Abstract
Objectives
Post-traumatic arthritis is a major cause of disability. Current clinical imaging modalities are unable to reliably evaluate articular cartilage damage prior to surface breakdown, when potentially reversible changes are occurring. Optical Coherence Tomography (OCT) is a nondestructive imaging technology that can detect degenerative changes in articular cartilage with an intact surface. This study tests the hypothesis that OCT detects acute articular cartilage injury following impact at energy levels resulting in chondrocyte death and microstructural changes, but insufficient to produce macroscopic surface damage.
Methods
Bovine osteochondral cores underwent OCT imaging and were divided into a control with no impact or were subjected to low (0.175 J) or moderate (0.35 J) energy impact. Cores were reimaged with OCT following impact and the OCT signal intensity quantified. A ratio of the superficial to deep layer intensities was calculated and compared before and after impact. Chondrocyte viability was determined one day after impact, followed by histology and polarized microscopy.
Results
Macroscopic changes to the articular surface were not observed following low and moderate impact. The OCT signal intensity ratio demonstrated a 27% increase (p=0.006) following low impact, and a 38% increase (p=0.001) following moderate impact. Cell death increased by 150% (p<0.001) and 200% (p<0.001) after low and moderate energy impacts, respectively. When compared to unimpacted controls, both Mankin histology and David-Vaudey polarized microscopy scores increased (p=0.036, p=0.002, respectively) following moderate energy impact.
Conclusions
This study shows that OCT detects acute cartilage changes after impact injury at levels insufficient to cause visible damage to the articular surface, but sufficient to cause chondrocyte death and microscopic matrix damage. This finding supports the utility of OCT to detect microstructural subsurface cartilage damage that is poorly visualized with conventional imaging.
Keywords: Optical Coherence Tomography (OCT), Post-traumatic Arthritis, Cartilage, Imaging, Osteoarthritis
INTRODUCTION
Post-traumatic osteoarthritis (pt-OA) is a significant cause of disability. Estimates indicate that more than five million Americans suffer from pt-OA severe enough to cause them to consult an orthopaedic surgeon (1). In addition, this ailment places an approximate 3 billion dollar burden on the health care system (1). Impact injury to articular cartilage sustained by traumatic injury is a significant cause of post-traumatic osteoarthritis (2). The rapid progression of osteoarthritis after such an injury is likely due to chondrocyte death and matrix degradation caused by traumatic impact to the articular cartilage (3-5). Despite a faster progression to debilitating osteoarthritis, pt-OA is indistinguishable from primary osteoarthritis (OA) in the late stages of disease and is manifested by progressive loss of cartilage, pain, inflammation and decreased joint mobility (6).
Current clinical modalities used to evaluate articular cartilage health, including radiography, arthroscopy and magnetic resonance imaging (MRI) have been unable to reliably detect early cartilage damage when the articular surface still appears grossly normal (7, 8). It is during this early stage when potentially reversible changes may occur (9, 10). While radiographs are widely used and provide a fast and low cost method of imaging, they are limited in that they can identify cartilage damage indirectly only after significant cartilage loss has already occurred (7). Arthroscopy reliably detects gross surface damage and cartilage softening; however, it is insensitive to early changes (11). MRI is a useful noninvasive imaging tool, but it is costly and lacks the resolution to detect microstructural changes within articular cartilage. Histological analysis is able to evaluate early changes occurring throughout full-thickness cartilage, but is not a practical tool for clinical use as it requires removal of tissue for evaluation. In orthopaedic trauma, the treatment of open joint injuries and injuries that require exposure of the articular surface allow for direct visual inspection and evaluation of the cartilage. Gross inspection of the articular surface, however, may not detect subsurface damage.
Optical Coherence Tomography (OCT) is an emerging imaging modality that may be able to detect early cartilage damage in samples that appear normal by gross inspection even after a traumatic injury. OCT captures cross-sectional echographs of infrared light and is able to acquire near real-time microscopic resolution images (<10μm axial resolution) of articular cartilage (12). This technology was designed to permit imaging of human articular cartilage with resolutions similar to low powered histology during arthroscopy (11, 13). Abnormalities in the OCT signal in arthroscopically normal appearing human cartilage has been associated with chondrocyte metabolic incompetence (9, 14).
