SUMMARY
Vole tuberculosis (TB; Mycobacterium microti) is an understudied endemic infection. Despite progressing slowly, it causes severe clinical pathology and overt symptoms in its rodent host. TB was monitored for 2 years in wild field voles in Kielder Forest, UK. The prevalence of characteristic cutaneous TB lesions was monitored longitudinally at 4 sites, with individuals live-trapped and repeatedly monitored. A prevalence of 5·2% of individuals with lesions was recorded (n=2791). In a cross-sectional study, 27 sites were monitored bi-annually, with TB assessed by post-mortem examination for macroscopic lesions, and by culture and histopathology. Seventy-nine voles (10·78%; n=733) were positive for mycobacteria, with the highest prevalence in spring (13·15%; n=327). TB prevalence varied, with between 0% and 50% of voles infected per site. Prevalence increased with age (mass), and apparent seasonality was due to a higher proportion of older animals in spring. Survival analysis supported this result, with cutaneous lesions only manifesting in the advanced stages of infection, and therefore only being found on older voles. The body condition of individuals with lesions declined at the time when the lesion was first recorded, when compared to individuals without lesions, suggesting there may be an acute phase of infection during its advanced stage. Although predicted survival following the appearance of a cutaneous lesion was lower than for uninfected individuals, this was not significant.
Keywords: endemic pathogen, Microtus agrestis, longitudinal, TB, survival, wildlife disease
INTRODUCTION
The majority of studies investigating wildlife disease have focussed on epidemic pathogens causing high levels of mortality, whereas most pathogens are endemic, persist in host populations and show relatively small fluctuations in prevalence (Anderson and May, 1979). This study focuses on endemic vole tuberculosis (TB), Mycobacterium microti, in its reservoir host, the field vole, Microtus agrestis L., in Kielder Forest, northern England. M. microti is a member of the Mycobacterium tuberculosis complex, which includes M. tuberculosis and M. bovis (van Soolingen et al. 1997, 1998). Despite being the first endemic infection to be investigated thoroughly in wild rodents (Wells and Oxon, 1937; Wells, 1946; Chitty, 1954), with up to a third of sampled field voles recorded as infected (Wells, 1946; Cavanagh et al. 2004), very little is known about the effects of vole TB on wild populations. TB is also an interesting disease of wild rodents because, unusually for an endemic infection, it is a chronic infection causing obvious and severe pathology in its host in its later stages, with potential implications for host survival and increased susceptibility to further infections. This also enhances its potential to exhibit delayed host density dependence in the prevalence of (serious) late-stage infections. Moreover, vole TB is a zoonotic infection. M. microti infections have been recorded in immunosuppressed and immunocompetent humans (van Soolingen et al. 1998; Niemann et al. 2000; Horstkotte et al. 2001), and prevalence of infection in humans is likely to be higher than has been currently reported (van Soolingen et al. 1998).
The present study, the first to examine the temporal dynamics of M. microti infection in a wildlife population, investigates the pathology and duration of M. microti infection in wild field voles by monitoring the presence of characteristic, externally visible cutaneous lesions. Wells (1946) found these especially in the subcutaneous tissue of the inter-scapular region, often with ulceration through the skin. It also examines temporal patterns in the prevalence of these lesions, and assesses whether they affected body condition or survival. However, as diagnosis of M. microti infection on the basis of external lesions alone underestimates M. microti prevalence (Cavanagh et al. 2004), patterns in prevalence were also evaluated in a cross-sectional study of voles examined post-mortem that included diagnosis of M. microti infection by the culture of material taken from potentially infected organs.
MATERIALS AND METHODS
Study area
The study took place in Kielder Forest, a man-made spruce forest occupying 620 km2, situated on the English-Scottish border (55°13′N, 2°33′W). Kielder Forest can be divided into 3 main catchments, lying in adjacent watersheds: Redesdale 10–15 km north-east of Kielder, Kielder, and Kershope 10–15 km to the southwest. Field voles inhabit grassy clear-cuts that represent 16–17% of the total area, but are completely absent from forested areas. Clear-cuts range in size from 5–100 ha. Field vole populations at Kielder fluctuate cyclically with a 3–4 year period (Lambin et al. 2000). Populations situated close together fluctuate in synchrony, but populations further apart are out of synchrony (Lambin et al. 1998; Bierman et al. 2006).
Longitudinal study
Trapping methods
Voles were trapped in 4 similar-sized clear-cuts, in 2 areas of the forest approximately 12 km apart, between May 2001 and July 2003. Kielder Site (KCS) and Plashett’s Jetty (PLJ) were situated 4 km apart within the Kielder catchment, with vole populations at low to increasing density during the study. Black Blake Hope (BHP) and Rob’s Wood (ROB) were 3·5 km apart within the Redesdale catchment, with voles at increasing and peak density.
