Abstract
Here we report the use of a multi-genome DNA microarray to elucidate the genomic events associated with the emergence of the clonal variants of H. influenzae biogroup aegyptius causing Brazilian Purpuric Fever (BPF), an important pediatric disease with a high mortality rate. We performed directed genome sequencing of strain HK1212 unique loci to construct a species DNA microarray. Comparative genome hybridization using this microarray enabled us to determine and compare gene complements, and infer reliable phylogenomic relationships among members of the species. The higher genomic variability observed in the genomes of BPF-related strains (clones) and their close relatives may be characterized by significant gene flux related to a subset of functional role categories. We found that the acquisition of a large number of virulence determinants featuring numerous cell membrane proteins coupled to the loss of genes involved in transport, central biosynthetic pathways and in particular, energy production pathways to be characteristics of the BPF genomic variants.
Keywords: Haemophilus, Brazilian Purpuric Fever, pathogen emergence, virulence, comparative genomics, microarray
INTRODUCTION
Haemophilus influenzae (Hi) is a species of gram-negative, rod-shaped bacteria that have primarily evolved to live symbiotically in the upper respiratory tract of humans. The loss of genes for the synthesis of key metabolic compounds (heme and nicotineamide adenine dinucleotide, NAD) renders H. influenzae unable to survive in environments outside its human host. This species is comprised of distinct evolutionary lineages that express one of six different capsular serotypes, a through f, along with a highly diverse group of non-encapsulated bacteria displaying a non-clonal relationship [1,2].
Despite the commensal life-style of most H. influenzae strains, they remain one of the five most frequent causes of death among pathogenic microorganisms [3]. Prior to the introduction of wide-spread vaccination in many countries, H. influenzae strains expressing the serotype b capsule ranked among the three leading causes of bacterial meningitis worldwide. This remains true in many developing countries. It is estimated that at least 3 million cases of serious disease and an estimated 400,000–700,000 deaths occur in young children worldwide per year due to H. influenzae infection [3]. Invasive infections are primarily associated with strains representing one (Division I) of two distinct serotype b lineages, that display a characteristic partial duplication of the capsular biosynthesis (cap) locus [4]. Division II H. influenzae serotype b strains and those expressing any of the other five capsular serotypes are infrequent causes of infection. The non-encapsulated (“non-typeable”) H. influenzae are regular inhabitants of the human pharynx, particularly during childhood, and are frequent causes of mucosal infections at sites contiguous with the upper respiratory tract, e.g. otitis media, sinusitis, lower respiratory tract infections, and conjunctivitis [1].
Occasionally, non-encapsulated H. influenzae strains cause invasive infections in the absence of apparent predisposing conditions in the patient. A striking example is the disease described as Brazilian purpuric fever (BPF), a fulminant life-threatening pediatric infection caused by particular clones of H. influenzae belonging to the biogroup aegyptius. BPF was first recognized in 1984, when 10 children in a Brazilian town of 20,000 persons died of an acute febrile illness associated with hemorrhagic skin lesions, septicemia, hypotensive shock, vascular collapse, and death. Alarmingly, the time of death from the time of symptom onset was within 48 hours. The disease is characteristically preceded by purulent conjunctivitis that is resolved prior to the onset of fever. Additional outbreaks occurred subsequently in Brazil and cases with clinical symptomology indistinguishable from those of BPF have been described in Australia and the United States. These infections were caused by strains distinct from the Brazilian isolates and therefore considered to be the result of independent evolutionary events [5–10]. Common to all BPF isolates from such infections is the biotype III classification to, one of eight described H. influenzae biotypes [1]. In contrast to typical strains of H. influenzae, BPF isolates lack the ability to ferment xylose. It remains largely unknown how these bacteria evade the innate immune system and why they cause clinical attributes more commonly associated with meningococcal disease. Phylogenetic analysis indicated that the Brazilian BPF clones were most closely related to the group formally known as H. aegyptius [6,7], a recognized cause of an acute purulent and contagious form of conjunctivitis occurring as seasonal endemics, particularly in hot climates, including southern portions of the US. According to traditional taxonomic criteria, bacteria referred to as H. influenzae, H. aegyptius, and H. influenzae biogroup aegyptius are all members of a single species; however, there are formal obstacles to their unification into a single species. H. aegyptius isolates do not cause invasive disease, however they do share extensive genetic traits in common with the BPF clones.