The ability to reliably detect early cartilage damage after a traumatic injury, prior to the onset of irreversible changes, could lead to the development and use of early treatments aimed at potentially reversing or preventing the progression of debilitating post-traumatic osteoarthritis. Furthermore, the ability to observe previously undetectable cartilage damage could also allow for a more accurate prediction of the potential for future arthritis development. We hypothesize that OCT is able to detect acute cartilage injury after impact at energy levels sufficient to produce significant chondrocyte death, but insufficient to produce visible surface damage. To test this hypothesis, cartilage was subjected to impact injury and evaluated using OCT, chondrocyte viability analysis and histology.
MATERIALS AND METHODS
Osteochondral Core Collection
Tibial plateau explants (n=8) were obtained from fresh bovine knees acquired from a local abattoir within four hours of sacrifice. Knees were opened under sterile conditions and the tibial plateaus were removed. A 6.5 mm osteochondral coring device (Smith & Nephew, Andover, MA) was used to harvest osteochondral cores from normal appearing cartilage regions on the tibial plateau. Explants were immediately transferred to 24-well plates filled with 2 ml/well of chondrocyte growth medium (DMEM/F12 with 10% FBS and 1% antibiotics). A total of 48 osteochondral cores were harvested. The cartilage thickness of each core was measured and the subchondral bone was trimmed to 1 mm.
Cartilage Impact
Cores were randomly divided into 3 experimental groups: 1) control (no impact), 2) low energy impact (0.175 J) and 3) moderate energy impact (0.35 J). A custom, computer controlled impact tower was used to impact freshly harvested and intact articular cartilage cores. Cores were impacted in a size-matched holder (6.5 mm) with a flat cylinder 6.15 mm in diameter. A weight of 356 g was used throughout the study and impact energy was controlled by adjusting the drop height. The load-deformation responses were recorded by oscilloscope to determine impact energy, stress rate, and average peak force.
Optical Coherence Tomography
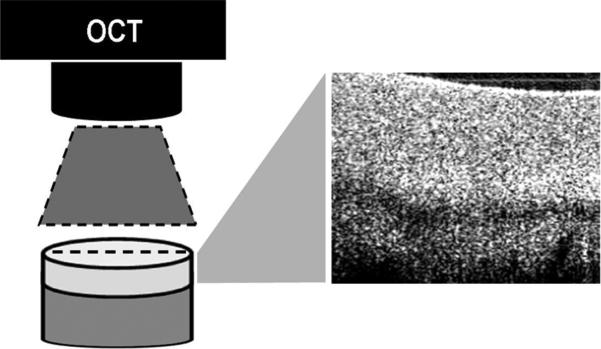
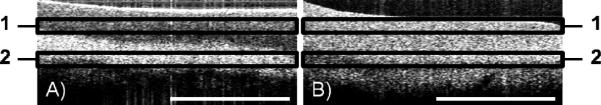
Following osteochondral core harvest, cores were scanned prior to impact using a custom OCT scanning system with a 1310 nm center wavelength (Bioptigen, Research Triangle Park, NC). A mark was made on each osteochondral core at the 12 o'clock position. The OCT scan line defined the cartilage section in the mid-sagittal plane running from the 12 o'clock mark to the 6 o'clock position and all cores were scanned in this position in order to preserve the orientation at which the OCT images were taken both before and after impact (Figure 1). Following impact, cores were incubated in 5% CO2 at 37°C in 1ml of chondrocyte growth medium (DMEM/F12 with 10% FBS and 1% antibiotics) for 12 hours. In preliminary studies (data not shown), a comparable increase in OCT signal intensity was observed both immediately after impact and 12 hours after impact. In order to ensure the OCT changes studied were not due to transient compaction of cartilage matrix by loading, cores were OCT imaged 12 hours after impaction to allow for appropriate recovery time. The mean OCT signal intensity of two distinct upper cartilage layers was obtained with a custom coded Matlab program (TheMathWorks, Natick, MA). In order to obtain the intensity measurement, edge artifacts were removed by manual segmentation and signal intensities greater than the 99th percentile and less than the 1st percentile were excluded. The superficial layer was defined as all pixels between 5 and 15 pixels (33-100 microns) below the articular surface. The deep cartilage layer was defined as all pixels between 35 and 45 pixels (232-300 microns) from the articular surface. An OCT signal intensity ratio was calculated by comparing the mean OCT signal intensity of the superficial layer to the deep layer. OCT signal intensity ratios were then compared before and after impact. Figure 2 shows an example of the OCT scan and image segmentation.