Populations were trapped in primary sessions every 28 days from March to November, and every 56 days from November to March. Each site had a permanent 0·3 ha live-trapping grid consisting of 100 Ugglan Special Mousetraps (Grahnab, Marieholm, Sweden), in optimal habitat dominated by Deschampsia caespitosa Beauv., Agrostis tenuis Sibth., and Juncus effusus L. Traps were set at 5 m intervals and baited with wheat and carrots. Traps were pre-baited with a slice of carrot and a few grams of oats 3 days before each trapping session, set at approximately 18:00 on the first day and checked 5 times (‘secondary sessions’) at roughly 12-h intervals at dawn and dusk.
Individuals were identified using subcutaneous microchip transponders (AVID plc, East Sussex, UK) injected into the skin at the back of the neck. Mass and sex were recorded at the time of first capture in each primary session. Animals with juvenile fur or in their first moult were classed as juveniles (Graham and Lambin, 2002). Animals were assigned to reproductive classes according to the external appearance of reproductive organs. A body condition (BC) ordinal score was calculated for each animal by estimating the degree of fat cover over the vertebral column and dorsal pelvic bones. Each area was scored on a scale between 1 and 5, and scores summed to give an overall BC score from 2 to 10 (Cavanagh, 2001).
Vole density estimates for each primary session were calculated via the closed population model MTH in the program CAPTURE (Otis et al. 1978) and using the estimator of Chao and Lee (1991).
Diagnosis of TB
The presence, size and location of characteristic tuberculous skin lesions denoting late-stage TB were noted in all animals. Lesions are known to be most commonly sited in the scapular region (Wells, 1946; Cavanagh et al. 2002, 2004), and thus in order to undertake a conservative estimate of TB prevalence, individuals were only recorded as positive for TB in the field if the lesion was observed between or on the shoulder blades and was > 1 mm in diameter, or if it was > 5 mm and situated else-where on the body. Lesions that did not meet these criteria, or which appeared atypical (i.e. resembled bite wounds, or were not ulcerated through the skin) were recorded as ‘suspect’ in the field, and their size and location noted, but because of their suspect nature, and because a swab for bacteriological analysis could not be taken without potentially damaging the animal concerned, they were omitted from analyses. Any effects of TB on voles are therefore likely to be an underestimate.
Effect of TB on survival
The goodness of fit (GOF) of a ‘global’ (most fully parameritized) model was assessed, as the capture-mark-recapture (CMR) models used assume that: (1) every marked animal in the population immediately after time (i) has the same probability of surviving to time (i+1); and (2) every marked animal present in the population at time (i) has the same probability of recapture (pi). Individual capture histories were classified by sex and site. GOF was assessed using tests implemented in the program RELEASE (Burnham et al. 1987) using a standard Cormack-Jolly Seber model applied to the each group. ‘Test 2′ in RELEASE tests for violation of assumption (2), and ‘Test 3′ tests for violation of assumption (1).
In examining the effect of TB on survival, the first step was to derive an optimal model accounting for individual variation in recapture and survival. The analysis was undertaken following Lebreton et al. (1992) using program MARK (White and Burnham, 1999). Full details have been given by Burthe et al. (2007), and the subscripts used for model parameters are listed in Table 1. Additive effects are denoted by a plus (+) sign, and interaction terms by an asterisk (*).
Table 1.
Subscripts used in survival models (program MARK)
(Details of which parameters were included in recapture models (p) and survival models (φ) are given in the right hand column.)
| Analysis stage | Subscript | Description | Parameter type |
|---|---|---|---|
| Group level | st | Site-effect (BHP, KCS, PLJ or ROB) | φ, p |
| sx | Sex-effect | φ, p | |
| t | Full time-dependence | φ, p | |
| Temporal | mo | Month (13 months/year) | φ, p |
| seas1 | Season (Nov–Feb, Mar–May, May–Jul, Aug–Oct) | φ, p | |
| seas2 | Season (winter Oct–Mar; summer Apr–Sept) | φ, p | |
| yr | Year (2001, 2002 or 2003) | φ, p | |
| Covariates | rf | Average rainfall during trapping session | p |
| temp | Average temperature during trapping session | p | |
| prevrf | Average rainfall during previous month | φ | |
| prevt | Average temperature during previous month | φ | |
| dens | Vole density during trapping session | φ, p | |
| n | Binary score of months with reduced number of secondary sessions |
p | |
| tb | Prevalence of TB lesions (population level) | φ, p | |
| Individual covariates | e | Binary covariate denoting individuals from grid edge | p |
| Time-specific individual covariates |
m | Trap-dependency effect | p |
| indtb | Presence of TB lesions (individual level) | φ |
The first captures of all individuals were removed (1514 in total) in order to overcome biases caused by transient individuals. This reduced the risk of confounding emigration and mortality as a cause of disappearance, but precluded the inclusion of age (juvenile/adult) as a parameter in model selection. The reduced data set of 1275 individual capture histories yielded a more robust analysis and was used to estimate the best ‘base’ recapture and survival model, with which possible effects of TB could be investigated. First, recapture was modelled in 3 stages. The first examined whether recapture rate varied with time, site or sex. The second investigated whether the temporal component of the recapture model could be adequately described by month, season or year. Effects of average rainfall and temperature over the 3-day trapping period, and of reduced trapping effort in some primary sessions (caused by inclement weather) were also investigated. The third stage then examined individual level covariates such as trap dependency and ‘edge’. An edge animal had ≥75% of its captures at the edge of the trapping grid. Trap dependency was a time-dependent individual covariate determined by whether or not an individual had been caught in the preceding primary session. The fits of the models were assessed by Akaike’s Information Criterion corrected for small samples (AICc) (Hurvich and Tsai, 1989). Models with a difference of AICc (ΔAICc) of less than 2 may be considered similar in their ability to account for the data (Sakamoto et al. 1986). According to the principle of parsimony, if 2 alternative models had indistinguishable AICc values (ΔAICc<2), the model with fewer parameters was selected.
