Abstract
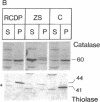
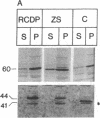
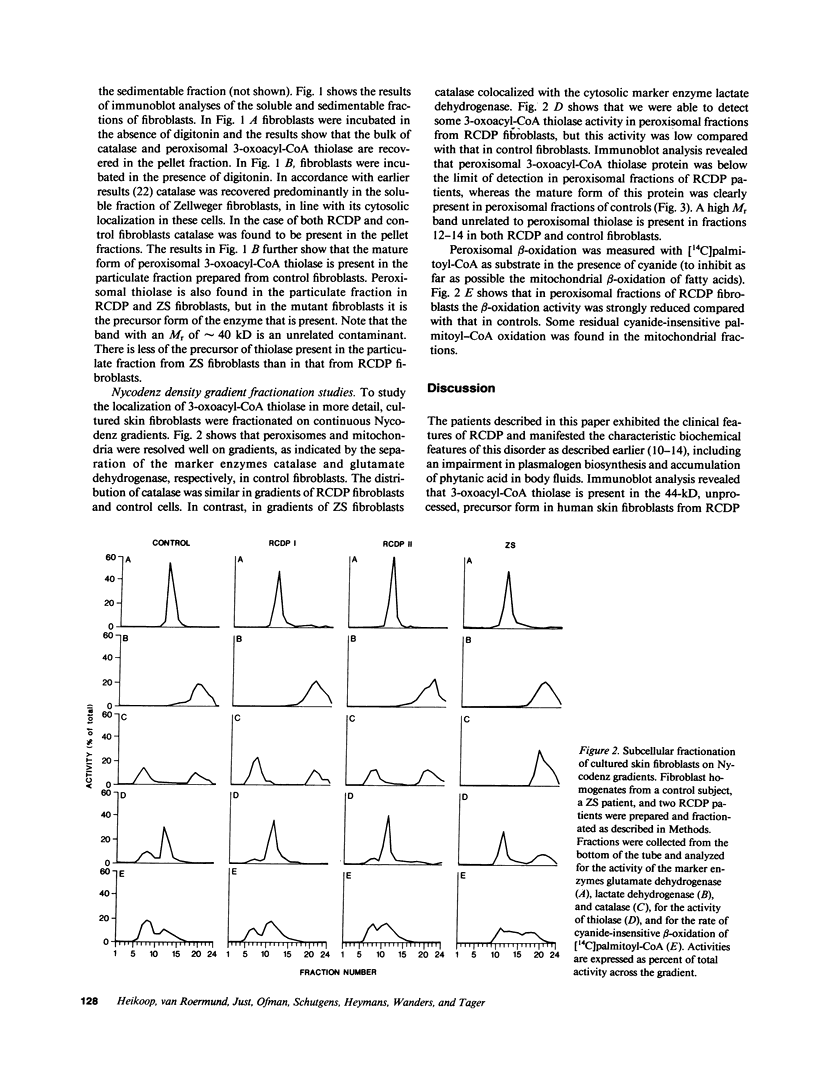
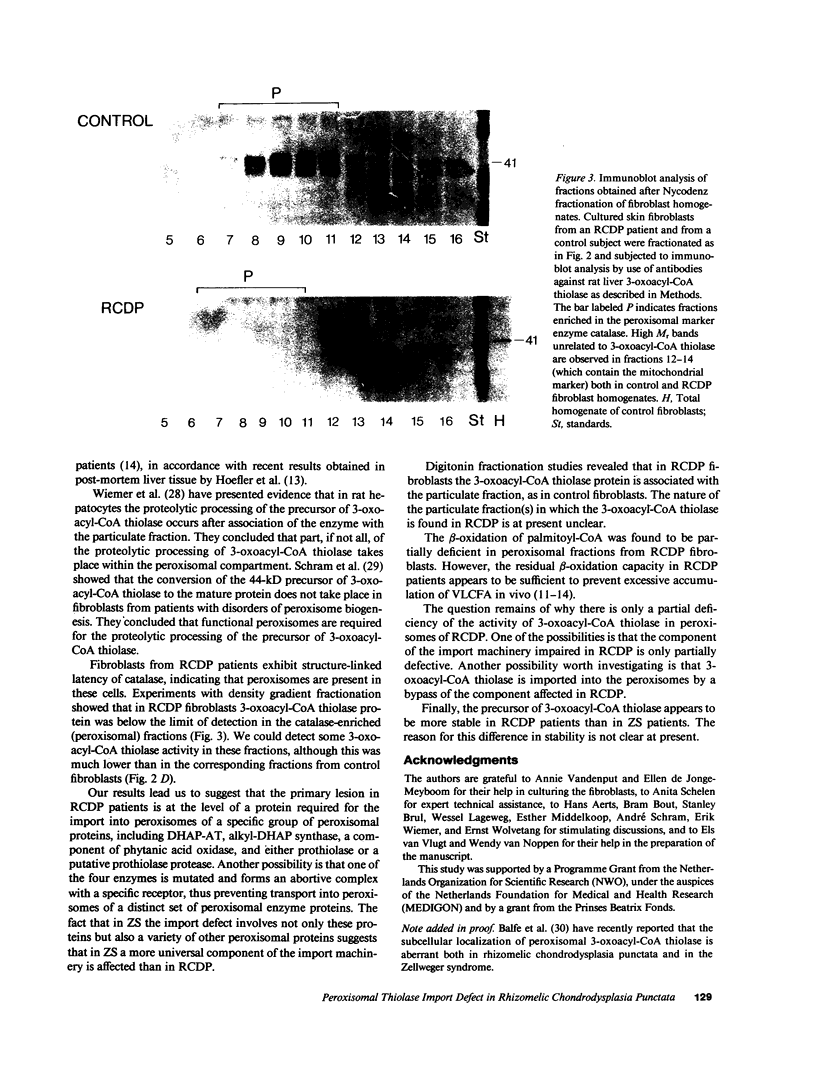
The rhizomelic form of chondrodysplasia punctata (RCDP) is a peroxisomal disorder characterized biochemically by an impairment of plasmalogen biosynthesis and phytanate catabolism. We have now found that the maturation of peroxisomal 3-oxoacyl-CoA thiolase is impaired in fibroblasts from RCDP patients. To establish the subcellular localization of the 3-oxoacyl-CoA thiolase precursor protein, cultured skin fibroblasts were fractionated on a continuous Nycodenz gradient. Only a small amount of 3-oxoacyl-CoA thiolase activity was present in the catalase-containing (peroxisomal) fractions of RCDP fibroblasts in comparison with control fibroblasts. Moreover, the amount of thiolase protein in immunoblots of the catalase-containing fractions was below the limit of detection. Finally, the beta-oxidation of [14C]palmitoyl-CoA was found to be reduced in these fractions. We conclude that the mutation in RCDP leads to a partial deficiency of 3-oxoacyl-CoA thiolase activity in the peroxisomes and, concomitantly, an impairment in the ability to convert the precursor of this protein to the mature form. The reduction of 3-oxoacyl-CoA thiolase activity results in a decrease in the rate of peroxisomal beta-oxidation of palmitoyl-CoA. However, the capacity of the peroxisomes to oxidize very-long-chain fatty acids must be sufficient to prevent excessive accumulation of these compounds in vivo.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balfe A., Hoefler G., Chen W. W., Watkins P. A. Aberrant subcellular localization of peroxisomal 3-ketoacyl-CoA thiolase in the Zellweger syndrome and rhizomelic chondrodysplasia punctata. Pediatr Res. 1990 Mar;27(3):304–310. doi: 10.1203/00006450-199003000-00023. [DOI] [PubMed] [Google Scholar]
- Danpure C. J., Jennings P. R., Watts R. W. Enzymological diagnosis of primary hyperoxaluria type 1 by measurement of hepatic alanine: glyoxylate aminotransferase activity. Lancet. 1987 Feb 7;1(8528):289–291. doi: 10.1016/s0140-6736(87)92023-x. [DOI] [PubMed] [Google Scholar]