Figure 1.
OCT scanner generates a 2D cross-sectional image (6.5mm x 2mm) along the scan line depicted by the laser aiming light (dashed line). The OCT scan line defines the cartilage section in the mid-sagittal plane running from the 12 o'clock mark to the 6 o'clock position and all cores were scanned in this position in order to preserve the orientation of the acquired OCT images both before and after impact.
Figure 2.
(A) OCT image of normal articular cartilage with corresponding superficial (1) and deep (2) analysis regions. (B) A significant alteration in OCT signal was seen after impact injury. Scale bar = 2mm.
Chondrocyte Viability
Following post-impact OCT imaging, cores were bisected in the midsagittal plane in line with the 12 o'clock to 6 o'clock line of OCT scanning, and two 500μm full-thickness cartilage sections (4 per core) were obtained. Cartilage/bone slices were incubated in 5% CO2 at 37°C in 1ml of chondrocyte growth medium (DMEM/F12 with 10% FBS and 1% antibiotics) for an additional 12 hours and then imaged. Cell viability was determined with live/dead fluorescent staining using 5-chloromethylfluorescein diacetate (CMFDA) CellTracker™ Green (Invitrogen, Eugene, Oregon) for living and propidium iodide (PI, Invitrogen) for dead cells. Each osteochondral slice was incubated with 2 μM of CMFDA and 1 μM of PI for 60 min at 37°C. Following incubation, stained sections were washed with HBSS and imaged using an epi-fluorescent stereo-microscope (MVX10 MacroView System, Olympus, Japan) and a confocal microscope (IX81 DSU, Olympus, Center Valley, PA). Images were analyzed with VIS Imaging Software (Visiopharm, Denmark). The total area of dead and living chondrocytes per slice was automatically segmented with Visiopharm and the percent area of dead cells was calculated (15).
Histologic Analysis
Osteochondral cores were bisected and one-half fixed, processed and paraffin-embedded for histologic analysis using standard techniques (11, 13), (16). Histologic sections were stained with hematoxylin/eosin (HE), safranin O/fast green (Saf-O), and picrosirius red (PSR). Sections stained with HE were graded blindly and evaluated for cartilage structure, proteoglycan distribution, and cellularity using the Mankin Score (17). Osteochondral sections stained with picrosirius red were assessed for collagen fiber orientation, organization, and disruption by polarized light microscopy (PLM). PLM images were acquired with two polarizers set perpendicular to each other and with sections offset by 45 degrees to obtain optimal birefringence. PLM images were assessed by classifying the collagen architecture as either normal or disrupted.
Qualitative Cartilage Matrix Evaluation
HE and PSR slides were qualitatively assessed in accordance with the previously published David-Vaudey matrix scale (27, 28). Table 1 describes the criteria used to assess the histologic and birefringence features and the corresponding matrix grade for the David-Vaudey (DV) scale (28).
Table 1.