Survival was then modelled similarly using the best recapture model. To investigate the effect of climatic variables on survival probabilities, the average daily temperature or the average daily rainfall over the entire 28-day period was included as weather variables. Finally, this best model of recapture and survival (the ‘base’ model) was used to investigate whether TB affected host survival and recapture probabilities.
Cutaneous lesions are reported to represent an advanced stage of TB, and to confirm this TB was included first as a binary individual covariate (tb) depending on whether that individual was ever recorded positive for a cutaneous lesion (i.e. TB individuals vs individuals that were never infected). In a second separate analysis, TB was modelled as a time-varying individual covariate of lesion presence (indtb) reflecting the detection of lesion in a given trapping occasion in order to investigate differential survival over the next trapping interval.
Effect of TB on body condition
The change in BC between primary sessions for voles positive for TB (n=47) was compared to voles without lesions (not including individuals suspect for lesions), by randomly assigning each positive individual a negative, control individual matched for trap session, sex and site for comparison, since Cavanagh (2001) found that only sex, site and season influenced individuals’ changes in BC. Individuals with TB lesions were included in the analysis if BC scores were available for the primary session when the lesion was first observed, and the preceding primary session. From within the appropriate control sets (size range 1–36, mean 13·6 per session/site/sex), individuals were selected at random. Forty-seven matched TB and non-TB changes in BC were compared using a 2-tailed Mann-Whitney U-test, and this was repeated 100 times with replacement. The median change in score for the TB group was also compared to the distribution of medians of the 100 control groups.
Statistical analysis of the risk of infection
Factors influencing whether an individual was positive for external tuberculous lesions were investigated using Generalised Linear Mixed Models (GLMM) with a logit link and binomial errors. Analyses were conducted using the lme4 (Bates and Sarkar, 2006) and Matrix (Bates and Maechler, 2006) packages in the R software available under the GNU license at http://www.r-project.org. As is common with parasitological data, individual observations were non-independent (individuals being sampled from the same site at the same time), and so site*trap-session was included as a random effect (Paterson and Lello, 2003). The same individuals could also be sampled repeatedly over time, potentially leading to pseudo-replication. In this study, however, individuals appeared in the data only 1·38 times on average. Consequently the variance component due to vole id was not estimable as a random effect in the GLMMs.
Population level covariates included the month, season, site and year of capture. The individual level covariates considered were sex and mass (as a crude proxy for age). Mass2 was also fitted in order to test for non-linear relationships between risk of TB and age. Biologically meaningful two-way interactions between individual level covariates and between individual level covariates and season were also included. Models were constructed following a step-down procedure, eliminating interactions first. The models were restricted using the AIC, with models with a ΔAIC of less than 2 considered similar in their ability to account for the data (Sakamoto et al. 1986).
Cross-sectional trapping
Trapping methods
Twenty-seven clear-cut sites were trapped: 6 in Redesdale, 12 in Kielder and 9 in Kershope, with 3 traps placed within a 1 m radius of each corner of a 15 m by 15 m trapping grid. Grids were trapped bi-annually (March and September) between September 2001 and March 2003. These times were chosen to reflect differences in population structure: March samples consisting of over-wintered, adult animals, and September samples occurring during the breeding season and including juveniles. Traps were pre-baited with a slice of carrot and a few grammes of oats 3 days before each trapping session, set overnight and then checked daily over 3 days. Animals were euthanized in the field by isoflurane inhalation.
Post-mortem examination of field voles
Mass, sex, BC and reproductive status of individuals were recorded post-mortem. The presence, size and location of characteristic cutaneous lesions or gross internal lesions denoting TB (Wells, 1946; Cavanagh et al. 2002) were recorded during dissection. Field voles from surveys 2–4 (March 2002–March 2003) with internal signs of TB, or abnormally large lymph nodes, had all organs and any lesion material removed and divided for culture and histology. Field voles with no visible signs of TB had their lymph nodes, particularly those from the facial and scapular regions, removed for culture in March 2002 and had their lungs and lymph nodes removed for culture in September 2002 and March 2003. No organs were cultured from the September 2001 survey. All suspect lesions were tested to confirm TB by PCR on clinical material for survey 1, by PCR on cultured material from subsequent surveys (Magdalena et al. 1998), and by histology. Samples for histology were fixed in 10% buffered formalin, processed by standard procedures for embedding in paraffin, and stained with haematoxylin-eosin (HE), Auramine-rhodamine (AR) and Ziehl-Neelson (ZN) stains.