- Fujiki Y., Rachubinski R. A., Mortensen R. M., Lazarow P. B. Synthesis of 3-ketoacyl-CoA thiolase of rat liver peroxisomes on free polyribosomes as a larger precursor. Induction of thiolase mRNA activity by clofibrate. Biochem J. 1985 Mar 15;226(3):697–704. doi: 10.1042/bj2260697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukami M. H., Flatmark T. Studies on catalase compartmentation in digitonin-treated rat hepatocytes. Biochim Biophys Acta. 1986 Oct 31;889(1):91–94. doi: 10.1016/0167-4889(86)90012-1. [DOI] [PubMed] [Google Scholar]
- Furuta S., Hashimoto T., Miura S., Mori M., Tatibana M. Cell-free synthesis of the enzymes of peroxisomal beta-oxidation. Biochem Biophys Res Commun. 1982 Mar 30;105(2):639–646. doi: 10.1016/0006-291x(82)91482-6. [DOI] [PubMed] [Google Scholar]
- Heymans H. S., Oorthuys J. W., Nelck G., Wanders R. J., Schutgens R. B. Rhizomelic chondrodysplasia punctata: another peroxisomal disorder. N Engl J Med. 1985 Jul 18;313(3):187–188. [PubMed] [Google Scholar]
- Hoefler G., Hoefler S., Watkins P. A., Chen W. W., Moser A., Baldwin V., McGillivary B., Charrow J., Friedman J. M., Rutledge L. Biochemical abnormalities in rhizomelic chondrodysplasia punctata. J Pediatr. 1988 May;112(5):726–733. doi: 10.1016/s0022-3476(88)80689-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazo O., Contreras M., Bhushan A., Stanley W., Singh I. Adrenoleukodystrophy: impaired oxidation of fatty acids due to peroxisomal lignoceroyl-CoA ligase deficiency. Arch Biochem Biophys. 1989 May 1;270(2):722–728. doi: 10.1016/0003-9861(89)90555-9. [DOI] [PubMed] [Google Scholar]
- Miura S., Mori M., Takiguchi M., Tatibana M., Furuta S., Miyazawa S., Hashimoto T. Biosynthesis and intracellular transport of enzymes of peroxisomal beta-oxidation. J Biol Chem. 1984 May 25;259(10):6397–6402. [PubMed] [Google Scholar]
- Poll-The B. T., Roels F., Ogier H., Scotto J., Vamecq J., Schutgens R. B., Wanders R. J., van Roermund C. W., van Wijland M. J., Schram A. W. A new peroxisomal disorder with enlarged peroxisomes and a specific deficiency of acyl-CoA oxidase (pseudo-neonatal adrenoleukodystrophy). Am J Hum Genet. 1988 Mar;42(3):422–434. [PMC free article] [PubMed] [Google Scholar]