David-Vaudey matrix grading scale. The matrix grading scale is based on the histologic and PLM characteristics to evaluate the disruption of cartilage matrix, developed by David-Vaudey et al. (28)
| Grade | Histologic Characteristics | PML Characteristics |
|---|---|---|
| 0 | No surface irregularities | Presence of birefringence in the surface and deep zone |
| 1 | Mild surface irregularities | Minor disruptions of birefringence in the surface |
| 2 | Significant surface fibrillation | Disruption of birefringence in the superficial zone |
| 3 | Significant surface degeneration and moderate transitional zone degeneration | Complete breakdown of the superficial birefringence |
| 4 | Significant structural degeneration extending well into the radial zone | Breakdown of birefringence in the superficial as well as deep zone |
Statistical Analysis
Statistical significance was determined using one-way ANOVA and the Bonferroni t-test with p<0.05 being significant. Linear regression analysis was used to assess the correlation between OCT signal intensity ratio and chondrocyte viability and between OCT signal intensity ratio and histology grade after impact.
RESULTS
Optical Coherence Tomography Analysis
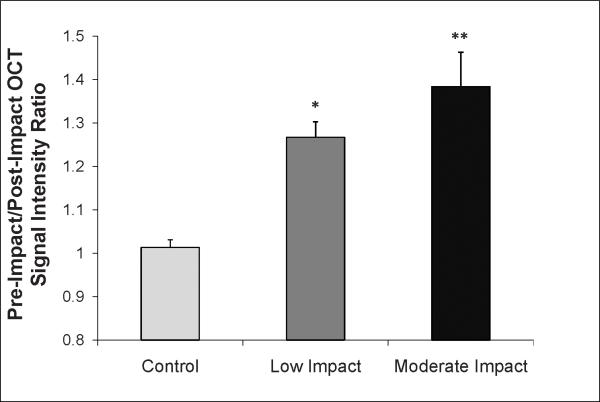
Impact injury caused a relative increase in the OCT signal intensity of the superficial layer and a relative decrease in the OCT signal intensity of the deep layer, leading to a net increase in the signal intensity ratio. ANOVA analysis demonstrated that the superficial to deep OCT signal intensity ratio increased with increasing energy of impact (p<0.001). Cores in the low impact energy group exhibited a 27% increase (p=0.006) in the signal intensity ratio following impact, while cores in the moderate energy impact group showed a 38% increase (p=0.001) following impact injury (Figure 3). No alteration in the OCT signal intensity ratio was found in unimpacted controls following incubation in the chondrocyte growth medium (p=0.81).
Figure 3.
The superficial to deep OCT signal intensity ratio demonstrated a significant 27% increase following low impact (*p=0.006) and a 38% increase following moderate impact injury (**p=0.001). Values reported are an average ± SD.
Chondrocyte Viability Analysis
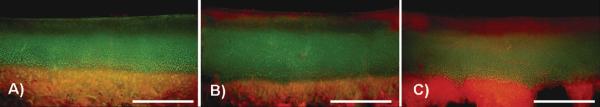
Chondrocyte viability (Figure 4) was assessed one day after impact injury and ANOVA analysis demonstrated increasing cell death with increasing levels of impact (p<0.001). The increase in cell death following impact was most pronounced in the superficial cartilage layers; however, a moderate increase in cell death was seen in deeper layers. In the low impact energy group, cell death increased by an average of 150% (p<0.001) compared to control samples. Cell death in the moderate energy impact group was 200% (p<0.001) greater than that of the control group.
Figure 4.
Representative fluorescence images of osteochondral core sections stained for live (green) and dead (red) cells. Few dead cells are present in the unimpacted control core (A), compared to increasing amounts in cores subjected to low (B), and moderate (C) energy impact. Scale bar = 2mm.
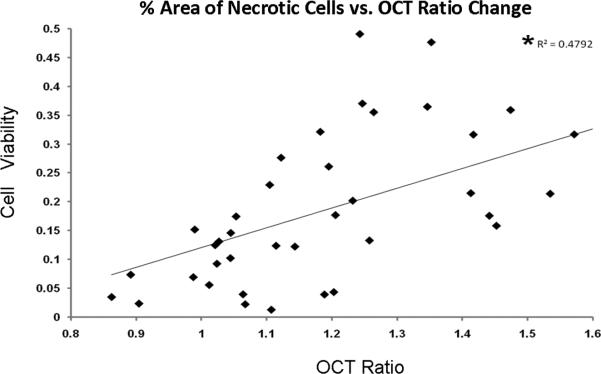
Optical Coherence Tomography and Chondrocyte Viability
A comparison between chondrocyte viability and OCT signal intensity ratio in individual cores demonstrated increased chondrocyte death in cores which had a higher OCT signal intensity ratio (Figure 5). Linear regression analysis demonstrated a significant correlation between chondrocyte viability and the OCT signal intensity ratio (R^2=0.48, p<0.001, t-stat = 5.9).