Decontamination and culture methods
Lymph nodes were pooled for culture if the animal had no sign of TB, and cultured separately if macroscopic lesions were observed. All other organ samples were cultured separately. To decontaminate, the tissue samples were homogenized in an equal volume of sterile PBS. The sample was agitated at room temperature overnight, with an equal volume of CPC decontaminant (5 g cetyl-pryridinium chloride, 10 g NaCl/1000 ml H2O). Decontaminated samples were centrifuged at 3000 g for 15 min. Sediment was re-suspended in 250 μl of DD-H2O, and 200 μl of this used to inoculate Lowenstein-Jensen pyruvate based culture slopes (SGS Laboratory Supplies). Slopes were incubated at 37 °C for a minimum of 12 weeks. Negative slopes were kept for a further 4 weeks for a final examination. Slopes with potential TB colonies were subcultured and subject to ZN staining and PCR (Magdalena et al. 1998) to confirm the presence of M. microti. The PCR is specific to the M. tuberculosis complex, and the size of the amplified DNA fragments detected varies amongst the different strains, with M. microti being 560 bp in size. In order to further confirm that the species was M. microti, positive cultures were subject to spoligotyping (Kamerbeek et al. 1997) and IS6110 RFLP typing (van Embden et al. 1993).
Statistical analysis
Factors influencing whether an individual was positive for M. microti were investigated using GLMMs with a logit link and binomial errors, with site*survey included as a random effect. As for the longitudinal data (above) the analyses were conducted in a 2-stage stepwise selection procedure. Population level covariates were catchment or site. The individual level covariates were sex, age (juvenile or adult, or mass as a proxy for age), maturity and BC score.
RESULTS
Patterns of external TB lesion prevalence (longitudinal study)
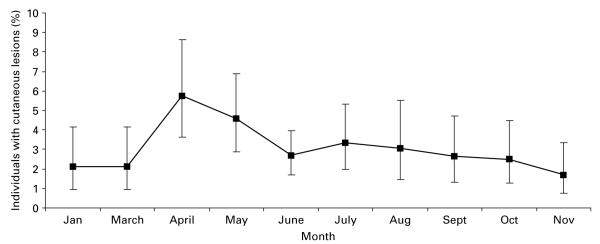
A total of 2791 voles were caught over the 2-year study period, with 108 individuals being positive for externally visible cutaneous TB lesions at some stage in their lives (prevalence 3·87%; 95% CI: 3·18–4·65). A further 60 individuals had suspect lesions, 58 of which were not captured again and hence could not be confirmed as having positive lesions and were not included in subsequent analyses. No juveniles (<18 g) were observed with external lesions, and prevalence was very low (1·31%, n=2287, 95% CI:0·89–1·87) for individuals of 18–25 g. Overall prevalence of lesions in adults was 7·01% in 2001 (4·90–9·66, n=485), 5·52% in 2002 (4·23–7·07, n=1081) and 5·43% in 2003 (4·06–7·10, n=948). Prevalence showed a seasonal pattern, with peaks of infection in late spring and early summer. April had the highest monthly prevalence when the 4 sites were considered together (Fig. 1). However, month and season fell out of the model once mass and year were included, reflecting covariation between season and the relative prevalence of animals of mass >18 g.
Fig. 1.
Prevalence of voles positive for cutaneous TB lesions (raw data) per month based on the total years combined. No trapping was undertaken in February.
The model with the lowest AIC included mass and mass2 of individuals, and year. TB lesion prevalence increased with the mass of individuals (b=0·500, s.e.=0·099, z=5·066, P<0.0001). However, the risk of TB decelerated with increasing weight, as indicated by a negative relationship with the squared mass of individuals (b=−0·007, s.e.=0·002, z=−4·377, P<0·0001) (Fig. 2). The prevalence of lesions also varied by year, with prevalence lowest in 2003 (b=−0·712, s.e.=0·262, z=−2·719, P<0.007). There was no significant difference in prevalence between males and females (ΔAIC=1 between the model with the lowest AIC and one including sex), or between sites (ΔAIC=1 between the model with the lowest AIC and one including site).
Fig. 2.
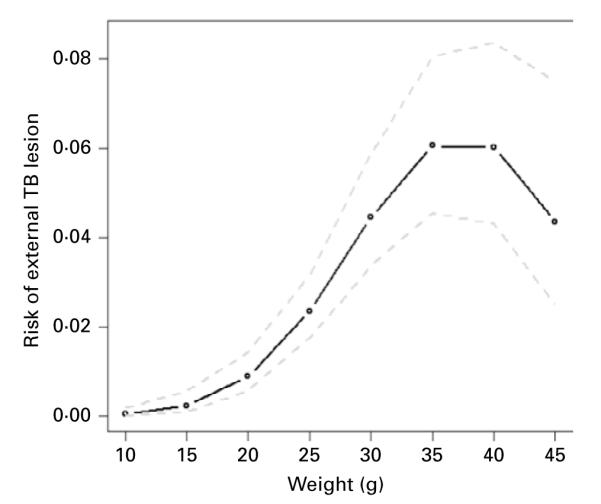
Predicted increase in risk of external tuberculosis lesions with increasing individual mass for voles in 2002 based on the model estimates. The dotted grey lines indicate the 95% confidence limits for the estimates.