The presence of a 24 MDa cryptic plasmid and the H. influenzae insertion sequence IS1016 have been previously considered unique features of the BPF isolates [11,12]. However, this plasmid does not encode recognizable virulence factors, drawing the significance of this observation into question [13]. H. influenzae strain KW20/Rd, a capsule-deficient derivative of an essentially non-pathogenic serotype d strain, was the first living organism to have its complete genome sequence determined [14]. More recently, three complete and 11 additional draft genome sequences of non-encapsulated H. influenzae strains were reported [15–17]. Comparative genomics of H. influenzae genomes reveal various lineage-specific genes, including virulence factors. However, the underlying evolutionary processes leading to emergence and the genetic basis of invasive potential remain unclear [8,9,12,14–19].
In this report we demonstrate the utility of a multi-genome microarray for evaluating the gene content and extent of genomic diversity of 21 Haemophilus influenzae group members, including 14 H. influenzae strains, four H. aegyptius, two H. influenzae biogroup aegyptius and one H. haemolyticus. These strains represent a wide phenotypic diversity and geographic coverage of the species. Among the query group analyzed were three isolates obtained from patients associated with Brazilian Purpuric Fever (BPF), a fulminant fatal pediatric disease. The multi-genome-array, unlike a single-genome DNA microarray, reveals both gene loss and gene complement patterns with respect to any query genome and enabled us to identify evolutionary events associated with the emergence of BPF clones from H. influenzae variants associated with conjunctivitis.
MATERIALS and METHODS
Bacterial Strains and DNA Techniques
The Haemophilus strains used in this study (Table 1) were grown and propagated as previously described [1]. All strains have been characterized extensively as part of a population genetic analysis [7] and were further analyzed by a multi-locus sequence typing (MLST) scheme developed for this species [2]. The majority of these strains were selected to represent major lineages with this taxon on the basis of a previously reported population genetic study that used both Multi Locus Enzyme Electrophoresis (MLEE) and MLST [7] (Figure 1). These strains represent a broad phylogenetic, phenotypic, and geographic coverage of the species (Table 1). The bacterial strains here referred to as H. influenzae, H. influenzae biogroup aegyptius (Hibae), and H. aegyptius (Hae) all belong to one species according to traditional taxonomic criteria. The formal obstacle to merging all into one species H. influenzae is that the name H. aegyptius has priority. Until this problem is formally resolved we use the separate names to indicate that, although genetically closely related, the three taxa represent distinct populations of bacteria with distinct pathogenic potential. Genomic DNAs (gDNA) were isolated as described [7]. Metabolic profiling of the strains was performed as described by Kilian [1] and Brenner et al. [9].
Table 1.
The strains used in the study.
| Strain Name |
Designation | Serotype | Country of Origin |
Biotype | Division | Virulence | Aberrant Traits | Alterative Designations |
References |
|---|---|---|---|---|---|---|---|---|---|
| HK1368 | H. influenzae | b | Denmark | I | I | meningitis | |||
| HK715 | H. influenzae | b | USA | I | II (ET26) | less virulent | 1066/1971 | [7] | |
| HK635 | H. influenzae | c | Papua N. Guinea | III | lung aspirate | 43/LA/Tarr | |||
| HK2122 | H. influenzae | Germany | III | septicemia | xylose negative | [69] | |||
| KW20/Rd | H. influenzae | Rough derivative of serotype d |
IV | ATCC51907 | [14] | ||||
| HK2067 | H. influenzae | f | USA | septicemia | GA1463 | [70] | |||
| HK1136 | H. influenzae | USA | II | ATCC9134 | |||||
| HK61 | H. influenzae | NTa | Denmark | I | Pharynx | Phylogenetically distant (MLEE, MLST, iga) |
[71] | ||
| HK1220 | H. influenzae | NT | Brazil | III | oropharynx, healthy subject |
60/86 | [7] | ||
| HK1210 | H. influenzae | NT | Denmark | II | meningitis | ||||
| HK1141 | H. influenzae | NT | UK | III | ATCC19418 (NCTC4560) |
||||
| HK295 | H. influenzae | NT | Egypt | III | conjunctivitis | NCTC8143T | [71] | ||
| HK389 | H. influenzae | NT | II | [71] | |||||
| HK1212 | H. influenzae | NT | Australia | III | BPF-like infection | xylose negative | 199/88 | [7,10] | |
| HK367 | H. aegyptius | NT | USA | III | conjunctivitis | NTCC8502 | |||
| HK1246 | H. aegyptius | NT | USA | III | conjunctivitis | Pittman 46 | |||
| HK1214 | H. aegyptius | NT | Brazil, Valparaiso |
III | meningitis, CSFb | plasmid negative | 677/90 | [9] | |
| HK1240 | H. aegyptius | NT | Brazil, Mato Grosso |
III | conjunctivitis | plasmid restriction 1947 |
105/91 | [9] | |
| HK1219 |
H. influenzae biogr. aegyptius |
NT | Brazil | III | Conjunctivitis, patient underwent intensive antibiotic treatment to prevent BPF |
Plasmid negative, closely related to HK870 by MLST and MLEE |
690/90 | [9,42] | |
| HK870 |
H. influenzae biogr. aegyptius |
NT | Brazil | III | septicemia | BPF case clone reference |
F3031 | [9] | |
| HK2111 | H. haemolyticus | NT | USA | COPDc | Hemolytic | 3P5H | [72] |
NT, non-typable.