Figure 5.
Linear regression analysis demonstrated a significant correlation between the percent cell viability and the OCT signal intensity ratio (p<0.001).
Gross Examination and Histologic Analysis
In both the low and moderate energy groups, impact injury did not cause visible damage to the articular surface. Mean mankin scores in articular cartilage subjected to low impact injury (1.5±1.08) were similar to non-impacted controls (0.75±0.75, p=0.44). In the moderate impact group, the mean Mankin score (2.33±1.86) was significantly increased compared to control (p=0.036), indicating increased micro-structural matrix damage.
The published David-Vaudey Scale (28) was used to assess PLM cartilage fiber structure. No difference was found between the average David-Vaudey matrix grade of non-impacted controls (0.67±0.49) and low energy impacted cores (0.89±0.60). The overall David-Vaudey matrix grade was significantly higher for samples subjected to moderate energy impact (2.00±1.09) compared to non-impacted controls (p=0.002), consistent with micro-structural collagen matrix damage following moderate impact injury (Figure 6).
Figure 6.
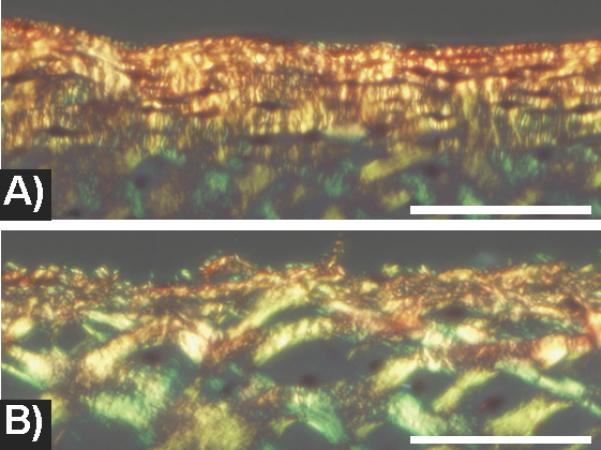
PLM microscopy images of (A) non-impacted and (B) moderate impacted articular cartilage. A) In the unimpacted specimen, polarized microscopy shows a dense layer of collagen fibrils in the superficial zone running parallel to the articular surface. The collagen fibrils in the transitional zone are regular and closely spaced. B) In the impacted specimen, the collagen fibers of the superficial zone are disrupted and attenuated. The collagen network in the transitional zone appears porous with irregular gaps between fibers. Scale bar = 125 microns.
Optical Coherence Tomography and Histology Grading
A comparison between OCT signal intensity ratio and Mankin score or the David-Vaudey matrix score in individual cores demonstrated higher OCT signal intensity ratio in cores with more severe matrix degeneration (higher Mankin score and higher David-Vaudey matrix score). Linear regression analysis demonstrated a significant correlation between the OCT signal intensity ratio and the total Mankin score (R^2=0.40, p=0.001, t-stat = 4.0) and the David-Vaudey matrix score (R^2=0.19, p=0.024, t-stat = 2.4).
DISCUSSION
This study shows that OCT is able to detect acute cartilage changes after impact injury at energy levels insufficient to cause visible damage to the articular surface. Our results demonstrate that the OCT signal intensity ratio is sensitive to the degree of impact injury in that the OCT changes correlated with increasing cartilage damage and injury as determined by laboratory assessments of chondrocyte death and microstructural changes. These findings support the potential utility of OCT to detect early cartilage damage.