Effect of TB on vole survival
Nineteen individuals caught on only 1 occasion had a TB lesion. These might have been transient animals, and hence no conclusion can be drawn regarding their fate. Of the remaining 89 infected voles, 41 were never caught again subsequent to having been first caught with a lesion. Based on the frequency of voles infected per number of trap sessions, the median length of time that an infected individual was caught with a cutaneous TB lesion present was 1 month, and the maximum was 6 months (5 primary sessions due to 56 days between winter trap sessions) (Table 2). Twenty-nine of the 108 individuals (26·85%; 95% CI: 18·78–36·24%) caught with positive external lesions were subsequently caught negative for lesions at a later primary session. Of these, 6 were subsequently caught positive for lesions again, after having been recorded as negative for several primary sessions.
Table 2.
The number of primary sessions that individuals were caught exhibiting an external tuberculous lesion
| Number of primary sessions an individual was caught with an external lesion present |
Number of individual voles |
|---|---|
| 1 | 76 |
| 2 | 25 |
| 3 | 4 |
| 4 | 1 |
| 5 | 2 |
Individuals positive for a cutaneous lesion (i.e. that had a lesion at some stage in their capture history) had higher overall survival than uninfected individuals. The untransformed β parameter estimate on the logit scale was 0·72 (95% CI: 0·44–1·01), significantly greater than zero. This indicates that only those with good lifetime survival can exhibit these lesions (i.e. it confirms that cutaneous lesions are an advanced stage of the disease).
In spite of a drop in deviance, the AICc of a model including the effect of the presence of a lesion in a primary session on immediately subsequent survival did not differ from that of a simpler model excluding this effect. Thus the hypothesis of an effect of TB on survival was not supported (Table 3). Nonetheless, predicted survival of individuals following the appearance of a cutaneous lesion was lower. The untransformed β parameter estimate on the logit scale was −0·36 (95% CI: −0·93–0·20) equating to a drop of 8·05% in monthly survival for individuals with TB compared to uninfected individuals for 1 month with median survival rates.
Table 3.
The effect of TB on survival of field voles, as overall lesion positive (i.e. individuals that were positive for a cutaneous lesion at some point in their capture history (tb)), and a time-specific individual TB covariate (indtb)
(The base model was φ(st * dens)+prevrf+mo+yr+sx p mo+yr+rf+n +m+edge. The most parsimonious model is highlighted in bold.)
| Model | Survival model | Recapture model | AICc | No. of parameters |
Deviance |
|---|---|---|---|---|---|
| Base model | (st*dens)+prevrf+mo+yr+sx | mo+yr+rf+n+m +edge |
4767.62 | 39 | 4688.51 |
| Investigating TB as an individual covariate |
(st*dens)+prevrf+mo+yr
+sx+tb |
mo+yr+rf+n+m
+edge |
4742.76 | 40 | 4661.59 |
| Investigating TB as a time-specific individual covariate |
(st*dens)+prevrf+mo+yr
+sx+indtb |
mo+yr+rf+n+m
+edge |
4767.66 | 40 | 4686.49 |
Effect of TB on body condition
Forty-seven infected individuals had BC information for the primary session when first caught with a cutaneous lesion, and for the preceding session. These showed a significant decline in BC between the primary session preceding infection and the primary session at which the lesions were first recorded, when compared to individuals of the same sex and from the same primary session and site. The median change in BC score was −1 for TB positive individuals and 0 for negative individuals. Of the 100 Mann-Whitney U-tests, 87 were significant, with 58 significant at the P<0·01 level. The median change in score for the positive individuals was less than all but 5 of the changes in the 100 control groups (Fig. 3).
Fig. 3.
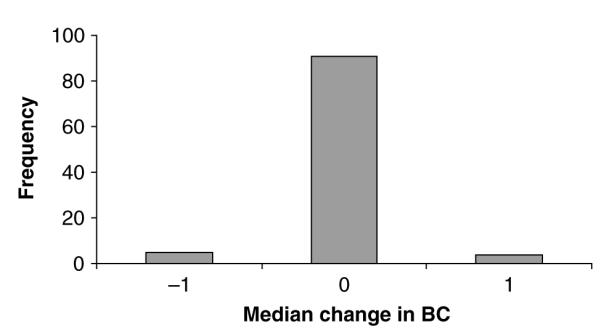
Frequency of median changes in BC for uninfected voles matched to the 47 TB voles in the 100 replicate Mann-Whitney U-tests. TB voles had a median change in BC of −1.
Risk of TB: cross-sectional study
The average prevalence (based on post-mortem examination for lesions and on culture of organs) for all sites and all surveys was 9·09% (n=733) and ranged between 1·78% (September 2001; n=169) and 13·15% (March 2003; n=327). However, prevalence varied between sites, with between 0% and 50% of voles infected. No voles of mass <17 g were ever positive regardless of the detection method used (Table 4).
Table 4.