- Poulos A., Sheffield L., Sharp P., Sherwood G., Johnson D., Beckman K., Fellenberg A. J., Wraith J. E., Chow C. W., Usher S. Rhizomelic chondrodysplasia punctata: clinical, pathologic, and biochemical findings in two patients. J Pediatr. 1988 Oct;113(4):685–690. doi: 10.1016/s0022-3476(88)80378-0. [DOI] [PubMed] [Google Scholar]
- Schrakamp G., Schalkwijk C. G., Schutgens R. B., Wanders R. J., Tager J. M., van den Bosch H. Plasmalogen biosynthesis in peroxisomal disorders: fatty alcohol versus alkylglycerol precursors. J Lipid Res. 1988 Mar;29(3):325–334. [PubMed] [Google Scholar]
- Schram A. W., Goldfischer S., van Roermund C. W., Brouwer-Kelder E. M., Collins J., Hashimoto T., Heymans H. S., van den Bosch H., Schutgens R. B., Tager J. M. Human peroxisomal 3-oxoacyl-coenzyme A thiolase deficiency. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2494–2496. doi: 10.1073/pnas.84.8.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schram A. W., Strijland A., Hashimoto T., Wanders R. J., Schutgens R. B., van den Bosch H., Tager J. M. Biosynthesis and maturation of peroxisomal beta-oxidation enzymes in fibroblasts in relation to the Zellweger syndrome and infantile Refsum disease. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6156–6158. doi: 10.1073/pnas.83.16.6156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimozawa N., Suzuki Y., Orii T., Hashimoto T. Immunoblot detection of enzyme proteins of peroxisomal beta-oxidation in fibroblasts, amniocytes, and chorionic villous cells. Possible marker for prenatal diagnosis of Zellweger's syndrome. Prenat Diagn. 1988 May;8(4):287–290. doi: 10.1002/pd.1970080407. [DOI] [PubMed] [Google Scholar]
- Tager J. M., Van der Beek W. A., Wanders R. J., Hashimoto T., Heymans H. S., Van den Bosch H., Schutgens R. B., Schram A. W. Peroxisomal beta-oxidation enzyme proteins in the Zellweger syndrome. Biochem Biophys Res Commun. 1985 Feb 15;126(3):1269–1275. doi: 10.1016/0006-291x(85)90322-5. [DOI] [PubMed] [Google Scholar]