CSF, cerebral spinal fluid.
COPD, chronic obstructive pulmonary disease.
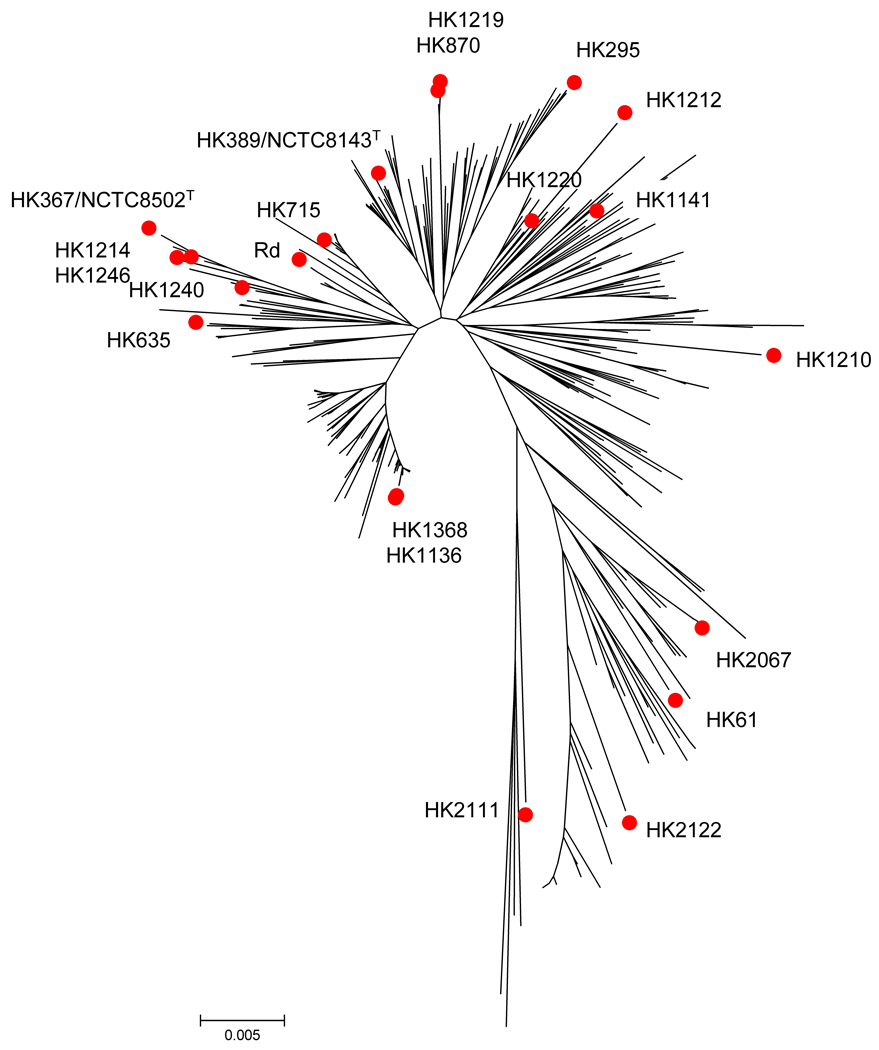
Figure 1.
MLST-based phylogenetic tree using H. influenzae sequences available in the MLST database (www.mlst.net). Red dots denote strains used in this study. Relationships are drawn using the minimum evolution algorithm applied to concatenated sequences of the seven MLST housekeeping genes and a fragment of hap [7]. The MLST-based calculated mean genetic distance within our collection is 0.026 ± 0.002, which is in agreement with the one calculated from all recognized sequence types (STs), i.e., 0.025 ± 0.002.