Prevalence of TB per mass category of individuals
| Mass | % infected (based on culture/ histopathology) |
Sample size |
|---|---|---|
| <17 g | 0.00 | 109 |
| 17–24 g | 9.62 | 312 |
| 25–34 g | 14.08 | 277 |
| >34 g | 20.69 | 29 |
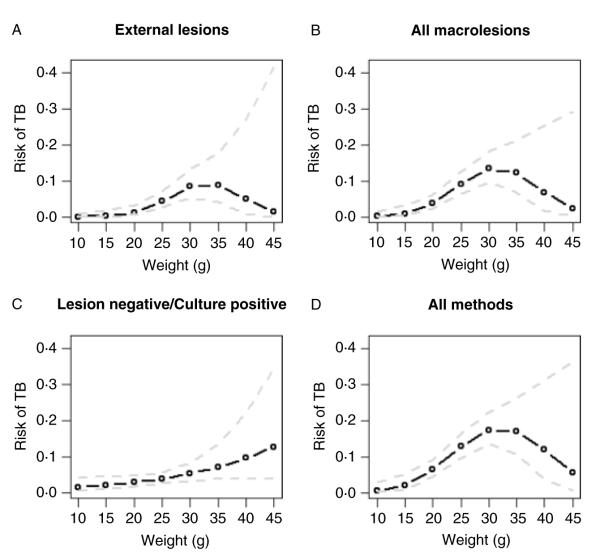
The model with the lowest AIC included mass and mass2 of individuals (AIC=461·4). The probability of TB increased with the mass of individuals (b=0·509, s.e.=0·199, z=2·554, P=0·011) however the risk of TB decelerated with increasing weight, as indicated by a negative relationship with the squared mass of individuals (b−0·008, s.e.=0·004, z= −2·172, P=0.030). There were also significant relationships between mass and mass2 and the probability of TB for the different methods of diagnosis (i.e. based on external lesions, external and internal macro-lesions or on being culture positive but lesion negative) but the slopes of these relationships varied (Fig. 4). The slope for external lesions, indicative of late-stage infection, was steepest and differed from the slope for individuals that were culture positive but lesion negative (with earlier stage infection). Season was not retained in the model once mass was included (AIC=461·4). No other covariates, or two-way interactions, were significant at the P<0·01 level.
Fig. 4.
Predicted increase in risk of infection with Mycobacterium microti with increasing individual mass for each dependent variable (A) presence of cutaneous lesions; (B) cutaneous and internal lesions detected post-mortem; (C) culture positive for mycobacteria but with no macroscopic lesions; (D) total infection based on all methods combined. The dotted lines are the 95% confidence limits on the predictions.
DISCUSSION
The prevalence of vole TB averaged 3·87% from the longitudinal study, and 9·09% from the cross-sectional study at Kielder Forest. Significant variation in lesion prevalence was observed between the 3-study years of the longitudinal survey, with the highest prevalence occurring in 2001 (7·01% in 2001; 5·52% in 2002; 5·43% in 2003). There was considerable spatial variation in prevalence from the cross-sectional surveys, with local site prevalence as high as 50%.
The range of prevalence of cutaneous lesions observed is comparable to that reported by Cavanagh et al. (2002) in the same area, where overall prevalence of external signs was approximately 2%, but reached levels of 8% in April. As shown by the cross-sectional study, the diagnosis of TB based on external lesions vastly underestimates TB prevalence. Wells (1946) recorded an overall prevalence of infection of 20·5% based on post-mortem examination for macro-lesions, and also found that prevalence was highest in spring and early summer, and varied between sites.
External lesions were only found on adult voles of mass >17 g, with prevalence generally increasing with the age of individuals. Indeed, apparent seasonality in TB prevalence actually reflected differences in the proportion of older animals present in the population, reaching up to 13·2% in March 2002. However, due to low numbers, and hence broad confidence intervals, for individuals of mass >30 g in weight, it is not possible to confirm whether risk of TB increases in the heaviest age classes. The relationship between age of individuals and the prevalence of macro-lesions (based on post-mortem) was stronger than that between age and prevalence for individuals that were lesion negative and culture positive. This suggests that older animals are slightly more likely than younger animals to have early stage infections (with no cutaneous lesions that have ulcerated through the skin), but are much more likely to have advanced stage TB. Although experimental work is necessary to elucidate this further, it does suggest that external lesions only manifest themselves during the more advanced stages of the disease, primarily encountered in older individuals.
Once mass was accounted for there was no significant difference in prevalence between males or females. This is counter to the hypothesis that one route of infection of TB in field voles is via skin abrasions caused by aggressive encounters, as might be expected based on the appearance of cutaneous lesions, and which would be expected to be more prevalent in males due to their more aggressive nature.
