Abstract
Aims
The DoUble-blind Atorvastatin AmLodipine (DUAAL) trial investigated whether atorvastatin decreases ischaemia by a vascular benefit, independent of low-density lipoprotein cholesterol lowering, in patients with coronary artery disease (CAD), both alone and in combination with the traditional anti-anginal therapy, amlodipine.
Methods and results
Randomized, double-blind, parallel-group, multicountry trial (2 weeks run-in and 24 weeks active therapy) comparing three treatments: amlodipine, atorvastatin, and amlodipine + atorvastatin; in 311 patients (78% male; mean age 62 years) with stable angina (≥2 attacks/week), CAD history, ≥3 transient myocardial ischaemia (TMI) episodes, and/or ≥15 min ischaemia on 48 h ambulatory electrocardiographic (AECG) monitoring. Efficacy variables were change in TMI by AECG, exercise ischaemia, angina diary data, and inflammatory biomarkers at Week 26. There was a comparable, highly significant decrease in TMI with amlodipine and atorvastatin, but no additional benefit for the combination. More than 50% of patients became TMI-free in all three groups and this was accompanied by a comparable, marked reduction in angina and nitroglycerin consumption. High-sensitivity C-reactive protein fell by 40% in patients receiving atorvastatin but there was no change with amlodipine. Adverse events were comparable among groups.
Conclusion
Atorvastatin was as potent an anti-ischaemic agent as amlodipine. Future studies of combination therapies will be instructive.
Clinical trial registration information: National clinical trial number: NCT00159718, protocol number A0531031 listed on http://clinicaltrials.gov/.
Keywords: Coronary artery disease, Transient myocardial ischaemia, Statin, Combination therapy
See page 2567 for the editorial comment on this article (doi:10.1093/eurheartj/ehq214)
Introduction
Symptomatic coronary artery disease (CAD) in patients with chronic stable angina is frequently treated by percutaneous coronary intervention (PCI), but recent evidence has not shown significant improvement in mortality with this approach vs. modern medical therapy.1 It is increasingly clear that cardiovascular outcomes depend on an understanding of the biology of atherosclerotic disease, which may involve vascular inflammation, endothelial dysfunction, and plaque instability.2 This has therefore become a target for novel therapies.
Transient myocardial ischaemia (TMI) during daily life has been linked to an increased risk of coronary events.3 The pattern of symptomatic and asymptomatic TMI has been used as a measure of disease activity and response to therapies.4,5 We have previously shown that the long-acting calcium channel blocker, amlodipine besylate, reduces TMI episodes over 24 h.4,6 Recently, there has been considerable interest in the potential vascular benefits of statins in addition to lowering of low-density lipoprotein cholesterol (LDL-C).7 In a study of 40 patients, lovastatin reduced ambulatory TMI in patients with CAD. A larger study of 341 low-risk CAD patients, AVERT, demonstrated that therapy with 80 mg of atorvastatin daily reduced the incidence of ischaemic at least to a similar level as that achieved by angioplasty followed by usual care.8 In patients with acute coronary syndromes and after myocardial infarction, the rapid outcome benefit and fall in inflammatory biomarkers achieved with statins suggest an effect on vascular biology.9,10 This has not been investigated in patients with stable CAD. The DoUble-blind Atorvastatin AmLodipine (DUAAL) study was designed to determine whether atorvastatin relieved TMI in a large cohort of symptomatic CAD patients and to compare the effect of atorvastatin with amlodipine. We investigated the time course of effects on TMI and inflammatory biomarkers as a guide to mechanism of action. Co-administration of amlodipine and atorvastatin has been shown to increase nitric oxide and reduce oxidative stress in vitro.11 We therefore explored whether there was an additive effect of combining atorvastatin with amlodipine.
Methods
Patient population
Men and women (aged 21–80 years) with clinically stable angina pectoris (≥2 attacks per week without change for ≥3 months prior to study entry) were recruited from 46 clinical centres in 13 countries (Appendix). Patients had to have a positive exercise test and documentation of CAD defined by ≥1 of the following: a positive thallium exercise test; a coronary arteriogram demonstrating >70% narrowing of ≥1 major coronary artery; documentation of previous myocardial infarction >2 months prior to the study; or history of coronary artery bypass surgery or PCI. Total cholesterol was required to be ≥200 mg/dL (≥5.2 mmol/L). Angiograms were mandatory for all females as false-positives can occur with other diagnostic procedures.
Exclusion criteria included congenital heart disease, uncontrolled hypertension, standing systolic blood pressure (SBP) <100 mmHg, bradyarrhythmias (heart rate <50 b.p.m.), greater than first-degree atrioventricular block, any medication known to interfere with the electrocardiogram (ECG), intolerance or hypersensitivity to amlodipine or atorvastatin, elevated creatine phosphokinase >3× upper limit of normal, active liver disease or hepatic dysfunction, impaired renal function, and total cholesterol ≥300 mg/dL (7.8 mmol/L). All patients gave written, informed consent as approved by the institutional review boards of the study sites.
Eligible patients underwent 48 h of continuous ambulatory ECG (AECG) monitoring and were included if they demonstrated ≥15 min of ischaemia by ST-segment depression and/or three or more ischaemic episodes. Patients were maintained on stable doses of all concomitant cardiovascular medications; only patients who had received calcium channel blockers within 2 weeks before screening and statins 4 weeks before screening were excluded. Sublingual nitroglycerin was permitted as required, but not for prophylaxis, and was recorded in patient diaries.