Gene Discovery: HK1212 Genomic Library Preparation and Screening
The gene discovery approach is summarized in Supplementary Figure 1s. Briefly, a partial Tsp509I digestion was performed with 3 µg HK1212 to generate a mass peak centered at ∼500 base pair fragments. Digested DNA was electrophoresed on 1% ultra pure agarose gel (Invitrogen, Carlsbad, CA), and fragments of an apparent MW ∼300–800 bp. were excised and recovered using the QIAquick gel extraction kit as per manufacturer’s instruction (QIAgen, Valencia, CA). These fragments were cloned into the EcoRI site of pUC19 [20] and transformed into ElectroMAX DH10B cells (Invitrogen, Calsbad, CA) by electroporation. High-throughput plasmid purification was performed at the J. Craig Venter Science Foundation, Joint Technology Center (Rockville, MD). To estimate the number of plasmids to screen, we assumed an average genomic insert size to be 500 bp and wished to set a 99% confidence limit of achieving 5X coverage of the HK1212 genome (1.8 Mb). Using the following equation: N = ln(1 - P)/ ln (1 - f), where: P = probability of cloning a given sequence, f = (average insert size)/(genome size), ln = natural log [21], indicated that the screening of 18000 recombinant plasmids were required. Plasmids were printed and screened using HK1212 and KW20/Rd genomic probes labeled with either Cy3 or Cy5. Replicate hybridizations were conducted with flip-dye replicates. The signal intensity ratios were used to indicate those plasmids that contained inserts that were significantly divergent or uniquely present in the HK1212 genome relative to KW20/Rd. Genomic DNA hybridizations were performed essentially as described at http://pfgrc.jcvi.org/index.php/microarray/protocols.html [22]. The slides were dried and then scanned using the GenePiX 4000B scanner (Axon Instruments, Union City, CA).
HK1212 Genomic DNA Sequence Analysis
All sequence reads from selected recombinant plasmids (see previous section) were assembled using the TIGR assembler [23]. The resulting contigs and singletons were concatenated into a pseudomolecule [17,24] and subjected to the JCVI’s automatic annotation pipeline, followed by manual curation. Glimmer [25] was used to identify open reading frames (ORFs). Proteins were also classified into putative functional role categories within the cell according to modified Riley’s schema [26]. HK1212 genomic sequences obtained were initially filtered for uniqueness using the blastn algorithm [27] against KW20/Rd. Sequences with nucleotide matches < 100 nucleotides and P-values > 10−5 were considered to be potentially unique. The sequences were further subjected to blastp [27] comparison against the non-redundant amino acid (NRAA) database. Sequences with a translated P-value match of < 10−5 were considered orthologous to previously characterized proteins while the remaining template sequences, i.e. those with no match or P-values > 10−5, were considered unique ORFs. In addition, we conducted gene cluster analysis to identify sequence variants that may represent paralogous (multi-gene) families using CAP3 [28]; sequences with < 94 % identity in the overlap region of > 30 bp were considered paralogous genes. Sequence alignments were also investigated through Clustal X [29].
DNA Microarray Design and Fabrication
ArrayOligoSelector (http://arrayoligosel.sourceforge.net [30]) was used to design 4,578 seventy base oligonucleotides (70-mer genomic markers) for the H. influenzae multigenome microarray. These oligonucleotides represent 4,578 putative ORFs identified from complete and partial genomic sequences of H. influenzae KW20/Rd [14], 86-028NP [15], R2846 (NCBI accession AADO00000000), R2866 (NCBI accession AADP00000000), HK1212, Haemophilus influenzae biogroup aegyptius genetic island [31], and plasmids pF3031 and pF3028 [13]. Table 1s summarizes the 70-mers according to the number of features they represent from each sequence source. An equal volume of the oligonucleotides 50 µM (Illumina, San Diego, CA) were combined with 100% DMSO or Corning Buffer (Corning, NY). Oligonucleotides were printed onto Ultra-gap glass slides (Corning, NY) using a Lucidea microarray printer (Amersham, Piscataway, NJ). Printed slides were UV-crosslinked using a Spectrolinker XL-1500UV cross-linker (Spectronics Corporation, Westbury, NY) at 25,000 µJ/cm2. Gene Discovery slides were generated by printing plasmid template DNA at a concentration of ∼200ng/µl.