This study found evidence that TB appears to have a negative impact on body condition of individuals, providing further evidence that the decrease in survival following the development of a cutaneous lesion is likely to be significant. Positive individuals showed a significantly greater reduction in BC between primary sessions than non-infected voles. Such a decline in BC associated with a chronic disease such as TB is not surprising considering the extent of pathological damage observed in individuals with advanced infection. Although laboratory experiments involve unnatural dosage levels and transmission routes, Manabe et al. (2002) found that Microtus species voles infected via an intraperitoneal injection of M. microti were visibly ill and moribund by 8-weeks post-infection, and all showed pathological signs of advanced disease. Cavanagh et al. (2002) found 1 field vole with advanced TB to have had all of its lung lobes containing tuberculous nodules of up to 10 mm in diameter. Evidence of heavy levels of infection in lungs and lymphatic tissue is further supported by the results of the cross-sectional part of this study.
In general, duration of advanced disease appeared to be very short, with the majority of individuals only caught once with an external TB lesion. There were some exceptions with 2 individuals being positive for 6 months. The survival analysis supported the finding that TB risk increases with age, with only individuals that have survived for a relatively long time reaching the advanced stage of TB where lesions are visible. Survival rates following the primary session where cutaneous lesions were present were lower compared to uninfected individuals, suggesting that TB reduces survival in the advanced stages of infection, but the result must be interpreted with caution, due to low numbers of infected animals reducing the power of the analysis to detect survival differences. However, any negative effect of TB on field vole survival is likely to have been under-estimated, due to individuals dying before being caught with overt cutaneous symptoms and being recorded as negative in the survival analyses, and due to individuals only caught once with TB lesions not being included in the analysis. Moreover, some positive individuals were subsequently recorded as being negative for cutaneous lesions, suggesting that lesions may be a transient stage in advanced infections. Classifying such individuals as being negative for TB would not only underestimate the effect of TB on survival in infected animals, but also lead to lower survival probabilities for apparently uninfected individuals that were actually infected.
In conclusion, utilizing complementary cross-sectional and longitudinal surveys reveals that this disease appears to adversely affect the condition and perhaps the survival of infected individuals. Further study of TB in natural populations, in conjunction with the development of superior diagnostic methods and laboratory studies, would provide further valuable insights into this relatively under-studied, and potentially zoonotic, disease.
Acknowledgments
This study was funded by the Natural Environmental Research Council (Studentship number NER/S/A/2000/03445 awarded to S.B.) and the Wellcome Trust (075202/Z/04/Z) and was licensed under Home Office project license PPL40/1813. The Forestry Commission provided access to sites. Gordon Brown, David Carslake, Jonathan Fairbairn, Matt Oliver, Laura Taylor, Gill Telford, David Tidhar, Rachel Yeates and many others provided fieldwork assistance. Richard Birtles and Richard Fox assisted with post-mortems. Dick van Soolingen, Annemarie van den Brandt, Petra de Haas and Kristin Kremer at RIVM in the Netherlands undertook spoligotyping and RFLP typing of the TB cultures.
REFERENCES
- Anderson RM, May RM. Population biology of infectious diseases: Part 1. Nature, London. 1979;280:361–367. doi: 10.1038/280361a0. [DOI] [PubMed] [Google Scholar]
- Bates D, Maechler M. Matrix: a Matrix Package for R. R Package Version 0.995-5. 2006 [Google Scholar]
- Bates D, Sarkar D. lme4: Linear Mixed-Effects Models using S4 Classes. R Package Version 0.995-2. 2006 [Google Scholar]
- Bierman SM, Fairbairn JP, Petty SJ, Elston DA, Tidhar D, Lambin X. Changes over time in the spatiotemporal dynamics of cyclic populations of field voles (Microtus agrestis L.) American Naturalist. 2006;167:583–590. doi: 10.1086/501076. [DOI] [PubMed] [Google Scholar]
- Burnham KP, Anderson DR, White GC, Brownie C, Pollock KH. Design and Analysis of Fish Survival Experiments Based on Release-Recapture Data. American Fisheries Society, Monograph 5; Bethesda, MD, USA: 1987. [Google Scholar]
- Burthe SJ, Telfer S, Begon M, Bennett M, Smith A, Lambin X. Cowpox virus infection in natural field vole, Microtus agrestis, populations: significant negative impacts on survival. Journal of Animal Ecology. 2007 doi: 10.1111/j.1365-2656.2007.01302.x. (in the Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh R. Ph.D. thesis. University of Liverpool; UK: 2001. Interactions between population dynamics, body condition and infectious diseases (cowpox virus and Mycobacterium microti) of wild rodents. [Google Scholar]
