Abstract
Nocardia farcinica is an emerging pathogen in immunocompromised hosts. Even though several species of Nocardia have been reported as causative pathogens of catheter-related blood stream infections (CRBSI), CRBSI caused by N. farcinica has not been reported. A 70-yr-old man with a tunneled central venous catheter (CVC) for home parenteral nutrition was admitted with fever for two days. Norcardia species was isolated from the blood through CVC and peripheral bloods and identified to N. farcinica by 16S rRNA and rpoB gene sequence analyses. This report emphasizes the rapid and correct identification of causative agents in infectious diseases in the selection of antimicrobial agents and the consideration of catheter removal.
Keywords: Nocardia farcinica, Catheter-related Blood Stream Infection (CRBSI), rpoB
INTRODUCTION
More than 70 taxonomical species have been recognized in the genus Nocardia. Of these, N. asteroides, N. nova, N. cavie, N. farcinica, N. brasiliensis, N. pseudobrasiliensis, N. otitidiscaviarum and N. transvalensis are known to cause infections in human. Predisposing conditions of Nocardia infection include solid tumors, hematologic malignancies, treatment with corticosteroids, bone marrow or solid organ transplantation, chronic pulmonary or renal diseases and acquired immunodeficiency syndrome (1).
Nocardia farcinica has been increasingly recognized as a causative microorganism of human infections (2). Unlikely other Nocardia spp., N. farcinica is characteristically resistant to multiple antimicrobial agents, including third-generation cephalosporins (3). Consequently, a patient who is infected by N. farcinica faces difficulty in treatment and the outcome is often death. Fortunately, N. farcinica rarely causes serious infections in immunocompetent hosts (1). Catheter-related bloodstream infection (CRBSI) caused by N. farcinica has not been reported to date. In this paper, we report the first case of CRBSI caused by N. farcinica, which was identified using 16S rRNA and rpoB gene sequences.
CASE REPORT
A 70-yr-old man with a two days history of fever was admitted to the Samsung Medical Center (SMC) in March 2005. The patient had been treated for atrial fibrillation and hypertension for the past several years. Two months previously, the patient had undergone an ileojejunal resection and right-sided hemicolectomy because of acute mesenteric infarction. Thereafter, the patient had been receiving home parenteral nutrition via a tunneled central venous catheter (CVC; Hickman catheter). The patient had no history of travel or contact with animals. Physical examination revealed a body temperature of 38.8℃. The right subclavian tunneled CVC displayed erythema and minimal purulent discharge at the exit site. Laboratory data including complete blood counts and blood chemistry were within normal limits and a chest radiograph was unremarkable. Three sets of blood samples for culture were drawn through the CVC (two sets) and a peripheral vein (one set). The patient was empirically treated with cefazolin (1 g every 8 hr intravenously). Later, ciprofloxacin (200 mg every 12 hr intravenously) was added to cover gram-negative rods. On day 5 of hospitalization, all the blood cultures yielded gram-positive rods. The bacteria grew at least 2 hr earlier in blood obtained from the CVC than blood obtained from the vein. By day 8, the bacteria had been identified as Nocardia species as described below. Cefazolin and ciprofloxacin were empirically substituted by parenteral trimethoprim-sulfamethoxazole (TMP-SMX, 15 mg/kg/day of trimethoprim) empirically after the identification of Nocardia spp. The CVC was removed because of persistent fever. Thereafter, the patient became afebrile and repeated blood cultures were negative. On day 17, the patient was switched from parenteral to oral TMPSMX. Oral TMP-SMX was continued for 6 weeks. At a 3-month follow-up after discontinuation of TMP-SMX, the patient was well with no evidence of recurrence.