Consistent with the OCT detected changes to the articular cartilage following impact, histological analysis of the samples showed an increase in Mankin score in cores subjected to moderate energy impact. An increase in matrix disruption in cores subjected to impact was also detected via polarized light microscopy and the David-Vaudey matrix grading scale. Consequently, there was a significant increase in both the Mankin score and David-Vaudey score in cores subjected to moderate energy impact as compared to non-impacted controls. These changes were observed in areas that exhibited a decrease in viability and in areas with changes in the OCT signal. Although these changes were not evident to gross inspection, Milentijevic et al. has shown that even shallow cracks in the articular surface can eventually progress to fully pronounced post-traumatic osteoarthritis, under in vivo conditions (18).
Although low energy impact injury did not cause a significant macroscopic change in the cartilage surface, chondrocyte death demonstrated a significant increase over unimpacted controls after both low and moderate energy impact. This finding is consistent with ex vivo studies which have shown detrimental effects to articular cartilage after a single impact (19-21). A recent study by Szczodry et al. demonstrated a significant increase in chondrocyte death at similar levels of impact without gross damage to the articular surface (15).
The ability of OCT to identify cartilage signal changes in the absence of visible surface damage is consistent with previous studies evaluating human articular cartilage (9), (22). During arthroscopic examination, Chu et al. found that alterations in the OCT signal in normal appearing cartilage was similar to changes in OCT birefringence associated with chondrocyte metabolic incompetence (9). In another study using human knee explants, OCT signal changes in normal appearing cartilage correlated with signs of degeneration and collagen matrix disorganization (22).
The polarized light microscopy data in this study further suggest that the OCT detectable changes may be related to alterations of the collagen architecture. Preliminary studies demonstrated a similar increase in OCT signal intensity both immediately after impact and 12 hours after impact, further suggesting that matrix structural alteration played a role in these changes. The differing amount of OCT signal changes observed between the superficial and deep layers of cartilage after impact may be due to differential loading patterns and is consistent with previous findings (23).
The positive correlation between the OCT signal intensity ratio, histology, and chondrocyte death has significant implications for the clinical utility of OCT in the evaluation of cartilage damage after acute injury. Based on our findings, the degree of chondrocyte death following traumatic injury could be predicted by the OCT signal intensity ratio, with higher ratios indicating greater injury. The signal intensity ratio could thus serve as a biomarker for acute cartilage injury. It is important to note that OCT does not directly detect necrotic and/or compromised cells. Rather, the increased chondrocyte death observed in impacted specimens compared to unimpacted controls in this study of fresh, healthy cartilage explants provided additional evidence of impact injury.
A potential limitation of this study was that bovine cartilage was used for analysis, instead of human articular cartilage. Although human tissue would have been preferred in order to best evaluate the clinical utility of OCT, the chondrocyte viability analysis performed in this study required the use of fresh cartilage. The amount of cartilage used in this study would be extremely difficult to obtain from normal human tissue. As such, fresh bovine cartilage, which has been previously used for impact injury experimentation (15), was used.
Moreover, there are potential differences between cartilage damage seen in chondral cores and whole joint preparations. Previous studies on bovine osteochondral explants indicated that cartilage failure occurred at 25 MPa (25). Articular cartilage in situ, however, is a remarkably resilient tissue and is capable of tolerating impact stresses of at least 55 MPa without significant damage (26). Despite its resilience, chondrocyte necrosis following impact injury to cartilage has been observed in whole joint preparation without fracture of the articular surface (18). This highlights the clinical need for methods to reliably detect early cartilage damage when the articular surface still appears grossly normal.