- Cavanagh R, Begon M, Bennett M, Ergon T, Graham IM, de Haas PEW, Hart CA, Koedam M, Kremer K, Lambin X, Roholl P, van Soolingen D. Mycobacterium microti infection (vole tuberculosis) in wild rodent populations. Journal of Clinical Microbiology. 2002;40:3281–3285. doi: 10.1128/JCM.40.9.3281-3285.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh R, Lambin X, Ergon T, Bennett M, Graham IM, van Soolingen D, Begon M. Disease dynamics in cyclic populations of field voles (Microtus agrestis): cowpox virus and vole tuberculosis (Mycobacterium microti) Proceedings of the Royal Society of London, B. 2004;271:859–867. doi: 10.1098/rspb.2003.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao A, Lee S. Technical Report 91-C-01. Institute of Statistics, National Tsing Hua University; Hsin-Chu, Taiwan, Republic of China: 1991. Estimating population size for continuous time capture-recapture models via sample coverage. [Google Scholar]
- Chitty D. Tuberculosis among wild voles: with a discussion of other pathological conditions among certain mammals and birds. Ecology. 1954;35:227–237. [Google Scholar]
- Graham IM, Lambin X. The impact of weasel predation on cyclic field-vole survival: the specialist predator hypothesis contradicted. Journal of Animal Ecology. 2002;71:946–956. [Google Scholar]
- Horstkotte MA, Sobottka I, Schewe CK, Schafer P, Laufs R, Rusch-Gerdes S, Niemann S. Mycobacterium microti llama-type infection presenting as pulmonary tuberculosis in a human immunodeficiency virus-positive patient. Journal of Clinical Microbiology. 2001;39:406–407. doi: 10.1128/JCM.39.1.406-407.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurvich CM, Tsai CL. Regression and time series model selection in small samples. Biometrika. 1989;76:297–307. [Google Scholar]
- Kamerbeek J, Schouls L, Kolk A, van Agterveld M, van Soolingen D, Kuijper S, Bunschoten A, Molhuizen H, Shaw R, Goyal M, van Emden J. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. Journal of Clinical Microbiology. 1997;35:907–914. doi: 10.1128/jcm.35.4.907-914.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambin X, Petty SJ, MacKinnon JL. Cyclic dynamics in field vole populations and generalist predation. Journal of Animal Ecology. 2000;69:106–118. [Google Scholar]
- Lambin X, Elston DA, Petty SJ, MacKinnon JL. Spatial asynchrony and periodic travelling waves in cyclic populations of field voles. Proceedings of the Royal Society of London, B. 1998;265:1491–1496. doi: 10.1098/rspb.1998.0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebreton JD, Burnham KP, Clobert J, Anderson DR. Modelling survival and testing biological hypotheses using marked animals: a unified approach with case studies. Ecological Monographs. 1992;62:67–118. [Google Scholar]
- Magdalena J, Vachee A, Supply P, Locht C. Identification of a new DNA region specific for members of Mycobacterium tuberculosis complex. Journal of Clinical Microbiology. 1998;36:937–943. doi: 10.1128/jcm.36.4.937-943.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manabe YC, Scott CP, Bishai WR. Naturally attenuated, orally administered Mycobacterium microti as a tuberculosis vaccine is better that subcutaneous Mycobacterium bovis BCG. Infection and Immunity. 2002;70:1566–1570. doi: 10.1128/IAI.70.3.1566-1570.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemann S, Harmsen D, Rusch-Gerdes S, Richter E. Differentiation of clinical Mycobacterium tuberculosis complex isolates by gyrB DNA sequence polymorphism analysis. Journal of Clinical Microbiology. 2000;38:3231–3234. doi: 10.1128/jcm.38.9.3231-3234.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis D, Burnham K, White G, Anderson D. Statistical inference from capture data on closed animal populations. Wildlife Monographs. 1978;62:1–133. [Google Scholar]
- Paterson S, Lello J. Mixed models: getting the best use of parasitological data. Trends in Parasitology. 2003;19:370–375. doi: 10.1016/s1471-4922(03)00149-1. [DOI] [PubMed] [Google Scholar]
- Sakamoto Y, Ishiguro M, Kitagawa G. Akaike Information Criterion Statistics. KTK Scientific Publishers; Tokyo: 1986. [Google Scholar]
- van Embden JDA, Cave MD, Crawford JT, Dale JW, Eisenach KD, Gicquel B, Hermans P, Martin C, McAdam R, Shinnick TM, Small PM. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. Journal of Clinical Microbiology. 1993;31:406–409. doi: 10.1128/jcm.31.2.406-409.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Soolingen D, Hoogenboezem T, de Haas PEW, Hermans PWM, Koedam MA, Teppema KS, Brennan PJ, Besra GS, Portaels F, Top J, Schouls LM, van Emden JDA. A novel pathogenic taxon of the Mycobacterium tuberculosis complex, Canetti: characterization of an exceptional isolate from Africa. International Journal of Systematic Bacteriology. 1997;47:1236–1245. doi: 10.1099/00207713-47-4-1236. [DOI] [PubMed] [Google Scholar]
- van Soolingen D, van der Zanden AGM, de Haas PEW, Noordhoek GT, Kiers A, Foudraine NA, Portaels F, Kolk AHJ, Kremer K, van Emden JDA. Diagnosis of Mycobacterium microti infections among humans by using novel genetic markers. Journal of Clinical Microbiology. 1998;36:1840–1845. doi: 10.1128/jcm.36.7.1840-1845.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells AQ. The murine type of tubercle bacillus (the vole acid-fast bacillus) Special Report Series in Medicine, Council of London. 1946;259:1–48. [Google Scholar]
- Wells AQ, Oxon DM. Tuberculosis in wild voles. Lancet. 1937;I:1221. [Google Scholar]
- White GC, Burnham KP. Program MARK: survival estimation from populations of marked animals. Bird Study. 1999;46:120–138. [Google Scholar]