Abstract
Objectives
To investigate the association between meniscal pathology and incident or enlarging bone marrow lesions (BMLs) in knee osteoarthritis (OA)
Methods
We studied subjects from the Multicenter Osteoarthritis Study (MOST) aged 50 to 79 either with knee OA or at high risk of the disease. Baseline and 30-months magnetic resonance images of knees (n=1344) were scored for subchondral BMLs. Outcome was defined as an increase in BML score in either the tibial or femoral condyle in medial and lateral compartments, respectively. We defined meniscal pathology at baseline as the presence of either meniscal lesions or meniscal extrusion. We estimated the risk of an increase in BML score in relation to meniscal status in the same compartment using a log linear regression model adjusted for age, sex, body mass index, physical activity level, and mechanical axis. In secondary analyses we stratified by ipsilateral tibiofemoral cartilage status at baseline and compartments with pre-existing BMLs.
Results
The adjusted relative risk of incident or enlarging BMLs ranged from 1.8; 95% confidence interval (95% CI) 1.3, 2.3 for mild medial meniscal pathology to 5.0; 95% CI 3.2, 7.7 for major lateral meniscal pathology (using no meniscal pathology in the same compartment as reference). Stratification by cartilage or BML status at baseline had essentially no effect on these estimates.
Conclusions
Knee compartments with meniscal pathology have a substantially increased risk of incident or enlarging subchondral BMLs over 30 months. Higher relative risks were seen in those with more severe and with lateral meniscal pathology.
Keywords: Bone marrow lesion, Menisci, Tibial, Knee, Osteoarthritis, Magnetic Resonance Imaging
The menisci are two wedge-shaped fibrocartilageous discs located one in each tibiofemoral compartment of the knee. They absorb shocks and distribute load over the joint cartilage covering the tibial plateau and femoral condyle [1–3]. When a meniscus is damaged by injury, degenerative processes, or removed by surgery there is an increased risk of developing knee osteoarthritis (OA) [4–7]. The knee is one of the most frequent locations of OA, causing pain and disability to a large proportion of the middle-aged and elderly [8]. The mechanism(s) by which meniscal injury increases OA risk is primarily assumed to be due to increased and altered biomechanical loading of the joint cartilage which initiates or accelerates a pathologic response involving several joint tissues [9–11]. Still, there is little evidence of the temporal sequence of events in this process and the contributing role of the various OA features.
Bone marrow lesions (BMLs) are often seen on magnetic resonance imaging (MRI) as ill-defined signal alterations adjacent to the subchondral plate [12]. They typically represent a number of noncharacteristic histologic abnormalities including bone marrow necrosis, bone marrow fibrosis, trabecular abnormalities, and bone marrow oedema [13]. In the acutely injured knee BMLs have been described as a footprint of the trauma, i.e., typically from the impact collision between the femoral condyle and tibial plateau [14, 15]. However, BMLs also seem to develop in areas subjected to chronic excess of focal loading such as occur when the limb is malaligned [16] and may also possibly be associated with the use of antiresorptive drugs [17]. BMLs have been linked with knee pain [18, 19], and are thus of particular interest as a target for therapeutic intervention or prevention. Also, BMLs are associated with cartilage loss and joint space narrowing [16, 20, 21], and predict prevalent and incident subchondral bone attrition in the same subregion [22]. In recent cross-sectional studies BMLs have been related to dynamic knee loading [23], and meniscal pathology has been associated with ipsilateral BMLs and increased bone mineral density [24, 25]. However, OA pathology including meniscal lesions, BMLs, hyaline cartilage loss and even synovitis often co-occurs so the contemporaneous occurrence of BMLs and meniscal lesions provides little insight into which comes first or whether one of these lesions might increase the risk of the other. Meniscal pathology may result in injury to other joint structures by focusing rather than distributing loading forces. Hypothetically, if there is increased stress on subchondral bone, BMLs may result. However, in theory both meniscal pathology and BMLs may be independent consequences of abnormal load transmission in the knee caused by e.g. malalignment or instability. To better identify the temporal sequence of joint injury leading to OA we examined the association between meniscal pathology at baseline and the development of BMLs among subjects of the Multicenter Osteoarthritis Study (MOST).
PATIENTS AND METHODS
The Multicenter Osteoarthritis Study
MOST is a large, prospective cohort study of individuals aged 50–79 years, in which the goal was to identify risk factors for incident and progressive knee OA.
Study subjects either had knee OA at baseline or were at high risk of developing the disease. Factors considered to contribute to a high risk of knee OA included being overweight or obese, having either frequent knee pain, aching, or stiffness on most of the preceding 30 days, a prior knee injury that made it difficult to walk for at least 1 week, or previous knee surgery. All 3,026 subjects were recruited from two communities in the US (Birmingham, Alabama and Iowa City, Iowa) through mass mailing of letters and study brochures, supplemented by media and community outreach campaigns. Subjects were excluded if they screened positive for rheumatoid arthritis [26], had ankylosing spondylitis, psoriatic arthritis, chronic reactive arthritis, a severe medical condition that made continued participation in the study unlikely, bilateral knee replacement surgery, inability to walk without the help of another person or walker, or were planning to move out of the area during the next 3 years.
The baseline and 30-month assessments followed the same protocol, and each included a telephone interview and clinic visit. Subjects completed a survey on physical activity, the Physical Activity Scale for the Elderly (PASE) [27], and were weighed and had their height measured.
Acquisition and grading of knee MRI scans
At baseline and 30-month follow-up, knee MRIs of all MOST participants who were willing and eligible were obtained with a 1.0T MR system (OrthOne; ONI, Wilmington, MA) with a circumferential transmit–receive extremity coil. MRIs were performed using sagittal and axial fat-suppressed fast spin-echo proton density–weighted sequences (repetition time [TR] 5,800/2,500 msec, time to echo [TE] 35 msec, slice thickness 3 mm, field of view [FOV] 14 cm, matrix 288 × 192 pixels), and coronal STIR sequence (TR 7,820 msec, TE 15 msec, slice thickness 3 mm, FOV 14 cm, matrix 256 × 256 pixels) [28]. Two musculoskeletal radiologists (AG and FWR), who were blinded to clinical and radiographic data and unaware of the study hypothesis, read the paired images separately with knowledge of time sequence. In this study we included all participants whose MRIs at baseline and 30-months follow-up had been read at the time of data analysis. These were selected for reading according to a protocol previously detailed [20].
MRI study variables
MRI findings of knee OA were assessed with the Whole-Organ MRI Score (WORMS) method [29]. Meniscal tear, maceration, and (or) destruction or resection of the anterior horn, body segment, and the posterior horn of the medial and lateral menisci, which in this study are collectively referred to as meniscal lesions, were graded separately on a 5-item ordered scale of 0–4, where 0 = intact, 1 = minor radial or parrot-beak tear, 2 =nondisplaced tear, 3 = displaced tear or partial maceration or destruction, and 4 = complete maceration, destruction, or resection (interobserver weighted κ= 0.80). The readers regarded an increased intrameniscal signal (often a linear signal within the meniscus) as a meniscal tear when it communicated with the inferior or superior margin, and (or) free edge of the meniscus on at least 2 slices.