- Wanders R. J., Heymans H. S., Schutgens R. B., Barth P. G., van den Bosch H., Tager J. M. Peroxisomal disorders in neurology. J Neurol Sci. 1988 Dec;88(1-3):1–39. doi: 10.1016/0022-510x(88)90203-1. [DOI] [PubMed] [Google Scholar]
- Wanders R. J., Kos M., Roest B., Meijer A. J., Schrakamp G., Heymans H. S., Tegelaers W. H., van den Bosch H., Schutgens R. B., Tager J. M. Activity of peroxisomal enzymes and intracellular distribution of catalase in Zellweger syndrome. Biochem Biophys Res Commun. 1984 Sep 28;123(3):1054–1061. doi: 10.1016/s0006-291x(84)80240-5. [DOI] [PubMed] [Google Scholar]
- Wanders R. J., Smit W., Heymans H. S., Schutgens R. B., Barth P. G., Schierbeek H., Smit G. P., Berger R., Przyrembel H., Eggelte T. A. Age-related accumulation of phytanic acid in plasma from patients with the cerebro-hepato-renal (Zellweger) syndrome. Clin Chim Acta. 1987 Jun 30;166(1):45–56. doi: 10.1016/0009-8981(87)90193-8. [DOI] [PubMed] [Google Scholar]
- Wanders R. J., van Roermund C. W., van Wijland M. J., Heikoop J., Schutgens R. B., Schram A. W., Tager J. M., van den Bosch H., Poll-Thé B. T., Saudubray J. M. Peroxisomal very long-chain fatty acid beta-oxidation in human skin fibroblasts: activity in Zellweger syndrome and other peroxisomal disorders. Clin Chim Acta. 1987 Jul 15;166(2-3):255–263. doi: 10.1016/0009-8981(87)90428-1. [DOI] [PubMed] [Google Scholar]
- Wanders R. J., van Roermund C. W., van Wijland M. J., Schutgens R. B., van den Bosch H., Schram A. W., Tager J. M. Direct demonstration that the deficient oxidation of very long chain fatty acids in X-linked adrenoleukodystrophy is due to an impaired ability of peroxisomes to activate very long chain fatty acids. Biochem Biophys Res Commun. 1988 Jun 16;153(2):618–624. doi: 10.1016/s0006-291x(88)81140-9. [DOI] [PubMed] [Google Scholar]
- Watkins P. A., Chen W. W., Harris C. J., Hoefler G., Hoefler S., Blake D. C., Jr, Balfe A., Kelley R. I., Moser A. B., Beard M. E. Peroxisomal bifunctional enzyme deficiency. J Clin Invest. 1989 Mar;83(3):771–777. doi: 10.1172/JCI113956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zellweger H. Peroxisomopathies: new developments. Dev Med Child Neurol. 1989 Apr;31(2):264–266. doi: 10.1111/j.1469-8749.1989.tb03989.x. [DOI] [PubMed] [Google Scholar]