Study design
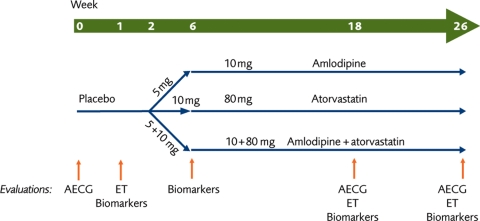
This was a parallel, randomized trial with a 2-week, single-blind, placebo run-in phase followed by a double-blind treatment period. Eligible patients were randomized in a 1:1:1 ratio using a computer-generated randomization code to receive treatment with amlodipine, atorvastatin, or both amlodipine and atorvastatin, once daily for 24 weeks (Figure 1).
Figure 1.
Study design. AECG, ambulatory electrocardiogram; ET, exercise test.
Study medication was administered in a double-blind, double-dummy design. Patients randomized to amlodipine received 5 mg/day, and at Week 6, the dose was increased to 10 mg/day. Similarly atorvastatin 10 mg was increased to 80 mg. In the combination arm, the same titration schedule was undertaken for both agents.
The primary efficacy endpoint was the number of ischaemic episodes during 48 h AECG monitoring at Week 26. Secondary assessments included changes from baseline to Week 18 in AECG ischaemic episode number, as well as changes from baseline to Weeks 18, and 26 in bicycle exercise test variables, inflammatory biomarkers, angina diary data, and lipid variables. Adverse events were also recorded.
Ambulatory electrocardiographic monitoring
Patients underwent 48 h AECG monitoring at Weeks 1, 18, and 26. A calibrated two-channel AECG recording electrocardiographic apparatus (Rozinn Electronics model 151, Rozinn Electronics Inc., Glendale, NY, USA) with a modified lead V3 and V5 or lead II was used. Recordings were analysed centrally by Cardiology Consultants (London, UK).
Episodes were identified by standard criteria. Episodes of symptomatic and asymptomatic TMI were not analysed separately by AECG monitoring. Chest pain was recorded in angina diaries. Recordings were analysed in a blinded fashion both with respect to the patient and study phase. For each episode of ST depression, time of onset, number, duration, and severity were recorded.
Bicycle exercise testing
Bicycle ergometer exercise tests were performed at Weeks 2, 18, and 26 using a standard graded increase in workload. Heart rate and blood pressure were measured. Total exercise time, reasons for stopping the exercise, and the times to onset of ≥0.1 mV ST-segment depression and to angina were recorded.
Blood assessments
High-sensitivity C-reactive protein, amyloid-A, and interleukin-6 levels were determined at screening and at Weeks 6, 18, and 26. Lipid levels, including total cholesterol, high-density lipoprotein cholesterol, LDL-C, and triglycerides, were measured at Weeks 2, 6, 18, and 26. Liver function tests were performed with central analysis.
Patient angina diaries
Angina diaries were completed daily by each patient from Weeks 0 to 26. Patients recorded the date, time, and duration of their angina episodes and the number of sublingual nitroglycerin tablets taken.
Statistical methods
The sample size was based on information from the Circadian Anti-ischemia Program in Europe (CAPE II) trial.6 A sample size of 300 (100 patients per treatment group) was calculated to have a power of 80% to detect a difference in means of 0.6 episodes with a standard deviation (SD) of 1.5 using a two-group t-test with a two-sided significance level of 0.05.
The efficacy analyses were performed on the modified intent-to-treat population, which was defined as all randomized patients with a baseline efficacy measurement and ≥1 follow-up measurement for the same variable. Missing measurements at post-baseline visits were extrapolated using the last observation carried forward (LOCF) method. Sensitivity analyses for the LOCF method were performed using only data collected after Week 6, when the dosage of study medication was increased. The results were identical to those presented in this article. A statistical significance level of 0.05 was used for all efficacy analyses without adjusting for multiplicity. Safety analyses were performed for all randomized patients who received ≥1 dose of any study medication.
For efficacy measurements with skewed distributions, including 48 h AECG monitoring data, and inflammatory biomarkers, the overall treatment differences for change from baseline were analysed using the Kruskal–Wallis test. If the overall treatment effect was significant, a pair-wise comparison between treatment groups was performed using the Wilcoxon rank-sum test. Within-treatment comparison was performed using the Wilcoxon signed-rank test.
For the efficacy measurements with approximate normal distributions including lipid profiles, angina diary data, and bicycle exercise test data, the overall treatment differences on the change from baseline were analysed using an analysis of covariance model with treatment as the main effect and baseline as a covariate. If the overall treatment effect was significant, the pair-wise between-treatment comparison was performed using least-squares means from the analysis of covariance model. Within-treatment comparison was performed using a paired t-test.
The planned total duration of the bicycle exercise test (including eight workload stages) was 16 min (960 s). If the onset of events, >1 mm ST depression or onset of angina, was not observed by the end of the test, the time to event was censored at that time point. A log-rank test (including all three treatment groups) for the main treatment effect was performed for the time to onset of >1 mm ST depression and onset of angina at Weeks 18 and 26. If the main treatment effect was significant, then all possible pair-wise treatment comparisons were performed using the log-rank test including the two corresponding treatment groups.
The association between the change from baseline to Week 18 and to Week 26 in the number of ischaemic episodes and the change from baseline to Week 18 and to Week 26 in age, high-sensitivity C-reactive protein, blood pressure, and total cholesterol were investigated using stepwise regression models.
Statistical analysis was performed using SAS Version 8 (SAS Institute Inc., Cary, NC, USA) according to the pre-specified statistical analysis plan by the sponsor (Pfizer Inc.), under the guidance and supervision of the independent steering committee.