In addition, medial and lateral meniscal extrusion were graded as 0 = absent, 1 = ≤50%, and 2 = >50% from the midposterior coronal slice where the medial tibial spine was depicted to its maximum extent (interobserver weighted κ= 0.60). The point of reference for meniscal extrusion was the tibial plateau osteochondral junction at the joint margin (excluding osteophytes) (29).
As the main exposure variable for our analyses we combined the two constructs: meniscal lesions and meniscal extrusion to create a 3-item ordered categorical variable referred to as meniscal pathology: none = intact meniscus and grade 0 meniscal extrusion, minor = minor or nondisplaced tear or meniscal extrusion grade 1, and major = displaced tear, maceration, destruction, resection, or meniscal extrusion grade 2. We assigned a compartment of the knee the highest grade of meniscal lesion from any of the 3 subregions of the meniscus. Because we had fewer knees in subset analyses, we created a dichotomous meniscal exposure variable into no pathology = intact meniscus and grade 0 meniscal extrusion vs. pathology = meniscal lesion or extrusion. We also performed secondary analyses addressing the two constructs of meniscal pathology, meniscal lesion and meniscal extrusion as exposure variable, separately.
Subchondral BML size was scored from 0 to 3 at baseline and 30-month follow-up in 5 tibiofemoral regions of the medial and lateral compartment, respectively according to WORMS method (10 subregions in total: anterior, central, and posterior surface of the tibial plateau and the central and posterior articular surface of the femur) [29]. In a modification of WORMS developed for longitudinal readings, a score of 0.5 for any of the BMLs was introduced to reflect a within-grade change (+0.5 or −0.5 reflecting within-grade enlargement or regression, respectively).
An increase from baseline to follow-up in the sum of BML grades in either the tibial or femoral condyle of the compartment by +0.5 or more was regarded as an incident BML (fig 1 and fig 2) or enlarging BML (fig 3). The weighted κ of inter-reader reliability for the readings of BMLs (comparing longitudinal change in each subregion) was 0.59 with 88% observed agreement (κ negatively affected by the uneven prevalence, i.e., vast majority of subregions showing no change). An additional validation of BML for the 1.0 T images has been performed for 53 knees that also had received a 1.5 T MRI with the same sequence protocol. The weighted κ of the reliability readings for BML scoring was 0.71. Sensitivity and specificity for BML assessment using the 1.5 T readings as a reference standard was 73% and 96%, respectively [20, 30].
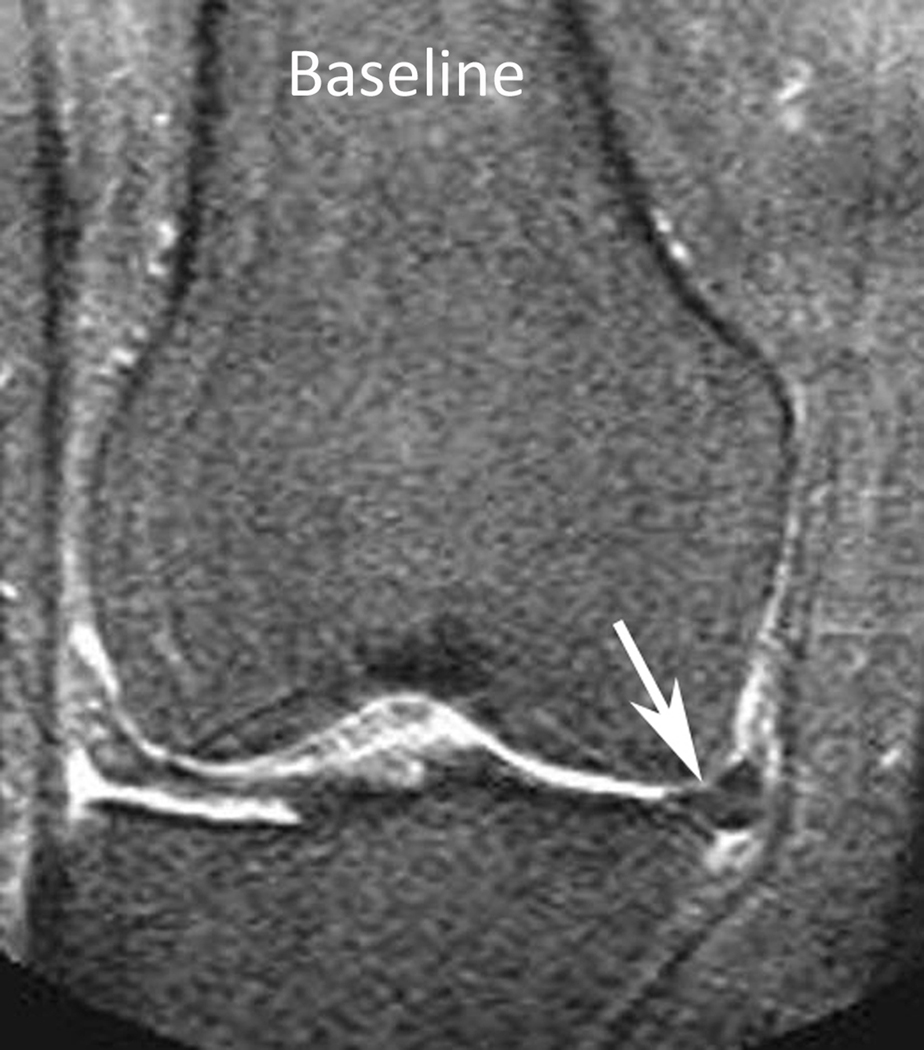
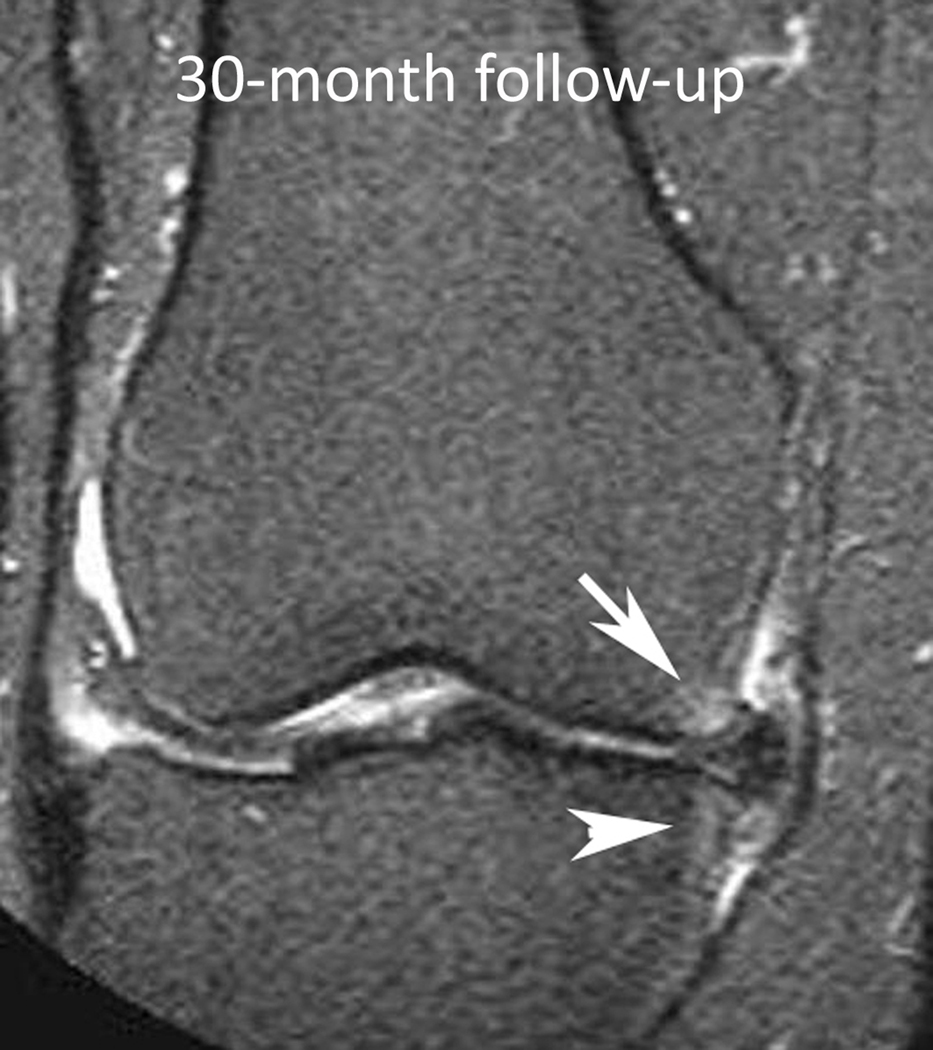
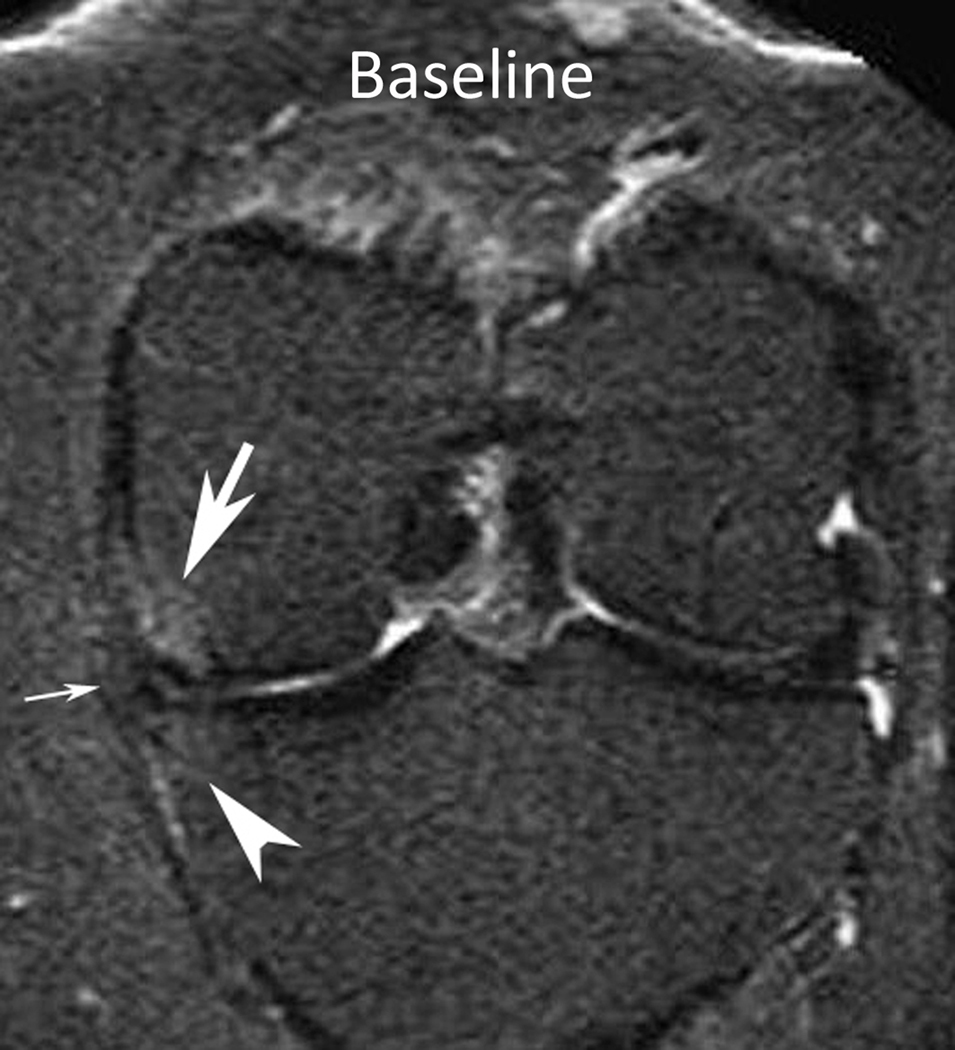
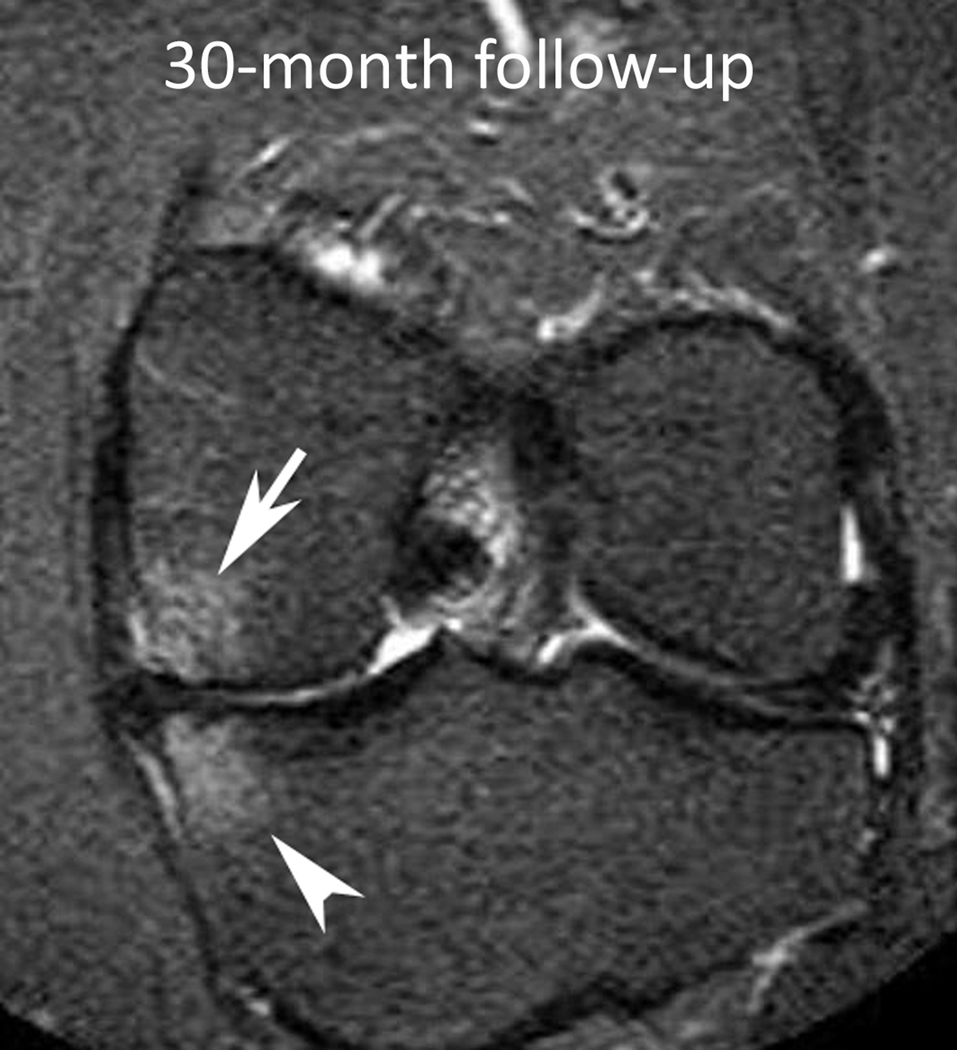
Figure 1.