Our findings support the utility of OCT as a nondestructive imaging modality to detect cartilage damage that may be poorly visualized with conventional imaging. We have previously shown that OCT can be used during arthroscopic surgery to show early cartilage degeneration (9, 14). This study demonstrates that OCT is able to detect cartilage damage after impact injury that is sufficient to cause significant chondrocyte death in the absence of visible surface breakdown. As current imaging modalities cannot reliably detect cartilage damage following impact injury insufficient to fracture the surface, when potentially reversible changes are occurring, the utilization of an improved method of detection is of critical importance. The addition of OCT in the clinical setting to evaluate cartilage after a traumatic injury could assist in identification of subsurface cartilage injuries and allow for the implementation of treatments prior to breakdown of the articular surface. By identifying cartilage damage previously unrecognizable subsurface cartilage damage, OCT could assist in the development of new chondroprotective treatments to prevent or delay the onset of osteoarthritis.
Acknowledgments
Funding provided by the National Institutes of Health (RO1 AR052784-CRC and P60 AR054731-CRC) and the Orthopaedic Research and Education Foundation (PAG00 19989-DMB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Brown TD, Johnston RC, Saltzman CL, et al. Posttraumatic osteoarthritis: a first estimate of incidence, prevalence, and burden of disease. Journal of orthopaedic trauma. 2006;20:739–744. doi: 10.1097/01.bot.0000246468.80635.ef. [DOI] [PubMed] [Google Scholar]
- 2.Gelber AC, Hochberg MC, Mead LA, et al. Joint injury in young adults and risk for subsequent knee and hip osteoarthritis. Annals of internal medicine. 2000;133:321–328. doi: 10.7326/0003-4819-133-5-200009050-00007. [DOI] [PubMed] [Google Scholar]
- 3.Borrelli J, Jr., Tinsley K, Ricci WM, et al. Induction of chondrocyte apoptosis following impact load. Journal of orthopaedic trauma. 2003;17:635–641. doi: 10.1097/00005131-200310000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Jeffrey JE, Gregory DW, Aspden RM. Matrix damage and chondrocyte viability following a single impact load on articular cartilage. Archives of biochemistry and biophysics. 1995;322:87–96. doi: 10.1006/abbi.1995.1439. [DOI] [PubMed] [Google Scholar]
- 5.Natoli RM, Scott CC, Athanasiou KA. Temporal effects of impact on articular cartilage cell death, gene expression, matrix biochemistry, and biomechanics. Annals of biomedical engineering. 2008;36:780–792. doi: 10.1007/s10439-008-9472-5. [DOI] [PubMed] [Google Scholar]
- 6.Furman BD, Olson SA, Guilak F. The development of posttraumatic arthritis after articular fracture. Journal of orthopaedic trauma. 2006;20:719–725. doi: 10.1097/01.bot.0000211160.05864.14. [DOI] [PubMed] [Google Scholar]
- 7.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Annals of the rheumatic diseases. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Recht MP, Resnick D. MR imaging of articular cartilage: current status and future directions. Ajr. 1994;163:283–290. doi: 10.2214/ajr.163.2.8037016. [DOI] [PubMed] [Google Scholar]
- 9.Chu CR, Izzo NJ, Irrgang JJ, et al. Clinical diagnosis of potentially treatable early articular cartilage degeneration using optical coherence tomography. Journal of biomedical optics. 2007;12:051703. doi: 10.1117/1.2789674. [DOI] [PubMed] [Google Scholar]
- 10.Chu CR, Coyle CH, Chu CT, et al. In Vivo Effects of Single Intra-Articular Injection of 0.5% Bupivacaine on Articular Cartilage. The Journal of bone and joint surgery. 2010;92(3):599–608. doi: 10.2106/JBJS.I.00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chu CR, Lin D, Geisler JL, et al. Arthroscopic microscopy of articular cartilage using optical coherence tomography. The American journal of sports medicine. 2004;32:699–709. doi: 10.1177/0363546503261736. [DOI] [PubMed] [Google Scholar]