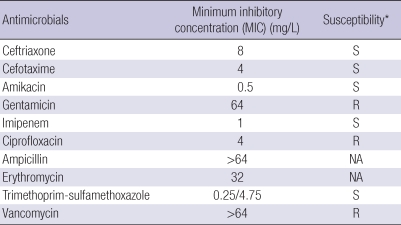
In vitro susceptibility testing was performed by broth microdilution method as described by the Clinical and Laboratory Standards Institute (CLSI) guidelines (4). The results are shown in Table 1. Conventional automated methods such as the VITEK II system (bioMerieux, Hazelwood, MO) were unable to identify this isolate ["SMC-A7077"] to a given Nocardia species. Thus, 16S rRNA and rpoB gene sequence analyses were performed by using primer sets of 16S-F3 (5'-CAG GCC TAA CAC ATG CAA GT-3')/16S-R3 (3'-GGG CGG WGT GTA CAA GGC-3') and MF (5'-CGA CCA CTT CGG CAA CCG-3')/MR (5'-TCG ATC GGG CAC ATC CGG-3'), respectively (5). DNA was amplified by polymerase chain reaction (PCR). Purification of the PCR product and sequencing were also performed as previously described. Determined sequences were compared with the public databases EzTaxon (http://www.eztaxon.org) and GenBank (http://www.ncbi.nlm.nih.gov/blast) using blast searches.
Table 1.
Results of antimicrobial susceptibility testing for isolate SMC-A7077
*S, susceptible; R, resistant.
NA, not available.
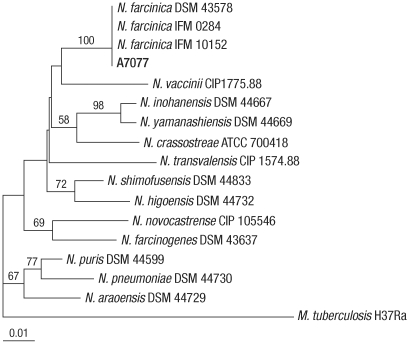
The 16S rRNA gene sequence (1,248 bp) of the isolate showed the greatest similarity (99.92%) to N. farcinica ATCC 3318T [Gen-Bank no. Z36936], followed by N. higoensis IFM 10084T (98.80%), N. shimofusensis IFM 10311T (98.72%) and N. asiatica IFM 0245T (98.72%). The rpoB gene sequence (296 bp) of isolate SMC-A7077 showed complete identity with those of several N. farcinica strains such as DSM 43578, IFM 0284 and IFM 10152. However, it showed similarity values of 96.59% with N. shimofusensis DSM 44733T, 95.56% with N. vaccinii CIP 1775.88T, and 95.22% with N. higoensis DSM 44732T. The phylogenetic relationships of isolate SMC-A7077 with other related Nocardia strains based on rpoB gene sequences are shown in Fig. 1. Based on 16S rRNA and rpoB gene sequence analyses, we concluded that the isolate SMC-A7077 was N. farcinica.
Fig. 1.
Phylogenetic tree of SMC-A7077 and closely related species of Nocardia based on partial rpoB gene sequences. The tree was reconstructed by the neighbor-joining method, and Mycobacterium tuberculosis H37Ra was used as an outgroup. Numbers on branching nodes are percentages of 1,000 bootstrap replications. Only values of ≥50% are shown. The scale bar represents one substitution per 100 nucleotides.
DISCUSSION
Species identification among Nocardia spp. is important to select appropriate antimicrobial agents because antimicrobial resistance profiles are different among Nocardia spp. For the identification of unidentified bacteria or isolates with ambiguous profiles, 16S rRNA gene sequences have been used. Although there are no universal criteria for species identification definition, isolates with sequence identity >99% are generally considered to belong to the same species (6). However, 16S rRNA gene is inappropriate for some genera including Nocardia due to high similarity. Other more variable housekeeping genes such as rpoB and gyrB have been suggested for alternate targets of bacterial identification (5).
Most CRBSIs emanate from the insertion site and skin is a prominent source of microbes causing bloodstream infection. The microbes that most commonly cause CRBSI associated with percutaneously inserted catheters are coagulase-negative staphylococci, S. aureus, Candida species, and enteric gram-negative bacilli (7). While N. asteroides, N. nova and N. cavie have been rarely associated with CRBSI in immunocompromised hosts, CRBSI caused by N. farcinica has not been reported (7-11). Although brain or intramuscular abscess, pneumonia or osteomyelitis by N. farcinica have been reported worldwide including Korea (12-16), bacteremic infection due to N. farcinica related with catheters has not been reported to our knowledge. In this report, we describe for the first time CRBSI caused by N. farcinica.