Results
Study population
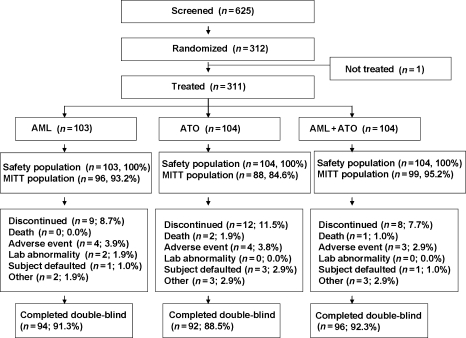
Of 625 patients who were screened, 312 were randomized and 311 (49.8%) received study drug (103 amlodipine, 104 atorvastatin, and 104 combination therapy) (Figure 2). Of these, 29 patients (9.3%) were excluded because of technical reasons and 94 (91.3%), 92 (88.5%), and 96 (92.3%) patients completed the study with technically acceptable AECG data and few missing data in the amlodipine, atorvastatin, and combination arms, respectively. Overall 4% or less of the patients had missing data on AECG, inflammatory biomarker, bicycle exercise test, lipids, and patient diary data at Week 18. An additional 5% or less of the patients had missing data at Week 26. In these individuals, the Week 18 values were carried forward in the analyses.
Figure 2.
Flow of participants through the trial. MITT, modified intent-to-treat.
Overall, five patients had a prior history of statin use (≥4 weeks before screening), two in the amlodipine arm, one in the atorvastatin arm, and two in the combination arm. However, no patient took a statin in the 4-week period before screening and we believe that such low prior usage is unlikely to influence the results. Discontinuations were similar across arms (Figure 2).
Patient baseline characteristics in the three arms were comparable (Table 1). The majority were receiving intensive cardiovascular drug treatment including β-blockers (80.7%), vasodilators (e.g. long-acting nitrates) (62.4%), and aspirin (81.7%) at entry, and minimal changes were made to background treatment during the study (Table 2).
Table 1.
Baseline characteristics
| Amlodipinea (n = 103) | Atorvastatina (n = 104) | Amlodipine + atorvastatina (n = 104) | |
|---|---|---|---|
| Age, mean (SD) (years) | 61.4 (9.0) | 62.5 (8.6) | 62.8 (8.7) |
| Weight, mean (SD) (kg) | 78.9 (10.9) | 81.0 (12.9) | 79.3 (11.9) |
| Diabetes, n (%) | 29 (28.2) | 23 (22.1) | 26 (25.0) |
| Hypertension, n (%) | 72 (69.9) | 83 (79.8) | 72 (69.2) |
| Hyperlipidaemia, n (%) | 75 (72.8) | 82 (78.8) | 84 (80.8) |
| Previous MI, n (%) | 53 (51.5) | 59 (56.7) | 62 (59.6) |
| TC, mean (SD) (mmol/L) | 6.13 (1.0) | 6.16 (1.0) | 5.93 (1.1) |
| LDL-C, mean (SD) (mmol/L) | 3.9 (1.2) | 4.0 (1.1) | 3.9 (0.9) |
| HDL-C, mean (SD) (mmol/L) | 1.2 (0.4) | 1.3 (0.3) | 1.3 (0.3) |
| TG, mean (SD) (mmol/L) | 2.1 (1.2) | 1.8 (0.8) | 1.9 (0.9) |
| SBP, mean (SD) (mmHg) | 131.7 (12.2) | 135.0 (13.0) | 131.9 (11.8) |
| DBP, mean (SD) (mmHg) | 80.0 (6.3) | 81.4 (6.8) | 80.4 (6.4) |
DBP, diastolic blood pressure; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; MI, myocardial infarction; SBP, systolic blood pressure; SD, standard deviation; TC, total cholesterol; TG, triglycerides.
aThere was no statistical difference between treatment groups for the baseline characteristics with the exception of TG (P < 0.05).
Table 2.
Antihypertensive and anti-anginal use before and during the study by treatment arm
| Amlodipine (n = 103) |
Atorvastatin (n = 104) |
Amlodipine + atorvastatin (n = 104) |
||||
|---|---|---|---|---|---|---|
| Before | During | Before | During | Before | During | |
| β-Blockers, n (%) | 79 (76.7) | 79 (76.7) | 89 (85.6) | 89 (85.6) | 83 (79.8) | 83 (79.8) |
| ACE-inhibitors, n (%) | 57 (55.3) | 57 (55.3) | 64 (61.5) | 65 (62.5) | 55 (52.9) | 55 (52.9) |
| Diuretics, n (%) | 17 (16.5) | 17 (16.5) | 25 (24.0) | 27 (26.0) | 21 (20.2) | 22 (21.2) |
| ARBs, n (%) | 2 (1.9) | 2 (1.9) | 4 (3.9) | 4 (3.9) | 3 (2.9) | 3 (2.9) |
| Other antihypertensive combinations, n (%) | 9 (8.7) | 4 (3.9) | 12 (11.5) | 7 (6.7) | 14 (13.5) | 8 (7.7) |
| Anti-anginal therapy, n (%) | 67 (65.0) | 67 (65.0) | 74 (71.2) | 74 (71.2) | 62 (59.6) | 60 (57.7) |
ACE, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Transient myocardial ischaemia by ambulatory electrocardiographic monitoring
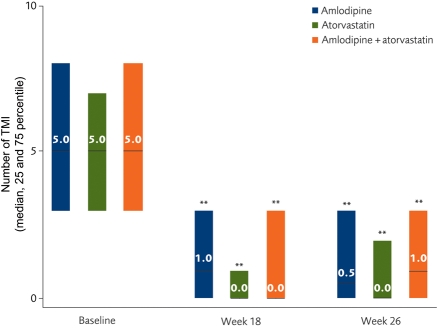
At Week 26, the median number of ischaemic episodes in all three groups decreased significantly (P < 0.001, within-treatment comparison) (Figure 3). There was no significant difference among the three treatment groups in the change from baseline to Week 26 in the number of ischaemic episodes (overall P = 0.922). Transient myocardial ischaemia episodes decreased by >66% in all cohorts, with >50% of patients becoming TMI-free at the final visit in all three groups (Figure 3). Additionally, the median duration of the TMI episodes decreased by >75% in all three groups. There were no differences in efficacy between the treatments, with virtual elimination of ischaemic events by Week 18; atorvastatin was as effective as amlodipine (Figure 3).
