined medium. J Reprod Fertil 17: 399–401 [DOI] [PubMed] [Google Scholar]
- Zeng F, Schultz RM (2005) RNA transcript profiling during zygotic gene activation in the preimplantation mouse embryo. Dev Biol 283: 40–57 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.