Baseline: Mid coronal STIR MRI shows extrusion of the body of the medial meniscus (arrow) without bone marrow lesion (BML). 30-month follow-up: Coronal STIR MRI at the same level 30 months later shows stable extruded medial meniscus and new BMLs (WORMS grade 1) of the central medial femur (arrow) and tibia (arrowhead). There are also new small marginal osteophytes of the medial femur (WORMS grade 1) and tibia (WORMS grade 2).
Figure 2.
Baseline: Sagittal fat-suppressed proton density-weighted MRI shows partial maceration of the posterior horn of the medial meniscus (arrow) without bone marrow lesions (BMLs). 30-month follow-up: Sagittal fat-suppressed proton density-weighted MRI at the same level 30 months later shows stable partially macerated medial meniscus and new large BMLs (WORMS grade 3) of the posterior medial femur (arrows) and anterior, central and posterior medial tibia (arrowheads). There is also new small joint effusion (WORMS grade 1).
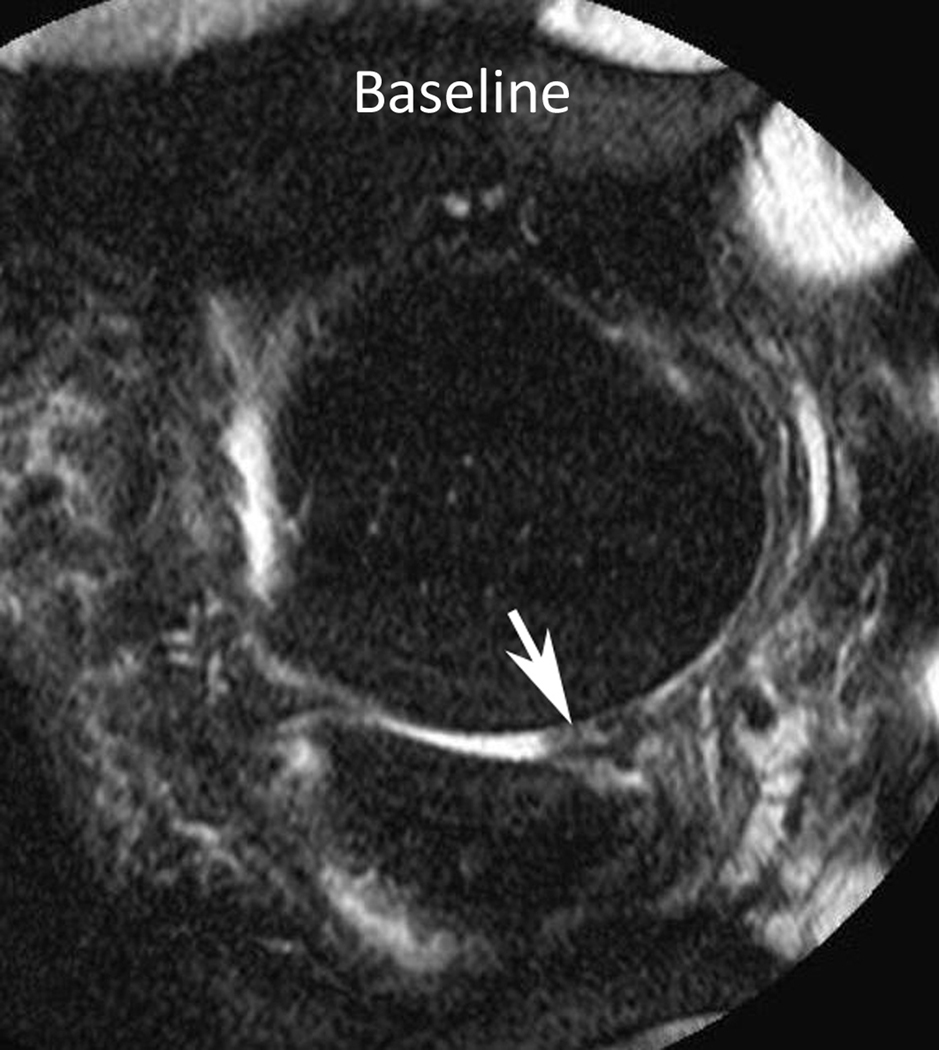
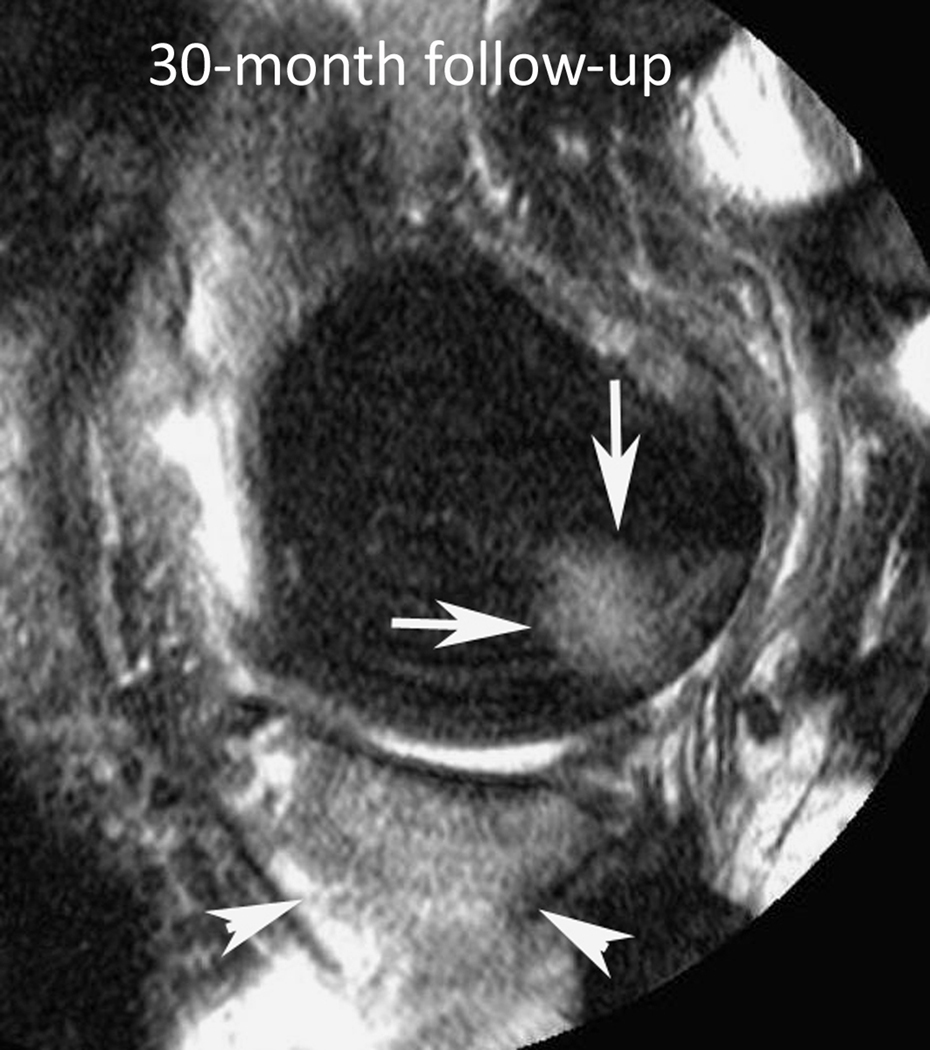
Figure 3.