Figure 3.
Median number of transient myocardial ischaemia episodes/week, recorded by ambulatory electrocardiographic 48 h monitoring. **P < 0.001 vs. baseline. TMI, transient myocardial ischaemia.
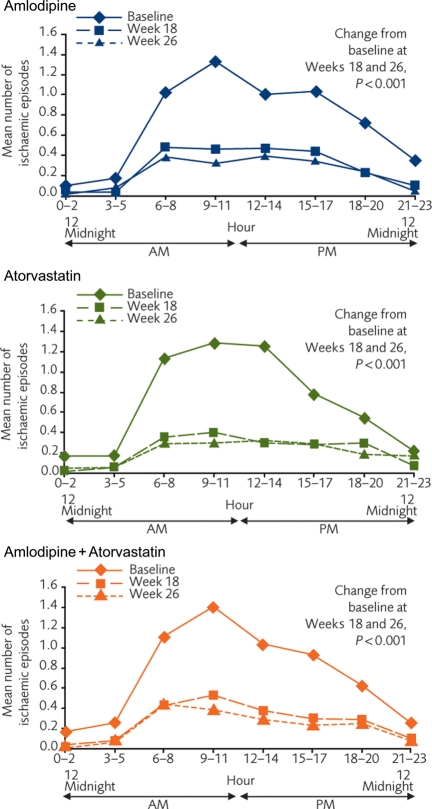
At baseline, the circadian pattern for TMI was observed in all three arms. By Week 18, there was a marked reduction in ischaemic events over 24 h in all three arms (P < 0.001 vs. baseline for all three cohorts), with little additional reductions recorded between Weeks 18 and 26 (Figure 4).
Figure 4.
Twenty-four-hour circadian patterns during ambulatory electrocardiographic monitoring for the three treatment groups.
Diary data
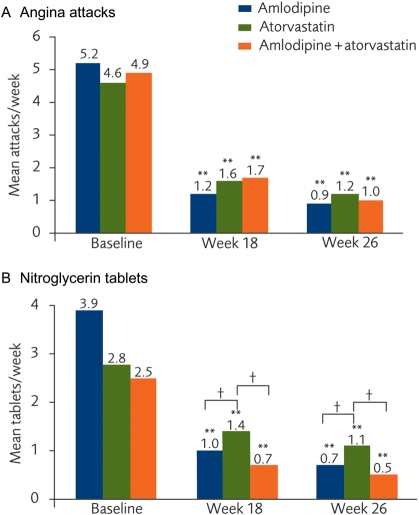
The mean number of diary reported angina attacks/week in all three arms decreased significantly from baseline by Week 18 (P < 0.001 for each arm) and the effects were maintained at Week 26 (P < 0.001 for each arm; Figure 5A). Similarly, the reported use of nitroglycerin tablets was reduced significantly during these periods (P < 0.001 for each arm, baseline vs. Week 18 and vs. Week 26; Figure 5B). The change in number of nitroglycerin tablets used reached significance between the amlodipine and atorvastatin arms (P = 0.05).
Figure 5.
(A) Mean angina attacks/week and (B) mean number of nitroglycerin tablets/week, based on patients' diaries. **P < 0.001 vs. baseline; †P < 0.05 change from baseline between groups.
Bicycle exercise testing
During the bicycle exercise test, many subjects did not have angina or ST depression and stopped the test because of fatigue. Since the time to angina or ST depression for many subjects was censored to their total exercise time, the exercise test result may not reflect the actual treatment effect on the time to angina or ST depression.
In the analysis of the time to onset of angina or the time to onset of ST depression using the log-rank test (Table 3), there were no statistically significant differences among the three treatment groups at the end of study. In each of the three treatment groups, 62–66% of patients had angina at baseline and this was reduced by ∼50% at Weeks 18 and 26. The proportion of patients with ST depression at baseline was between 33 and 40% and this was reduced by ∼25–50% at Weeks 18 and 26.
Table 3.
Time to onset of angina and ST depression during bicycle exercise testing at baseline and at Weeks 18 and 26
| Amlodipine (A) (N = 93) | Atorvastatin (B) (N = 91) | Amlodipine + atorvastatin (C) (N = 99) | Overall P-valuea | Pair-wise P-valuesb |
|||
|---|---|---|---|---|---|---|---|
| A vs. B | A vs. C | B vs. C | |||||
| Time to onset of angina (s)c | |||||||
| Baseline | |||||||
| n | 93 | 91 | 99 | ||||
| Median (s) | 500 | 485 | 536 | ||||
| Angina (%) | 62.4 | 61.5 | 65.7 | 0.817 | 0.524 | 0.837 | 0.668 |
| Week 18 | |||||||
| n | 93 | 91 | 97 | ||||
| Median (s) | 520 | 552 | 555 | ||||
| Angina (%) | 33.3 | 36.3 | 29.3 | 0.475 | 0.839 | 0.252 | 0.315 |
| Week 26 | |||||||
| n | 93 | 91 | 99 | ||||
| Median (s) | 509 | 554 | 540 | ||||
| Angina (%) | 32.3 | 29.7 | 26.3 | 0.444 | 0.365 | 0.243 | 0.714 |
| Time to onset of ST depression (s)c | |||||||
| Baseline | |||||||
| n | 92 | 88 | 97 | ||||
| Median (s) | 524 | 487 | 540 | ||||
| ST depression (%) | 33.3 | 42.9 | 40.4 | 0.673 | 0.333 | 0.653 | 0.711 |
| Week 18 | |||||||
| n | 92 | 88 | 95 | ||||
| Median (s) | 517 | 536 | 548 | ||||
| ST depression (%) | 29.0 | 38.5 | 20.2 | 0.026 | 0.341 | 0.079 | 0.006 |
| Week 26 | |||||||
| n | 92 | 88 | 97 | ||||
| Median (s) | 517 | 540 | 540 | ||||
| ST depression (%) | 25.8 | 31.9 | 25.3 | 0.649 | 0.583 | 0.709 | 0.366 |
N, number of subjects with a baseline and at least one post-baseline observation; n, number of subjects with data at a given visit.