- 12.Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science (New York, NY. 1991;254:1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan Y, Li Z, Xie T, et al. Hand-held arthroscopic optical coherence tomography for in vivo high-resolution imaging of articular cartilage. Journal of biomedical optics. 2003;8:648–654. doi: 10.1117/1.1609201. [DOI] [PubMed] [Google Scholar]
- 14.Chu CR, Williams A, Tolliver D, et al. Clinical Optical Coherence Tomography of Early Cartilage Degeneration in Persons with Degenerative Meniscal Tears. Arthritis and rheumatism. 2010 doi: 10.1002/art.27378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szczodry M, Coyle CH, Kramer SJ, et al. Progressive chondrocyte death after impact injury indicates a need for chondroprotective therapy. The American journal of sports medicine. 2009;37:2318–2322. doi: 10.1177/0363546509348840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rohde RS, Studer RK, Chu CR. Mini-pig fresh osteochondral allografts deteriorate after 1 week of cold storage. Clinical orthopaedics and related research. 2004:226–233. doi: 10.1097/01.blo.0000138955.27186.8e. [DOI] [PubMed] [Google Scholar]
- 17.Mankin HJ, Dorfman H, Lippiello L, et al. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. The Journal of bone and joint surgery. 1971;53:523–537. [PubMed] [Google Scholar]
- 18.Milentijevic D, Rubel IF, Liew AS, et al. An in vivo rabbit model for cartilage trauma: a preliminary study of the influence of impact stress magnitude on chondrocyte death and matrix damage. Journal of orthopaedic trauma. 2005;19:466–473. doi: 10.1097/01.bot.0000162768.83772.18. [DOI] [PubMed] [Google Scholar]
- 19.Green DM, Noble PC, Ahuero JS, et al. Cellular events leading to chondrocyte death after cartilage impact injury. Arthritis and rheumatism. 2006;54:1509–1517. doi: 10.1002/art.21812. [DOI] [PubMed] [Google Scholar]
- 20.Huser CA, Davies ME. Calcium signaling leads to mitochondrial depolarization in impact-induced chondrocyte death in equine articular cartilage explants. Arthritis and rheumatism. 2007;56:2322–2334. doi: 10.1002/art.22717. [DOI] [PubMed] [Google Scholar]
- 21.Jeffrey JE, Aspden RM. Cyclooxygenase inhibition lowers prostaglandin E2 release from articular cartilage and reduces apoptosis but not proteoglycan degradation following an impact load in vitro. Arthritis research & therapy. 2007;9:R129. doi: 10.1186/ar2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bear DM, Williams A, Chu CT, et al. Optical Coherence Tomography (OCT) Grading Correlates with MRI T2 Mapping and Extracellular Matrix Content. J Orthop Res. 2009;28(4):546–52. doi: 10.1002/jor.20998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Milentijevic D, Torzilli PA. Influence of stress rate on water loss, matrix deformation and chondrocyte viability in impacted articular cartilage. Journal of biomechanics. 2005;38:493–502. doi: 10.1016/j.jbiomech.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 24.Borrelli J, Jr., Silva MJ, Zaegel MA, et al. Single high-energy impact load causes posttraumatic OA in young rabbits via a decrease in cellular metabolism. J Orthop Res. 2008;27:347–352. doi: 10.1002/jor.20760. [DOI] [PubMed] [Google Scholar]
- 25.Borrelli J, Jr., Torzilli PA, Grigiene R, et al. Effect of impact load on articular cartilage: development of an intra-articular fracture model. Journal of orthopaedic trauma. 1997;11:319–326. doi: 10.1097/00005131-199707000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Borrelli J, Jr., Zhu Y, Burns M, et al. Cartilage tolerates single impact loads of as much as half the joint fracture threshold. Clinical orthopaedics and related research. 2004:266–273. doi: 10.1097/01.blo.0000136653.48752.7c. [DOI] [PubMed] [Google Scholar]
- 27.Williams A, Qian Y, Bear D, et al. Assessing degeneration of human articular cartilage with ultra-short echo time T2* mapping. Osteoarthritis and Cartilage. 2010;18:539–546. doi: 10.1016/j.joca.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.David-Vaudey E, Ghosh S, Ries M, et al. T2 relaxation time measurements in osteoarthritis. Magnetic Resonance Imaging. 2004;22:673–682. doi: 10.1016/j.mri.2004.01.071. [DOI] [PubMed] [Google Scholar]