Baseline: Mid coronal STIR MRI shows extrusion of the partially macerated body of the medial meniscus (small arrow) with small (WORMS grade 1) bone marrow lesions (BMLs) of the central medial femur (arrow) and tibia (arrowhead). 30-month follow-up: Coronal STIR MRI at the same level 30 months later shows enlarging BMLs (WORMS grade 2) of the central medial femur (arrow) and tibia (arrowhead).
Cartilage signal and morphology was also graded semiquantitatively according to WORMS from grade 0 to 6 in the articular surface regions (interobserver weighted κ= 0.78) (28). We defined a grade of 2 (partial thickness focal defect <1 cm in greatest width) or higher as a cartilage lesion in any of the following 5 tibiofemoral regions in the medial and lateral compartment, respectively: anterior, posterior, and central surface of the tibial plateau and the central and posterior articular surface of the femur.
Acquisition and grading of knee radiographs
At the baseline clinic visits, subjects underwent weight-bearing posteroanterior knee radiography, using a fixed flexion protocol [31, 32]. One musculoskeletal radiologist and 1 of 2 rheumatologists graded all films according to the Kellgren/Lawrence (K/L) scale [33]; discrepancies were adjudicated by a panel of 3 readers. Readers were blinded to MRI findings and clinical data. The two person interobserver reliability for determining K/L grade ranged from κ= 0.77 to 0.80. Radiographic tibiofemoral OA at baseline was considered present if the knee had K/L grade ≥2 on the posteroanterior film.
Full-limb radiographs of both legs were obtained at baseline. The mechanical axis was defined as the angle formed by the intersection of a line from the centre of the head of the femur to the centre of the femoral notch in the knee, and a second line from the centre of the talus to the centre of the tibial spines in the knee (for interobserver agreement intraclass correlation coefficient was 0.99).
Statistical analysis
To evaluate the relation of severity of medial and lateral meniscal pathology to incident or enlarging BML(s) in the ipsilateral compartment, we used contingency tables for crude analyses and calculated adjusted relative risks (RRs) with a log linear regression model using the Poisson assumption and robust variance estimates. We used generalized estimating equations to account for correlation between two knees from the same subject and controlled for age, sex, body mass index, physical activity level, and mechanical axis (categorised) at baseline. We performed secondary analyses stratified by cartilage and baseline BML status in the ipsilateral compartment using our binary meniscal exposure variable. In an additional analysis we evaluated the association between baseline meniscal pathology and regression or resolution of a BML (i.e., decrease in sum of BML grades by 0.5 or more in a condyle) in compartments with pre-existing BML. All tests were performed using SAS for Windows, version 9.1 (SAS Institute, Cary, NC). P values less than or equal to 0.05 were considered statistically significant.
RESULTS
The study sample consisted of 1344 knees from 1204 persons (60.5% women) who had baseline and 30-month follow-up MRIs read at the time of analysis. The mean (SD) age of subjects was 62.2 (7.9) with a mean (SD) body mass index of 30.0 (4.8) and 585 (43.5%) knees had radiographic tibiofemoral OA at baseline. BMLs were found in 490 medial compartments (36.5%) and 245 lateral compartments (18.2%) at baseline. At the 30-month visit 279 of 1344 knees (20.8%) had an incident and (or) enlarging subchondral BML in the tibial and (or) femoral condyle of the medial compartment. The corresponding share for the lateral compartment was 125 out of 1342 (9.3%, missing data for 2 knees). Meniscal pathology at baseline was more frequent in knee compartments which had incident or enlarging BMLs (table 1).
Table 1.
Characteristics at baseline according to outcome, incident or enlarging bone marrow lesion (BML) in either the femoral or tibial condyle of the same compartment as the meniscal exposure variable.
| Incident or enlarging BML in medial compartment |
||
|---|---|---|
| Yes (n=280) | No (n=1064) | |
| Age, mean ± SD years | 62.7 ± 8.2 | 61.9 ± 7.8 |
| Female knee, n (%) | 160 (57) | 639 (60) |
| Body mass index, kg/m2, mean ± SD kg/m2 | 30.3 ± 4.7 | 29.9 ± 4.8 |
| Physical activity level (PASE), mean ± SD | 177 ± 85 | 182 ± 90 |
| Radiographic tibiofemoral osteoarthritis, n (%) | 171 (61) | 414 (39) |
| Medial tibiofemoral JSN grade ≥1, n (%) | 186 (67) | 341 (32) |
| Mechanical axis, n (%) | ||
| varus (<179°) | 183 (66) | 468 (44) |
| neutral (179°–180°) | 65 (24) | 354 (34) |
| valgus (>180°) | 28 (10) | 231 (22) |
| Medial meniscal pathology, n (%) | 209 (75) | 534 (50) |
| meniscal lesion, n (%) | 162 (58) | 346 (33) |
| meniscal extrusion, n (%) | 174 (64) | 439 (41) |
| Medial BML, n (%) | 155 (56) | 335 (31) |
| Cartilage lesion in medial compartment, n (%) | 239 (86) | 672 (63) |
| Incident or enlarging BML in lateral compartment |
||
|---|---|---|
| Yes (n=125) | No (n=1217) | |
| Age, mean ± SD years | 62.6 ± 8.5 | 62.0 ± 7.8 |
| Female knee, n (%) | 83 (66) | 714 (59) |
| Body mass index, kg/m2, mean ± SD kg/m2 | 29.1 ± 4.7 | 30.1 ± 4.8 |
| Physical activity level (PASE), mean ± SD | 170 ± 73 | 183 ± 90 |
| Radiographic tibiofemoral osteoarthritis, n (%) | 74 (59) | 510 (42) |
| Lateral tibiofemoral JSN grade ≥1, n (%) | 47 (38) | 94 (8) |
| Mechanical axis, n (%) | ||
| varus (<179°) | 43 (35) | 608 (51) |
| neutral (179°–180°) | 34 (27) | 383 (32) |
| valgus (>180°) | 47 (38) | 212 (18) |
| Lateral meniscal pathology, n (%) | 62 (50) | 163 (13) |
| meniscal lesion, n (%) | 48 (38) | 116 (10) |
| meniscal extrusion, n (%) | 40 (33) | 94 (8) |
| Lateral BML, n (%) | 53 (42) | 192 (16) |
| Cartilage lesion in lateral compartment, n (%) | 100 (80) | 532 (44) |
SD = standard deviation, PASE = Physical Activity Scale for the Elderly (the higher score the higher the physical activity level), JSN = joint space narrowing.