CGH Data Analysis
Microarray data were analyzed as previously described [22]. Briefly, raw microarray images were analyzed using SpotFinder version 2.2.2 of TM4, Microarray Software Suite [32]. Spot florescence intensities corresponding to Cy3 and Cy5 were normalized using a log mode centering; spots less than 20000 relative fluorescence units were filtered out from further analysis. The Histogram Mode Centering (HMC) algorithm [33] was used initially to normalize the signal data and fit the log-ratio frequencies from the main peak to a Gaussian distribution function. We utilized an analytical approach by estimating the best Gaussian fit through minimizing the sum of squared residuals between raw histogram data and a theoretical Gaussian curve. The standard deviation value that resulted from the model was used to calculate the percentiles corresponding to each log-ratio based on the z-score table. These calculated percentile values from the z-score table were used later to infer gene presence or absence in both reference and query genomes. We used conservative confidence limits to assign each gene represented in the array as present or absent in the reference and/or query genomes. These criteria have been visually summarized in Supplementary Figure 2s. Briefly, a gene was considered present with no significant sequence divergence, when the signal log-ratio of the corresponding 70-mer fell in the range of 5th - 95th percentiles of the Gaussian fit model used for the normalization (see above). This group of genes was designated as shared (SH). Because we always calculated signal ratios as reference/query, a coding DNA sequence was considered uniquely present in the query (absent in the reference), if its 70-mer log-ratio percentile was ≤ 2.5. Alternatively, when the log-ratio percentile for a 70-mer was ≥ 97.5, the gene represented by that 70-mer was considered uniquely present in the reference (absent in the query). Areas within percentile values 2.5–5 or 95–97.5 were considered as borderline statistical confidence limits. Based on the hybridization signals, these two groups of features are considered to have some degree of sequence variability tending towards either the query or reference genome. Therefore, ORFs whose calculated 70-mer log ratio percentiles fell in ranges of 2.5–5 or 95–97.5 were designated as divergent. Despite some noticeable sequence variability trends, ORFs classified into these two groups were considered present in both the reference and query genomes.
CGH Data Clustering
In order to minimize bias and noise due to the individual log-ratio values when investigating the global gene presence/absence patterns among the query strains, the final data set were assigned one of three different values: 0 = absent, 0.5 = divergent and 1 = present. A similar approach has been previously reported [34–36]. Hierarchical clustering of the query genomes based on the CGH data was conducted on both the Multiple Experiment Viewer (MeV) of TM4 Software and MrBayes as previously described [32,36–39]. Due to the type of the data, we applied non-parametric statistical analysis using Pearson Correlation algorithm from the MeV. MrBayes software, which has been developed for the Bayesian estimation of phylogeny [38], was used to further refine phylogenetic inferences.
Allelic Grouping and Gene Designations
Our 70-mer design allowed us to distinguish subtle sequence differences among many sequence variants of orthologous and paralogous genes. In order to avoid confounding the final gene calls (presence, absence, divergence), or the total gene complement estimates, we identified and clustered related orthologous sequence variants into “allelic groups.” Each allelic group, to the best of our ability, includes putative sequence variants of a group of orthologous coding DNA sequences (CDSs). Clustering of the orthologous sequences into allelic groups was based primarily on 70-mer-to-gene and protein-to-protein relationships. Allelic grouping was further refined by taking into consideration predicted features’ functional roles as well as their corresponding Clusters of Orthologous Groups (COGs) assignments. In order to avoid under-estimating the total gene counts, allelic groups having more than one or more paralogous gene member were sub-divided so that each paralogous variant had its own sub-group when unambiguous data permitted.
Statistical Approach for Marker Association Analysis
We used Fisher’s exact test for conducting marker association analysis (MAA) between the genomic attributes i.e. gene presence or absence, and groups of strains that possessed particular phenotypes or clades [40,41]. Markers, and consequently their corresponding ORFs, were considered “characteristic for,” “associated with,” or “prevalent in” a particular group inferred by MAA using p<0.05 as a cut-off value for statistical significance.
RESULTS
We wished to establish the genetic basis for the emergence of H. influenzae causing BPF (Table 1). We initially screened 20 strains by CGH using a single-genome-based DNA microarray and conducted phylogenomic cluster analysis (Supplementary Figure 3s). Based on that cluster analysis, strain HK1212 appeared the closest to the node of a divergent clade comprising all three BPF-associated isolates, as well as all Hae strains. This finding suggested that HK1212 represented one of the deepest branching of the BPF clade. All three BPF isolates i.e. HK1212, HK870 and HK1219 displayed very similar gene loss patterns with respect to KW20/Rd. Strains HK870 and HK1219 had been isolated during the BPF outbreak in Brazil. Strain HK1212 was isolated in central Australia in 1986 from a child with characteristic symptomology of Brazilian Purpuric Fever (BPF, [5,10]). We hypothesized that the HK1212 genome contains additional or unique genes that contribute to its virulence, including its ability to evade innate immune factors, despite lacking a capsule.