N. farcinica is increasingly recognized as a human pathogen in infections of the lung, brain, skin, wounds and kidney (12). Resistance to antimicrobial agents occurs more frequently in N. farcinica than in other Nocardia spp. N. farcinica is usually susceptible to TMP-SMX, amikacin, imipenem and ciprofloxacin, but is often resistant to third-generation cephalosporin, ampicillin, erythromycin and gentamicin. TMP-SMX has been selected as the first choice for the treatment in patients with infections by Nocardia spp. N. farcinica strains resistant to multiple antibiotics including TMP-SMX have been recently reported (3). Considering the possibility of TMP-SMX resistance, some authors have proposed amikacin in combination with imipenem or amoxicillin-clavulanate as the first-line therapy (9, 12). Fluoroquinolones have emerged as one of the potentially attractive candidates for the treatment of infections by N. farcinica (17). In some reports, infections caused by multidrug-resistant N. farcinica in immunocompromized hosts were successfully treated with linezolid (2, 16, 18).
Identification of clinical isolates using conventional automated methods usually is reported as the genus Nocardia ('Nocardia species'), rather than to the species level. Given the aforementioned antibiotic resistance concerns posed by N. farcinica, it is prudent to suggest that molecular identification or antimicrobial susceptibility test for Nocardia species should be performed when 'Nocardia species' is isolated from a specimen culture to ensure proper treatment. Appropriate duration of antimicrobial treatment in patients with Nocardia CRBSI is unclear. Often, antimicrobial therapy is continued for several months after apparent cure because of a concern of high relapse rates of nocardiosis. While it has been suggested that pulmonary or systemic nocardiosis in immunocompromized hosts should be treated for 6-12 months (12), another report suggested that prolonged antibiotics may not be necessary in nocardial CRBSI without distant metastatic infection after removal of catheter (8). Presently, the patient was successfully treated with parenteral TMP-SMX for 10 days and a subsequent 6-week course of oral formulation after removal of the CVC.
Specific recommendations for catheter removal in nocardial CRBSI have not been forthcoming. A report suggested that nocardial bacteremia can be successfully treated without removing vascular catheters (11), while other case was relapsed after management of keeping CVC (19). Three cases were treated with removal of CVC (10, 20). In this report, the patient became afebrile after CVC removal and administration of parenteral TMP-SMX. Removal of the catheter is not absolutely indicated but should be seriously considered prompt removal of catheter when a patient with CRBSI caused by Nocardia species does not respond to appropriate antimicrobial agent(s).
The present novel report of CRBSI caused by N. farcinica indicates that N. farcinica should be included as a causative microorganism of CRBSI. Molecular identification for Nocardia species as well as antimicrobial susceptibility test will be helpful for proper treatment with antimicrobials because antimicrobial susceptibility patterns are variable between Nocardia spp.
ACKNOWLEDGMENTS
We thank Ms. Ji Young Choi (Sungkyunkwan University School of Medicine) for her technical assistance.
Footnotes
This study was supported by a grant from the Korea Health 21 R & D Project, Ministry of Health, Welfare & Family affairs, Republic of Korea (Grant No. A084063).
References
- 1.Christidou A, Maraki S, Scoulica E, Mantadakis E, Agelaki S, Samonis G. Fatal Nocardia farcinica bacteremia in a patient with lung cancer. Diagn Microbiol Infect Dis. 2004;50:135–139. doi: 10.1016/j.diagmicrobio.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 2.Rupprecht TA, Pfister HW. Clinical experience with linezolid for the treatment of central nervous system infections. Eur J Neurol. 2005;12:536–542. doi: 10.1111/j.1468-1331.2005.01001.x. [DOI] [PubMed] [Google Scholar]