aBased on extended log-rank test for overall comparison among three treatment groups.
bBased on log-rank test for pair-wise comparison between two treatment groups.
cIf a subject did not have angina or ST depression, then the time to onset of angina or time to onset of ST depression was censored at the total exercise time.
Inflammatory biomarkers
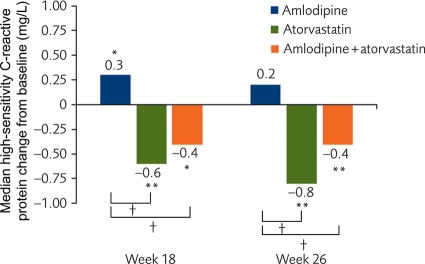
At Week 26, there was no significant change in high-sensitivity C-reactive protein in the patients who received amlodipine monotherapy. In contrast, high-sensitivity C-reactive protein fell significantly at Weeks 18 and 26 for atorvastatin or amlodipine + atorvastatin (Figure 6). There were no or minimal changes in amyloid-A or interleukin-6 at either time point for any arm. The median for interleukin-6 was 4.99 for all treatment arms at baseline and at the two subsequent visits. The interpretation of these results is limited by the large number of subjects with interleukin-6 below the minimum level of detection for the assay. Likewise, baseline values for amyloid-A were low and changes for amyloid-A were modest (range −0.01 to −0.05) and non-significant.
Figure 6.
Change from baseline in high-sensitivity C-reactive protein by treatment group. *P < 0.05 vs. baseline; **P < 0.001 vs. baseline; †P < 0.001 for change from baseline between treatment groups.
Lipids and blood pressure
As expected, there were significantly greater decreases in total cholesterol, LDL-C, and triglycerides at Week 26 in patients who received atorvastatin (monotherapy or in combination) compared with amlodipine monotherapy (P < 0.001). Low-density lipoprotein cholesterol reduction to Week 26 [mean (SD)] in the amlodipine cohort was −0.39 (46.4) mg/dL [−0.01 (1.2) mmol/L] compared with −77.3 (38.7) mg/dL [−2.0 (1.0) mmol/L] with atorvastatin monotherapy and −81.2 (38.7) mg/dL [−2.1 (1.0) mmol/L] for amlodipine + atorvastatin. Similarly, in this population with well-controlled blood pressure, reductions in SBP were slightly greater with amlodipine. This did not reach statistical significance [mean (SD) reduction in SBP from baseline to Week 26 with amlodipine, −9.12 (14.0) mmHg; atorvastatin, −6.85 (14.8) mmHg; amlodipine + atorvastatin, −8.54 (12.4) mmHg; P = 0.42]. Results from a stepwise regression model show that there was no relationship between LDL-C and blood pressure reduction and changes in measures of TMI.
Relationship between transient ischaemia and high-sensitivity C-reactive protein
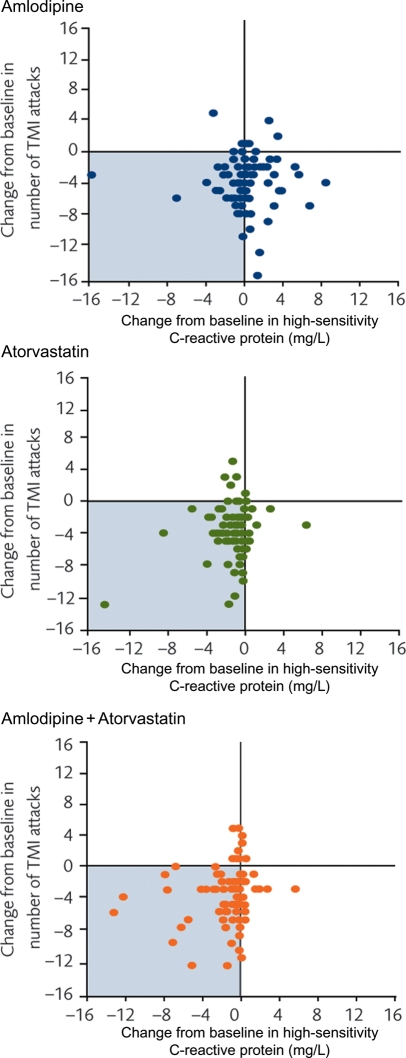
In the patients who received atorvastatin, a reduction in TMI was accompanied by a decrease in high-sensitivity C-reactive protein [median −0.8 mg/L, P < 0.001 (Pearson's correlation coefficient 0.17, P = 0.13) in the atorvastatin arm; and −0.4 mg/L, P < 0.001 (Pearson's correlation coefficient 0.21, P = 0.05) for amlodipine + atorvastatin]. This was not observed in those patients who received amlodipine monotherapy, in whom there was no systematic change in high-sensitivity C-reactive protein associated with ischaemia reduction [median 0.2 mg/L, P = 0.113 (Pearson's correlation coefficient −0.02, P = 0.84); Figure 7].