Initially we screened a genomic library derived from HK1212 to identify novel sequences encoded in its genome. The sequencing of unique DNA segments resulted in the identification of a large number of HK1212 ORFs that were then combined with existing DNA sequence available in public databases allowing the design of a H. influenzae “species” DNA microarray. We utilized this array to screen a diverse set of Hi strains for their genomic content. The data generated from this comparative genomic hybridization approach allowed us to conduct a phylogenomic analysis for understanding the evolutionary premises and steps that are associated with the emergence of BPF clones.
Identification of Unique Genomic Regions in Strain HK1212, a BPF-like isolate
We developed a directed sequencing strategy we refer to as Gene Discovery (GD) that, like subtractive hybridization, enables the identification and rapid characterization of strain-specific sequences present in microbial genomes. The method (Supplementary Figure 1s) exploits the use of DNA microarrays to discriminate between HK1212 unique and divergent genomic fragments to those shared by HK1212 and KW20/Rd genomes.
A total of 18,000 recombinant plasmids derived from an HK1212 genomic library were printed onto glass slide DNA microarrays. The genomic library was intentionally biased toward small DNA inserts to allow the identification of unique fragments whose length did not exceed the average length of eubacterial ORFs. DNA microarray hybridizations were conducted in replicate using flip-dye labeled genomic DNA probes (HK1212 and KW20/Rd). Hybridization signals displaying a HK1212/KW20/Rd log2-ratio of 2.0 or greater were identified and the 3,309 corresponding plasmid DNAs were subjected to DNA Sanger sequencing via two end reads using vector-based primers.
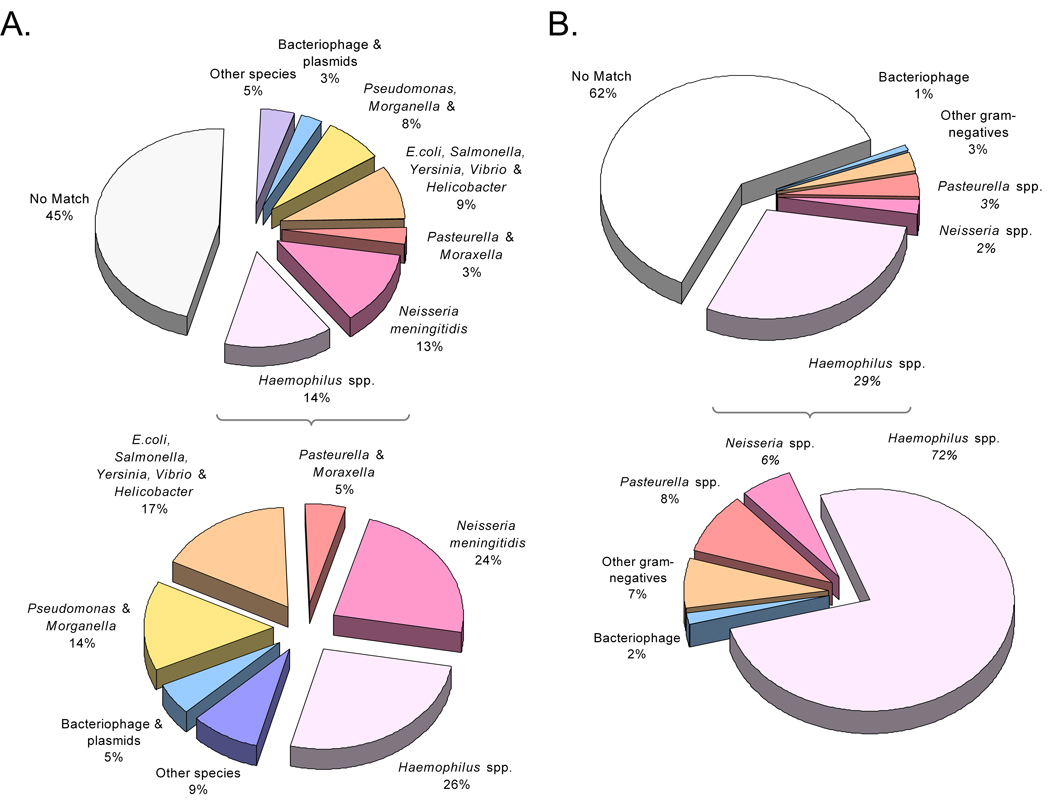
The sequence reads were initially compared to the KW20/Rd genome to identify unique genomic sequences (i.e. present in HK1212 but absent in KW20/Rd). Out of 6,618 reads that were obtained from paired-end sequencing of selected plasmids, 3,334 were considered unique. The remaining 3,284 reads represented orthologous sequences present in both genomes with varying degrees of nucleotide divergence. Figure 2A summarizes the results of the blastx searches of the non-redundant amino acid (NRAA) database.