Missing values: medial (n=3) and lateral (n=3) tibiofemoral JSN grade, mechanical axis (n=15), medial (n=11) and lateral (n=10) meniscal extrusion, incomplete BML status in one condyle in medial compartment (n=1), cartilage lesion in medial (n=2) and lateral (n=1) compartment.
In the crude analysis of the severity of meniscal pathology, there was an increased risk of incident or enlarging BML(s) in the ipsilateral compartment if having minor or major meniscal pathology (using no meniscal pathology as the reference category). The multivariable model did not materially alter the estimates of risk from the crude analysis. (table 2).
Table 2.
Relative risk (RR) of incident or enlarging bone marrow lesion(s) (BML) in the ipsilateral compartment by different severity of meniscal pathology.
| Incident or enlarging | Crude RR | Adjusted RR | |
|---|---|---|---|
| BML*† | (95% CI) | (95% CI)§ | |
| Medial meniscus pathology | |||
| None | 71/601 (12%) | 1.0 (ref) | 1.0 (ref) |
| Minor | 100/426 (23%) | 2.0 (1.5, 2.6) | 1.8 (1.3, 2.3) |
| Major | 109/317 (34%) | 2.9 (2.2, 3.8) | 2.4 (1.8, 3.2) |
| Lateral meniscus pathology | |||
| None | 63/1117 (6%) | 1.0 (ref) | 1.0 (ref) |
| Minor | 34/141 (24%) | 4.3 (2.9, 6.3) | 4.0 (2.7, 5.9) |
| Major | 28/84 (33%) | 5.7 (3.9, 8.5) | 5.0 (3.2, 7.7) |
ref = reference category, 95% CI = 95% confidence interval.
Missing value for 2 knees for the lateral compartment.
Chi-square test for trend P <0.001 for both medial and lateral compartment.
Poisson regression (using generalized estimating equations) adjusted for age, sex, body mass index, physical activity level, and mechanical axis at baseline.
We repeated the analysis using our binary meniscal exposure variable and stratified by cartilage status at baseline. Meniscal pathology remained strongly associated with incident or enlarging BML irrespective of whether there was a cartilage lesion, and the strongest estimates of relative risk were still obtained for the lateral compartment (table 3).
Table 3.
Crude and adjusted relative risk (RR) for incident or enlarging bone marrow lesion(s) (BML) in the same compartment as meniscal pathology. The analyses are stratified by ipsilateral cartilage status at baseline*.
| Cartilage lesion at baseline | No cartilage lesion at baseline |
|||
|---|---|---|---|---|
| Incident or enlarging BML† | Incident or enlarging BML | |||
| Medial meniscus pathology | Yes | No | Yes | No |
| Yes | 190 | 425 | 18 | 108 |
| No | 49 | 247 | 22 | 283 |
| Crude RR (95% CI) | 1.8 | (1.4, 2.4) | 1.9 | (1.0, 3.4) |
| Adjusted RR (95% CI)§ | 1.6 | (1.2, 2.1) | 2.0 | (1.1, 3.6) |
| Lateral meniscus pathology | Yes | No | Yes | No |
| Yes | 64 | 119 | 8 | 34 |
| No | 48 | 401 | 17 | 650 |
| Crude RR (95% CI) | 2.8 | (1.9, 3.9) | 7.6 | (3.5, 16.5) |
| Adjusted RR (95% CI)§ | 2.5 | (1.7, 3.7) | 7.9 | (3.7, 17.1) |
Numbers are n if not otherwise stated, 95% CI = 95% confidence interval.
Missing value for medial compartment for 2 knees and lateral compartment for 1 knee.
Missing value for 2 knees for the lateral compartment.
Poisson regression (using generalized estimating equations) adjusted for age, sex, body mass index, physical activity level, and mechanical axis at baseline.
Using our binary exposure variable, we also performed secondary analyses for knee compartments without any BML at baseline and for those with pre-existing BMLs, separately. The adjusted RR of developing a new BML was 1.5; 95% CI 1.1, 2.1 for medial meniscal pathology and 4.3; 95% CI 2.7, 6.8 for lateral (vs. no meniscal pathology). The corresponding adjusted RRs for worsening of compartments with pre-existing BML(s) was 2.4; 95% CI 1.4, 3.8 for medial meniscal pathology and 2.6; 95% CI 1.6, 4.2 for lateral meniscal pathology.
The overall results remained essentially the same when analyzing the two constructs meniscal lesion and meniscal extrusion separately as the exposure variables. Meniscal pathology was not associated with regression or resolution of pre-existing BMLs (data not shown).
DISCUSSION
This prospective cohort study provides firm evidence that meniscus pathology is a risk factor for both incident and enlarging subchondral BMLs. BMLs are frequently seen in knee OA, a disorder heavily influenced by excess focal loading of the joint and the response of joint tissues to such abnormal biomechanical stress. Thus, we shed light on the pathway where abnormal biomechanical loading patterns created by meniscal pathology lead to increased focal stress on articular cartilage often resulting in cartilage loss [34, 35], bone alterations including trabecular bone changes [36], increased bone mineral density [25], and possible development of BMLs.
The crude estimates of relative risk in our primary model were slightly higher than the adjusted estimates suggesting that there was confounding not accounted for in unadjusted estimates, i.e., factor(s) associated with both meniscal pathology and the development of BMLs. Further, cartilage loss is a key feature of OA which has been linked to change in BML size [20]. We chose to stratify additional analyses by ipsilateral cartilage status rather than adjustment as cartilage lesions may be a consequence of meniscal pathology, thus a potential intermediate variable on the causal pathway from meniscal pathology to the development of BMLs [34, 35]. Our stratified analyses firmly suggest meniscal pathology to be an independent and strong risk factor of incident or enlarging BML irrespective of cartilage status.
We obtained stronger estimates of relative risk for lateral meniscal pathology on the development of BML(s) in line with the greater fraction of load in the lateral compartment transmitted through the lateral meniscus [3] [37]. Lateral meniscectomy has been associated with a higher relative risk of developing radiographic OA than medial meniscectomy [5, 38–41]. Importantly, the risk of developing lateral compartment BMLs in absence of meniscal pathology appears to be lower compared to the medial compartment, driving the more elevated risk ratios.
An important limitation of our study is that the meniscal exposure variable and the outcome were both MRI-based and assessed by the same readers which could potentially bias the results towards finding a positive association (the MRIs were universally scored for all OA features included in WORMS). However, when the MRIs were read the readers were unaware of the study hypothesis. Also, blinding of BML or meniscal status on MRI would be very challenging. Potential bias working in the other direction is that we can not exclude a ceiling effect because large BMLs (in particular) already at baseline have little tendency to further enlarge. The number of knee compartments with large BMLs at baseline were limited and predominantly associated with meniscal pathology (data not shown).