- 3.Hansen G, Swanzy S, Gupta R, Cookson B, Limaye AP. In vitro activity of fluoroquinolones against clinical isolates of Nocardia identified by partial 16S rRNA sequencing. Eur J Clin Microbiol Infect Dis. 2008;27:115–120. doi: 10.1007/s10096-007-0413-2. [DOI] [PubMed] [Google Scholar]
- 4.CLSI. Susceptibility testing of Mycobacteria, Nocardiae, and other aerobic actinomycetes: approved standard. Pennsylvania: Wayne; 2003. CLSI document M24-A. [PubMed] [Google Scholar]
- 5.Oh WS, Ko KS, Song JH, Lee MY, Ryu SY, Taek S, Heo ST, Kwon KT, Lee JH, Peck KR, Lee NY. Catheter-associated bacteremia by Mycobacterium senegalense in Korea. BMC Infect Dis. 2005;5:107. doi: 10.1186/1471-2334-5-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stackbrandt E, Ebers J. Taxonomic parameters revisited: tarnished gold standards. Microbiol Today. 2006;33:152–155. [Google Scholar]
- 7.Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O'Grady NP, Raad II, Rijnders BJ, Sherertz RJ, Warren DK. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49:1–45. doi: 10.1086/599376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng YH, Huang WT, Tsao CJ. Venous access port-related nocardia bacteremia with successful short-term antibiotics treatment. J Chin Med Assoc. 2004;67:416–418. [PubMed] [Google Scholar]
- 9.Hitti W, Wolff M. Two cases of multidrug-resistant Nocardia farcinica infection in immunosuppressed patients and implications for empiric therapy. Eur J Clin Microbiol Infect Dis. 2005;24:142–144. doi: 10.1007/s10096-005-1285-y. [DOI] [PubMed] [Google Scholar]
- 10.Lee AC, Yuen KY, Lau YL. Catheter-associated nocardiosis. Pediatr Infect Dis J. 1994;13:1023–1024. [PubMed] [Google Scholar]
- 11.Lui WY, Lee AC, Que TL. Central venous catheter-associated Nocardia bacteremia. Clin Infect Dis. 2001;33:1613–1614. doi: 10.1086/323557. [DOI] [PubMed] [Google Scholar]
- 12.Torres OH, Domingo P, Pericas R, Boiron P, Montiel JA, Vazquez G. Infection caused by Nocardia farcinica: case report and review. Eur J Clin Microbiol Infect Dis. 2000;19:205–212. doi: 10.1007/s100960050460. [DOI] [PubMed] [Google Scholar]
- 13.Park I, Yim H, Kwan LS, Yu S, Cho J, Kim H, Shin GT. A case of a kidney transplant recipient with pulmonary Cytomegalovirus and Nocardia coinfection with Cytomegalovirus nephropathy. Korean J Nephrol. 2009;28:161–165. [Google Scholar]
- 14.Sim SH, Park HC, Kim CJ, Jeon JH, Kim EC, Oh MD, Kim NJ, Choe KW. A case of Nocardia farcinica brain abscess in the patient receiving steroid treatment. Infect Chemother. 2008;40:301–304. [Google Scholar]
- 15.Baek YH, Kim YJ, Lee HH, Youm JY, Kwon OW, Kim JH, Kim SH, Kang CN, Kim SH, Choi TY, Bae SC. A case of intramuscular abscess caused by Nocardia farcinica in a patient with lupus nephritis concurrent with pulmonary tuberculosis. J Korean Rheum Assoc. 2006;13:327–332. [Google Scholar]
- 16.Rivero A, García-Lázaro M, Pérez-Camacho I, Natera C, del Carmen Almodovar M, Camacho A, Torre-Cisneros J. Successful long-term treatment with linezolid for disseminated infection with multiresistant Nocardia farcinica. Infection. 2008;36:389–391. doi: 10.1007/s15010-008-7236-7. [DOI] [PubMed] [Google Scholar]
- 17.Fihman V, Berçot B, Mateo J, Losser MR, Raskine L, Riahi J, Loirat P, Pors MJ. First successful treatment of Nocardia farcinica brain abscess with moxifloxacin. J Infect. 2006;52:e99–e102. doi: 10.1016/j.jinf.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Moylett EH, Pacheco SE, Brown-Elliott BA, Perry TR, Buescher ES, Birmingham MC, Schentag JJ, Gimbel JF, Apodaca A, Schwartz MA, Rakita RM, Wallace RJ., Jr Clinical experience with linezolid for the treatment of Nocardia infection. Clin Infect Dis. 2003;36:313–318. doi: 10.1086/345907. [DOI] [PubMed] [Google Scholar]
- 19.Miron D, Dennehy PH, Josephson SL, Forman EN. Catheter-associated bacteremia with Nocardia nova with secondary pulmonary involvement. Pediatr Infect Dis J. 1994;13:416–417. doi: 10.1097/00006454-199405000-00018. [DOI] [PubMed] [Google Scholar]
- 20.Kontoyiannis DP, Jacobson KL, Whimbey EE, Rolston KV, Raad II. Central venous catheter-associated Nocardia bacteremia: an unusual manifestation of nocardiosis. Clin Infect Dis. 2000;31:617–618. doi: 10.1086/313941. [DOI] [PubMed] [Google Scholar]