Figure 2.
Putative sources of the novel genomic content of HK1212 based on blast analysis. A. Summary of HK1212 sequence database searches using blastx. Species-associated sequences with the best blastx scores are shown with (top) and without (bottom) those with no match (hypotheticals). B. Same as A, except the summary represents HK1212 features (partial ORF) analysis using blastp. Significant blast scores to sequences from non-typable H. influenzae and H. aegyptius strains constitute the majority of “Haemophilus spp.” fraction.
All sequence reads were then assembled using TIGR assembler [23] resulting in 1,302 contigs and 70 singletons. Contig sizes ranged from 138 – 3,740 bp in length, with an average coverage of 3.2x. Annotation of the contigs produced 1,459 putative ORFs or partial ORFs (pORFs), which are also referred to as “features.” Supplementary Table 2s summarizes the characteristics of all novel HK1212 features discovered. Among the 1,459 features, 439 were novel in HK1212 genome with respect to the H. influenzae KW20/Rd genome while 217 were unique i.e. no match to any hitherto sequenced genomes. The vast majority (90 %) of the remaining novel features found in HK1212 reflected best hits to sequences from Haemophilus spp. and other members of the Pasteurellaceae family (Figure 2B).
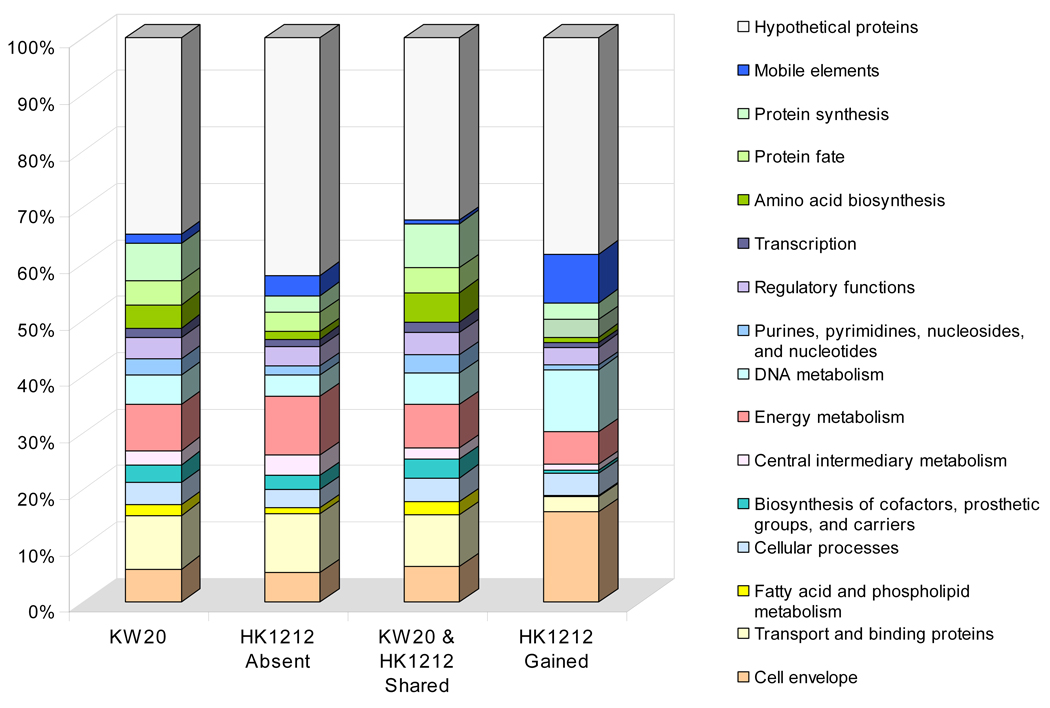
Analysis of the predicted functional role categories of those sequences unique to strain HK1212 genome relative to KW20/Rd is shown in Figure 3. This comparison revealed significantly differential profiles between missing and unique gene functions encoded in the HK1212 relative to KW20/Rd. The group of genes encoding proteins of unknown functions (hypothetical proteins) constituted the largest gene set among HK1212 unique ORFs (38%); six out of 253 hypothetical proteins had matches in the EMBL’s phage sequence database. Among the remaining groups of features with predicted functions we noticed that missing gene functions in HK1212 relative to KW20/Rd and their corresponding cellular role categories were not equally compensated by gained gene functions. While ORFs predicted to be involved in cellular transport, and energy metabolism constituted the majority of missing functions, those encoding mobile elements, cell envelope proteins, DNA metabolism, protein synthesis, protein fate, and amino acid biosynthesis made up the majority of HK1212 novel genomic content. We also searched the KEGG database to identify metabolic pathways that may be incomplete in HK1212 i.e. affected by gene absence in its genome. We focused our analysis on pathways containing ≥3 genes, and conservatively considered a pathway to be incomplete if ≥2 genes were absent. Results of this survey indicated that over nine pathways may be incomplete in HK1212 relative to HK20/Rd affecting the metabolism of: sucrose, glyoxylate and dicarboxylate, pentose and glucuronate interconversions, ascorbate and aldarate, butanoate, pantothenate and CoA biosynthesis, glycine, serine and threonine, lysine degradation, and vitamin B6. Some of the affected pathways may have downstream effect on others with which they are linked. For example, besides the absence of all ABC transporter components involved in xylose uptake, HK1212 is missing three genes involved in intracellular xylose metabolism – two xylulose kinases (HI1112, HI1027) and a xylose isomerase (HI1112); these three enzymes are primarily involved in pentose and glucuronate interconversions pathway. However, xylose metabolism is linked to several other biochemical pathways involved in the metabolism of pentose phosphate, riboflavin, ascorbate and aldarate, and some amino sugars and nucleotide sugars; one of these three genes – xylulose kinase (HI1112), is also involved in fructose and mannose metabolism.