We created a 3-item ordered categorical scale of pathology as a proxy for diminishing meniscal function. It was not within our scope trying to identify details of particular type(s), extent and site(s) of meniscal pathology at the highest risk of developing BMLs. However, based on our results there is a dose-response relationship, where, e.g., complete destruction of the body and posterior horn of a medial meniscus severely compromising medial meniscal function, is expected to be worse than a minor meniscal tear in an otherwise healthy meniscus. The finding is in parallel to the dose-response relationship seen with respect to the increased risk of developing radiographic tibiofemoral OA when having increasing severity of meniscal lesions [4], and all in line with the intricate biomechanical properties of the menisci to maintain a healthy knee.
In our study BMLs were only assessed at two time points 30 months apart, so it is unclear how fast and how often BMLs develop and regress. More time points and future studies of the association with patient-relevant outcomes such as knee pain will provide further information on the natural course of BML(s) and the impact of meniscal pathology in knee OA. Further studies will also be required to study the association between meniscal pathology and the development of BMLs in younger individuals.
We need to focus research efforts into the early stages in the development of meniscal pathology of degenerative character to understand its impact on the natural course of knee OA. We also need to consider prevention of meniscal degenerative processes. Corrective surgeries of meniscal pathology, i.e., meniscal repair or transplant, are still unproven in the long term, but do represent a feasible approach in selected cases. However, knee surgery is hardly the universal answer to the one third with meniscal lesions in the general population over 50 years of age who are at high risk of OA in the knee [4, 42].
In conclusion, this comprehensive study is important because, for the first time, it provides evidence of a potent effect of meniscal pathology on both development and enlargement of subchondral BMLs in the ipsilateral knee compartment. Higher relative risks were seen in those with more severe or lateral meniscal pathology. Simply put, over a 30-month period compartments with medial meniscal pathology have about a 2-fold increased risk of developing BMLs than medial compartments without meniscal pathology have. The corresponding increase in risk for lateral meniscal pathology (less frequent) and the development of BMLs in the lateral compartment is about 4 to 5-fold.
ACKNOWLEDGEMENTS
We would like to thank all MOST staff and study participants at Birmingham, Alabama and Iowa City, Iowa, and the UCSF MOST coordinating center, San Francisco, California, and in particular Ke Wang, Boston University School of Medicine, for statistical assistance.
FUNDING
The Multicenter Osteoarthritis (MOST) Study is a cooperative epidemiological study of knee OA funded by the National Institute on Aging (NIA): Felson – 1 U01 AG18820; Torner – 1 U01 AG18832; Lewis – 1 U01 AG18947; Nevitt – 1 U01 AG19069. Dr Englund was supported by Arthritis Foundation.
Footnotes
COMPETING INTERESTS
AG is president of Boston Imaging Core Lab, LLC (BICL), Boston, Massachusetts, USA, a company providing radiological image assessment services; and shareholder of Synarc Inc. FWR is vice president of BICL. None of the other authors have declared any conflict of interest.
LICENCE STATEMENT
The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non-exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd and its Licensees to permit this article (if accepted) to be published in Annals of the Rheumatic Diseases and any other BMJPGL products to exploit all subsidiary rights, as set out in our licence
REFERENCES
- 1.Fukubayashi T, Kurosawa H. The contact area and pressure distribution pattern of the knee. A study of normal and osteoarthrotic knee joints. Acta Orthop Scand. 1980;51:871–879. doi: 10.3109/17453678008990887. [DOI] [PubMed] [Google Scholar]
- 2.Seedhom BB, Hargreaves DJ. Transmission of the load in the knee joint with special reference to the role of the meniscus. Part I+II. Eng Med. 1979;4:207–228. [Google Scholar]
- 3.Walker PS, Erkman MJ. The role of the menisci in force transmission across the knee. Clin Orthop Relat Res. 1975:184–192. doi: 10.1097/00003086-197506000-00027. [DOI] [PubMed] [Google Scholar]
- 4.Englund M, Guermazi A, Roemer FW, et al. Meniscal tear in knees without surgery and the development of radiographic osteoarthritis among middle-aged and elderly persons: The multicenter osteoarthritis study. Arthritis Rheum. 2009;60:831–839. doi: 10.1002/art.24383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Englund M, Lohmander LS. Risk factors for symptomatic knee osteoarthritis fifteen to twenty-two years after meniscectomy. Arthritis Rheum. 2004;50:2811–2819. doi: 10.1002/art.20489. [DOI] [PubMed] [Google Scholar]
- 6.Fairbank TJ. Knee joint changes after meniscectomy. J Bone Joint Surg Br. 1948;30:164–170. [PubMed] [Google Scholar]
- 7.Jørgensen U, Sonne-Holm S, Lauridsen F, et al. Long-term follow-up of meniscectomy in athletes. A prospective longitudinal study. J Bone Joint Surg Br. 1987;69:80–83. doi: 10.1302/0301-620X.69B1.3818740. [DOI] [PubMed] [Google Scholar]
- 8.Lopez AD, Mathers CD, Ezzati M, et al. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367:1747–1757. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 9.Song Y, Greve JM, Carter DR, et al. Meniscectomy alters the dynamic deformational behavior and cumulative strain of tibial articular cartilage in knee joints subjected to cyclic loads. Osteoarthritis Cartilage. 2008;16:1545–1554. doi: 10.1016/j.joca.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 10.Allaire R, Muriuki M, Gilbertson L, et al. Biomechanical consequences of a tear of the posterior root of the medial meniscus. Similar to total meniscectomy. J Bone Joint Surg Am. 2008;90:1922–1931. doi: 10.2106/JBJS.G.00748. [DOI] [PubMed] [Google Scholar]
- 11.Lee SJ, Aadalen KJ, Malaviya P, et al. Tibiofemoral contact mechanics after serial medial meniscectomies in the human cadaveric knee. Am J Sports Med. 2006;34:1334–1344. doi: 10.1177/0363546506286786. [DOI] [PubMed] [Google Scholar]
- 12.Roemer FW, Frobell R, Hunter DJ, et al. MRI-detected subchondral bone marrow signal alterations of the knee joint: terminology, imaging appearance, relevance and radiological differential diagnosis. Osteoarthritis Cartilage. 2009;17:1115–1131. doi: 10.1016/j.joca.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 13.Zanetti M, Bruder E, Romero J, et al. Bone marrow edema pattern in osteoarthritic knees: correlation between MR imaging and histologic findings. Radiology. 2000;215:835–840. doi: 10.1148/radiology.215.3.r00jn05835. [DOI] [PubMed] [Google Scholar]
- 14.Yu JS, Cook PA. Magnetic resonance imaging (MRI) of the knee: a pattern approach for evaluating bone marrow edema. Crit Rev Diagn Imaging. 1996;37:261–303. [PubMed] [Google Scholar]