Figure 7.
Change in number of transient myocardial ischaemia episodes during ambulatory electrocardiographic monitoring and high-sensitivity C-reactive protein in the three groups. TMI, transient myocardial ischaemia; AECG, ambulatory electrocardiogram.
Safety and adverse events
The incidence of all-causality adverse events was similar in all three arms with similar low discontinuation rates. Approximately 24% of patients experienced an adverse event. The majority of events (75.4%) were mild or moderate, and only 4.2% of subjects discontinued due to an adverse event. Peripheral oedema was the only treatment-related adverse event that occurred in >2% of patients, and this occurred exclusively in those who received amlodipine (6.8% in the monotherapy arm and 10.6% in the combination arm, P = 0.46). There was one case of myalgia reported in each group with no elevation in creatine phosphokinase levels. Transitory elevations in alanine aminotransferase (>3× upper limit of normal) were observed in 1% of participants (one subject per arm). No hepatic adverse events were reported.
Discussion
This study demonstrated that in patients with stable angina and CAD, atorvastatin virtually eliminated episodes of TMI during normal daily life, both with and without symptoms. Atorvastatin was as effective as the well-established anti-anginal therapy, amlodipine. Because of the powerful effect of each agent alone, it was not possible to test whether combination therapy with both amlodipine and atorvastatin offered additional effectiveness over the individual agents. However, the impact of atorvastatin and amlodipine on the inflammatory marker high-sensitivity C-reactive protein differed, suggesting that the anti-ischaemic effects were not achieved by the same mechanisms.
Vascular effects of amlodipine and statins
We have previously demonstrated reduction in TMI in patients with stable CAD using amlodipine monotherapy and in combination with other agents.4,6 This is most likely due to direct arterial vasodilatation with improved coronary flow and systemic after-load reduction.12,13 There is increasing interest, however, in targeting further the dysfunctional vascular biology of atherosclerosis, as this may underlie both the disturbances of coronary blood flow in myocardial ischaemia and more serious adverse clinical outcomes.14,15 Statins show a variety of vascular effects in addition to LDL-C lowering, but the clinical relevance of these ‘pleiotropic’ actions has been controversial.7,16,17 We now show, in a large randomized trial, that atorvastatin reduces one of the most common clinical manifestations of atherosclerosis, TMI with and without accompanying angina pectoris, in association with high-sensitivity C-reactive protein lowering. Our objective measurements of TMI, based on ST-segment depression during AECG monitoring, were mirrored by similar reductions in both angina and nitroglycerin use recorded in independent patient diary data. These rapid changes are unlikely to be due solely to beneficial remodelling of the underlying arterial stenoses and, furthermore, were not related to degree of LDL-C lowering. The substantial reductions in ischaemic activity achieved by the treatments provide mechanistic support for the findings of the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial, in which angina and myocardial ischaemia are significantly reduced from baseline in the medically managed groups.1
Effects of atorvastatin
Previous studies in patients with acute coronary syndromes or after MI have shown similar rapid benefits in ischaemic events or mortality using high-dose atorvastatin.9,10 Patients were titrated rapidly to the maximum dose of 80 mg so that we were unable to determine whether a lower dose would have been effective in reducing myocardial ischaemia, as has been reported for clinical outcomes.18,19 We included a combination arm to explore the potential benefit for dual therapy with amlodipine and atorvastatin and powered our study on the basis of a modest anti-ischaemic effect from atorvastatin. The unexpectedly large reduction in TMI observed with atorvastatin (52% of patients were rendered ischaemia-free), however, precluded the possibility of investigating potential additive or synergistic actions of the two drugs in combination.
Mechanisms of action
Although the benefit on TMI was seen with both amlodipine and atorvastatin, the underlying mechanisms are likely to differ. High-sensitivity C-reactive protein was reduced only in the atorvastatin arms (as previously recorded in other populations), and no change was seen accompanying TMI reduction with amlodipine.20 This clearly does not imply a causal role for C-reactive protein in the pathogenesis of TMI or angina.
Statins are known to reduce vascular inflammation and oxidative stress and improve endothelial function.7 All of these processes may contribute to disturbances in coronary flow regulation during normal daily life and lead to TMI.14,21 Our study was not designed to examine the quantitative relation between the degree of LDL-C lowering or inflammatory markers and change in activity of TMI. We cannot therefore draw conclusions about the relative contribution of cholesterol lowering and ‘pleiotropic’ effects on the substantial improvement in ischemia seen with atorvastatin therapy.
Patient population and study design
Our trial included patients similar to those frequently encountered by physicians in clinical practice. They were aged 39–82 years, most were already receiving standard anti-anginal and anti-platelet medications, and had well-controlled blood pressure.4,6 We designed our study to compare the effects of two drugs with different actions and their combination. We did not include a placebo arm for comparison with the active therapies, as it was considered unethical by the investigator sites. Previous studies have reported substantial reduction in ischaemia and angina in placebo groups of patients experiencing angina and coronary disease due in part to ‘regression to the mean’. We set the threshold for ischaemic activity required for inclusion in the study at a lower level than in previous studies using AECG monitoring to reduce the likelihood of the improvements being due to this phenomenon.
All ECG analyses were conducted by core lab staff who were blinded to therapy assignment and treatment phase. Patients were recruited on the basis of TMI in normal daily life, which was also the primary outcome measure. As in our previous studies, interpretable data were obtained on episodes of ST depression from the majority of patients, with only 9.3% of randomized subjects excluded for technical reasons.4,6 We also undertook bicycle tests as a secondary measure, but this proved difficult to standardize and interpret largely due to the patients' good total exercise time at baseline and the variations between centres in the quality of records and conduct of the test.