- 15.Frobell RB, Le Graverand MP, Buck R, et al. The acutely ACL injured knee assessed by MRI: changes in joint fluid, bone marrow lesions, and cartilage during the first year. Osteoarthritis Cartilage. 2009;17:161–167. doi: 10.1016/j.joca.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 16.Felson DT, McLaughlin S, Goggins J, et al. Bone marrow edema and its relation to progression of knee osteoarthritis. Ann Intern Med. 2003;139:330–336. doi: 10.7326/0003-4819-139-5_part_1-200309020-00008. [DOI] [PubMed] [Google Scholar]
- 17.Carbone LD, Nevitt MC, Wildy K, et al. The relationship of antiresorptive drug use to structural findings and symptoms of knee osteoarthritis. Arthritis Rheum. 2004;50:3516–3525. doi: 10.1002/art.20627. [DOI] [PubMed] [Google Scholar]
- 18.Felson DT, Chaisson CE, Hill CL, et al. The association of bone marrow lesions with pain in knee osteoarthritis. Ann Intern Med. 2001;134:541–549. doi: 10.7326/0003-4819-134-7-200104030-00007. [DOI] [PubMed] [Google Scholar]
- 19.Lo GH, McAlindon TE, Niu J, et al. Bone marrow lesions and joint effusion are strongly and independently associated with weight-bearing pain in knee osteoarthritis: data from the osteoarthritis initiative. Osteoarthritis Cartilage. 2009;17:1562–1569. doi: 10.1016/j.joca.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roemer FW, Guermazi A, Javaid MK, et al. Change in MRI-detected subchondral bone marrow lesions is associated with cartilage loss: the MOST Study. A longitudinal multicentre study of knee osteoarthritis. Ann Rheum Dis. 2009;68:1461–1465. doi: 10.1136/ard.2008.096834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hunter DJ, Zhang Y, Niu J, et al. Increase in bone marrow lesions associated with cartilage loss: a longitudinal magnetic resonance imaging study of knee osteoarthritis. Arthritis Rheum. 2006;54:1529–1535. doi: 10.1002/art.21789. [DOI] [PubMed] [Google Scholar]
- 22.Roemer FW, Neogi T, Nevitt MC, et al. Subchondral bone marrow lesions are highly associated with, and predict subchondral bone attrition longitudinally: the MOST study. Osteoarthritis Cartilage. 2010;18:47–53. doi: 10.1016/j.joca.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bennell KL, Creaby MW, Wrigley TV, et al. Bone marrow lesions are related to dynamic knee loading in medial knee osteoarthritis. Ann Rheum Dis. 2010 February 4; doi: 10.1136/ard.2009.118182. Published Online First. [DOI] [PubMed] [Google Scholar]
- 24.Lo GH, Hunter DJ, Nevitt M, et al. Strong association of MRI meniscal derangement and bone marrow lesions in knee osteoarthritis: data from the osteoarthritis initiative. Osteoarthritis Cartilage. 2009;17:743–747. doi: 10.1016/j.joca.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lo GH, Niu J, McLennan CE, et al. Meniscal damage associated with increased local subchondral bone mineral density: a Framingham study. Osteoarthritis Cartilage. 2008;16:261–267. doi: 10.1016/j.joca.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karlson EW, Sanchez-Guerrero J, Wright EA, et al. A connective tissue disease screening questionnaire for population studies. Ann Epidemiol. 1995;5:297–302. doi: 10.1016/1047-2797(94)00096-c. [DOI] [PubMed] [Google Scholar]
- 27.Washburn RA, Smith KW, Jette AM, et al. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 28.Roemer FW, Guermazi A, Lynch JA, et al. Short tau inversion recovery and proton density-weighted fat suppressed sequences for the evaluation of osteoarthritis of the knee with a 1.0 T dedicated extremity MRI: development of a time-efficient sequence protocol. Eur Radiol. 2005;15:978–987. doi: 10.1007/s00330-004-2608-6. [DOI] [PubMed] [Google Scholar]
- 29.Peterfy CG, Guermazi A, Zaim S, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12:177–1790. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 30.Roemer FW, Lynch JA, Niu J, et al. A comparison of dedicated 1.0 T extremity MRI vs. large-bore 1.5 T MRI for semiquantitative whole organ assessment of osteoarthritis: the MOST study. Osteoarthritis Cartilage. 2010;18:168–174. doi: 10.1016/j.joca.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kothari M, Guermazi A, von Ingersleben G, et al. Fixed-flexion radiography of the knee provides reproducible joint space width measurements in osteoarthritis. Eur Radiol. 2004;14:1568–1573. doi: 10.1007/s00330-004-2312-6. [DOI] [PubMed] [Google Scholar]
- 32.Peterfy C, Li J, Zaim S, et al. Comparison of fixed-flexion positioning with fluoroscopic semi-flexed positioning for quantifying radiographic joint-space width in the knee: test-retest reproducibility. Skeletal Radiol. 2003;32:128–132. doi: 10.1007/s00256-002-0603-z. [DOI] [PubMed] [Google Scholar]
- 33.Kellgren JH, Lawrence JS. Radiological assessment of osteoarthritis. Ann Rheum Dis. 1957;16:494–501. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berthiaume MJ, Raynauld JP, Martel-Pelletier J, et al. Meniscal tear and extrusion are strongly associated with progression of symptomatic knee osteoarthritis as assessed by quantitative magnetic resonance imaging. Ann Rheum Dis. 2005;64:556–563. doi: 10.1136/ard.2004.023796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hunter DJ, Zhang YQ, Niu JB, et al. The association of meniscal pathologic changes with cartilage loss in symptomatic knee osteoarthritis. Arthritis Rheum. 2006;54:795–801. doi: 10.1002/art.21724. [DOI] [PubMed] [Google Scholar]
- 36.Podsiadlo P, Dahl L, Englund M, et al. Differences in trabecular bone texture between knees with and without radiographic osteoarthritis detected by fractal methods. Osteoarthritis Cartilage. 2008;16:323–329. doi: 10.1016/j.joca.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 37.Pena E, Calvo B, Martinez MA, et al. Why lateral meniscectomy is more dangerous than medial meniscectomy. A finite element study. J Orthop Res. 2006;24:1001–1010. doi: 10.1002/jor.20037. [DOI] [PubMed] [Google Scholar]
- 38.Allen PR, Denham RA, Swan AV. Late degenerative changes after meniscectomy. Factors affecting the knee after operation. J Bone Joint Surg Br. 1984;66:666–671. doi: 10.1302/0301-620X.66B5.6548755. [DOI] [PubMed] [Google Scholar]
- 39.Hede A, Larsen E, Sandberg H. The long term outcome of open total and partial meniscectomy related to the quantity and site of the meniscus removed. Int Orthop. 1992;16:122–125. doi: 10.1007/BF00180200. [DOI] [PubMed] [Google Scholar]
- 40.Johnson RJ, Kettelkamp DB, Clark W, et al. Factors effecting late results after meniscectomy. J Bone Joint Surg Am. 1974;56:719–729. [PubMed] [Google Scholar]
- 41.Yang N, Nayeb-Hashemi H, Canavan PK. The Combined Effect of Frontal Plane Tibiofemoral Knee Angle and Meniscectomy on the Cartilage Contact Stresses and Strains. Ann Biomed Eng. 2009;37:2360–2372. doi: 10.1007/s10439-009-9781-3. [DOI] [PubMed] [Google Scholar]
- 42.Englund M, Guermazi A, Gale D, et al. Incidental meniscal findings on knee MRI in middle-aged and elderly persons. N Engl J Med. 2008;359:1108–1115. doi: 10.1056/NEJMoa0800777. [DOI] [PMC free article] [PubMed] [Google Scholar]