Future studies
Medical therapy strategies are now moving towards concurrent reduction in multiple risk factors, such as blood pressure and LDL-C, because of emerging evidence for outcome benefit for this approach over individual risk-factor control.22 This may be due to benefits on the common vascular consequences of risk factors. We now show additional benefits of statin therapy beyond lipid lowering in a frequently encountered patient population, with substantial reduction in symptomatic and asymptomatic ischaemia. Although we did not demonstrate additional benefits with amlodipine and atorvastatin combination therapy, future studies in patients with more severe ischaemia, not controlled by a single agent, as well as of lower-dose combination treatment, will be instructive.
Funding
This study was sponsored by Pfizer Inc. Editorial/logistic support was provided by Dr Fiona Nitsche and Karen Burrows of UBC Scientific Solutions and funded by Pfizer Inc. Funding to pay the Open Access publication charges for this article was provided by Pfizer Inc.
Conflict of interest: J.E.D. has been on the speaker bureau for Pfizer Inc., AstraZeneca, Merck Sharp & Dhome, Sanofi Aventis, Novartis, and Takeda. He has received honorarium for consultancy work from Pfizer Inc., Novartis, Sanofi Aventis, Roche, and Danone. P.S. has received consulting fees from Pfizer Inc. and Servier. E.T.'s spouse owns stocks in a company with Pfizer stock options. J.B. has received consulting fees from Merck, Boehringer, Pfizer Inc., Servier, Novartis, Sanofi-Synthelabo, Aventis, Zentiva, and Teva. C.Y., H.S., and B.B. are employees of Pfizer Inc. J.B. was an employee of Pfizer Inc. at the time the study was conducted.
Acknowledgements
The authors had full access to and take responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Appendix
List of participating investigators
Jure Mirat (Croatia†), Mijo Bergovec (Croatia†), Martina Lovric-Bencic (Croatia), Dinko Vitezic (Croatia), Vjeran Nikolic-heizler (Croatia), Jan Pavlas (Czech Republic), Zdenek Lorenc (Czech Republic), Ondrej Jerabek (Czech Republic), Filip Malek (Czech Republic), Vera Bartunkova (Czech Republic), Olar Pullisaar (Estonia), Ullar Soopold (Estonia), Olga Taaler (Estonia), Juri Kaik (Estonia), Aladar Ronaszeki (Hungary*), Geza Lupkovics (Hungary), Istvan Berenyl (Hungary), Istvan Edes (Hungary), Gyula Kurta (Hungary*), Gad Keren (Israel†), Roberto Corinaldesi (Italy†), Antonio Barsotti (Italy†), Alessandro Boccanelli (Italy†), Angelo Branzi (Italy†), Andrejs Erglis (Latvia), Maija Keisa (Latvia), Arcils Gersamija (Latvia*), Ellinor Aaser (Norway*), Marius Troseid (Norway*), Helge Istad (Norway*), Barbara Kusnierz (Poland), Waldemar Banasiak (Poland†), Andrzej Cieslinski (Poland), Grzegorz Opolski (Poland), Michel Tendera (Poland), Radoi Mariana (Romania*), Carmen Ginghina (Romania), Dan Dominic (Romania), Andrej Dukat (Slovakia), Jan Ardo (Slovakia), Vasil Hricak (Slovakia), Patrick J. Commerford (South Africa*), Anton Frans Doubell (South Africa), Sabahattin Umman (Turkey), Mahmut Sahin (Turkey), and Mustafa Akin (Turkey). *Centres screened only or screened and dispensed single-blind placebo but did not randomize any subjects. †Centres received drug but did not screen, dispense single-blind placebo, or randomize any subjects.
References
- 1.Shaw LJ, Berman DS, Maron DJ, Mancini GB, Hayes SW, Hartigan PM, Weintraub WS, O'Rourke RA, Dada M, Spertus JA, Chaitman BR, Friedman J, Slomka P, Heller GV, Germano G, Gosselin G, Berger P, Kostuk WJ, Schwartz RG, Knudtson M, Veledar E, Bates ER, McCallister B, Teo KK, Boden WE. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation. 2008;117:1283–1291. doi: 10.1161/CIRCULATIONAHA.107.743963. doi:10.1161/CIRCULATIONAHA.107.743963. [DOI] [PubMed] [Google Scholar]
- 2.Libby P. Inflammation and cardiovascular disease mechanisms. Am J Clin Nutr. 2006;83:456S–460S. doi: 10.1093/ajcn/83.2.456S. [DOI] [PubMed] [Google Scholar]
- 3.Deedwania PC, Carbajal EV. Silent ischemia during daily life is an independent predictor of mortality in stable angina. Circulation. 1990;81:748–756. doi: 10.1161/01.cir.81.3.748. [DOI] [PubMed] [Google Scholar]
- 4.Deanfield JE, Detry JM, Lichtlen PR, Magnani B, Sellier P, Thaulow E. Amlodipine reduces transient myocardial ischemia in patients with coronary artery disease: double-blind Circadian Anti-Ischemia Program in Europe (CAPE Trial) J Am Coll Cardiol. 1994;24:1460–1467. doi: 10.1016/0735-1097(94)90140-6. [DOI] [PubMed] [Google Scholar]
- 5.Deedwania PC, Carbajal EV. Prevalence and patterns of silent myocardial ischemia during daily life in stable angina patients receiving conventional antianginal drug therapy. Am J Cardiol. 1990;65:1090–1096. doi: 10.1016/0002-9149(90)90319-v. doi:10.1016/0002-9149(90)90319-V. [DOI] [PubMed] [Google Scholar]
- 6.Deanfield JE, Detry JM, Sellier P, Lichtlen PR, Thaulow E, Bultas J, Brennan C, Young ST, Beckerman B. Medical treatment of myocardial ischemia in coronary artery disease: effect of drug regime and irregular dosing in the CAPE II trial. J Am Coll Cardiol. 2002;40:917–925. doi: 10.1016/s0735-1097(02)02050-8. doi:10.1016/S0735-1097(02)02050-8. [DOI] [PubMed] [Google Scholar]
- 7.Robinson JG. Models for describing relations among the various statin drugs, low-density lipoprotein cholesterol lowering, pleiotropic effects, and cardiovascular risk. Am J Cardiol. 2008;101:1009–1015. doi: 10.1016/j.amjcard.2007.11.060. doi:10.1016/j.amjcard.2007.11.060. [DOI] [PubMed] [Google Scholar]
- 8.Pitt B, Waters D, Brown WV, van Boven AJ, Schwartz L, Title LM, Eisenberg D, Shurzinske L, McCormick LS. Aggressive lipid-lowering therapy compared with angioplasty in stable coronary artery disease. Atorvastatin versus Revascularization Treatment Investigators. N Engl J Med. 1999;341:70–76. doi: 10.1056/NEJM199907083410202. doi:10.1056/NEJM199907083410202. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz GG, Olsson AG, Ezekowitz MD, Ganz P, Oliver MF, Waters D, Zeiher A, Chaitman BR, Leslie S, Stern T. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA. 2001;285:1711–1718. doi: 10.1001/jama.285.13.1711. doi:10.1001/jama.285.13.1711. [DOI] [PubMed] [Google Scholar]
- 10.Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, Joyal SV, Hill KA, Pfeffer MA, Skene AM. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–1504. doi: 10.1056/NEJMoa040583. doi:10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 11.Mason RP, Kubant R, Heeba G, Jacob RF, Day CA, Medlin YS, Funovics P, Malinski T. Synergistic effect of amlodipine and atorvastatin in reversing LDL-induced endothelial dysfunction. Pharm Res. 2008;25:1798–1806. doi: 10.1007/s11095-007-9491-1. doi:10.1007/s11095-007-9491-1. [DOI] [PubMed] [Google Scholar]
- 12.Saino A, Pomidossi G, Perondi R, Gregorini L, Rimini A, Alessio P, Zanchetti A, Mancia G. Effects of amlodipine on coronary hemodynamics and vascular responses to sympathetic stimulation in patients with coronary heart disease. J Cardiovasc Pharmacol. 1994;24:875–882. [PubMed] [Google Scholar]
- 13.Silke B, Verma SP, Zezulka AV, Sharma S, Reynolds G, Jackson NC, Guy S, Taylor SH. Haemodynamic and radionuclide effects of amlodipine in coronary artery disease. Br J Clin Pharmacol. 1990;29:437–445. doi: 10.1111/j.1365-2125.1990.tb03662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yeung AC, Vekshtein VI, Krantz DS, Vita JA, Ryan TJ, Jr, Ganz P, Selwyn AP. The effect of atherosclerosis on the vasomotor response of coronary arteries to mental stress. N Engl J Med. 1991;325:1551–1556. doi: 10.1056/NEJM199111283252205. [DOI] [PubMed] [Google Scholar]
- 15.Anderson TJ. Prognostic significance of brachial flow-mediated vasodilation. Circulation. 2007;115:2373–2375. doi: 10.1161/CIRCULATIONAHA.107.697045. doi:10.1161/CIRCULATIONAHA.107.697045. [DOI] [PubMed] [Google Scholar]
- 16.Wang CY, Liu PY, Liao JK. Pleiotropic effects of statin therapy: molecular mechanisms and clinical results. Trends Mol Med. 2008;14:37–44. doi: 10.1016/j.molmed.2007.11.004. doi:10.1016/j.molmed.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halcox JP, Deanfield JE. Beyond the laboratory: clinical implications for statin pleiotropy. Circulation. 2004;109(21 Suppl. 1):II42–II48. doi: 10.1161/01.CIR.0000129500.29229.92. [DOI] [PubMed] [Google Scholar]
- 18.Sever PS, Dahlöf B, Poulter NR, Wedel H, Beevers G, Caulfield M, Collins R, Kjeldsen SE, Kristinsson A, McInnes GT, Mehlsen J, Nieminen M, O'Brien E, Ostergren J. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial—Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361:1149–1158. doi: 10.1016/S0140-6736(03)12948-0. doi:10.1016/S0140-6736(03)12948-0. [DOI] [PubMed] [Google Scholar]
- 19.Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, Thomason MJ, Mackness MI, Charlton-Menys V, Fuller JH on behalf of the Cards Investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685–696. doi: 10.1016/S0140-6736(04)16895-5. doi:10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- 20.Genser B, Grammer TB, Stojakovic T, Siekmeier R, Maerz W. Effect of HMG CoA reductase inhibitors on low-density lipoprotein cholesterol and C-reactive protein: systemic review and meta-analysis. Int J Clin Pharmacol Ther. 2008;46:497–510. doi: 10.5414/cpp46497. [DOI] [PubMed] [Google Scholar]
- 21.Deanfield JE, Selwyn AP. Character and causes of transient myocardial ischemia during daily life. Implications for treatment of patients with coronary disease. Am J Med. 1986;80:18–24. doi: 10.1016/0002-9343(86)90448-1. [DOI] [PubMed] [Google Scholar]
- 22.Stamler J, Neaton JD. The Multiple Risk Factor Intervention Trial (MRFIT)—importance then and now. JAMA. 2008;300:1343–1345. doi: 10.1001/jama.300.11.1343. doi:10.1001/jama.300.11.1343. [DOI] [PubMed] [Google Scholar]