Figure 3.
Comparative sequence analysis of shared, presume absent and gained cellular role categories in HK1212 relative to the H. influenzae KW20/Rd reference genome. Absent and shared role categories were determined by CGH using a single genome array based on H. influenzae KW20/Rd. Gained functions were identified based on the Gene Discovery (GD) approach.
Identification of Virulence Factors in H. influenzae HK1212
Among the 439 HK1212-specific features (relative to KW20/Rd), we identified 80 (18%) predicted to encode virulence factors (Table 2). Virulence factors that facilitate host-pathogen interaction in the genome of HK1212 are proteins that are involved in invasion and cytadherence. Among the other virulence factors in H. influenzae HK1212 that do not directly facilitate host-pathogen interaction we include those that are involved in iron acquisition immune evasion proteins that contribute to phase or antigenic variation, and those that contribute to maintenance of cell envelope stability. Interestingly, the acquired repertoire of putative virulence factors in the HK1212 genome was dominated by genes encoding proteins predicted to facilitate the host-pathogen interaction, i.e. cytadherence and invasion. We found several features with sequence identity to known H. influenzae and Neisseria spp. virulence factor sequences including two BPF invasins bpf001 and bpf002 [12], iga1 protease [7,42], Haemophilus adhesion and penetration protein — hap [43], adherence-related high molecular weight proteins hmwA and hmwC [44], las/lav (virulence associated protein A (vapA-) homologs, see below), fimbriae and fimbrial ushers, precursors of hemoglobin/haptoglobin binding protein A (hgbA), and heme/hemopexin utilization protein [14,45]. Some of the features encoding fimbriae, fimbrial ushers and haemaglutinins were found in multiple contigs and showed significant sequence divergence from their homologs within the novel gene set suggesting that they may represent paralogous families, a finding that is consistent with previous reports [48]
Table 2.
Summary of novel features in HK1212 encoding putative virulence determinants
| Feature Name | Minimum number of predicted paralogous groups |
|---|---|
| Adhesins (emaA, xadA) A | |
| Adhesins/autotransporters (hsf, las, lav, pII, hep_hap) A | |
| bpf001V | |
| bpf002V | |
| Fimbriae gene cluster HifABCDEA | 2* |
| Other fimbriae and pili homologs A | 2 |
| Other fimbriae- and pili- usher homologs A | 2 |
| hmwAA | |
| hmwCA | |
| Haemaglutinins A | 2 |
| uspA1A | |
| licABC‡+E | |
| lex2AB‡+E | |
| HgbF | |
| slpAS,A | |
| plp4A |
Contributes to cytadherence
Contributes to invasion
Contributes to iron acquisition
Contributes to stability of membrane structures
Evidence for hifB only
These gene loci are involved in LPS biosynthesis and degradation.
One of the genes in this group contains tetrameric repeats that may contribute to phase or antigenic variation.
Contributes to immune evasion.
In an attempt to identify additional virulence associated genes, we examined features with significantly disparate GC content, those containing tetrameric repeats (Table 2s), or with significant sequence identity to other members of Pasteurellaceae family or unrelated pathogens that share the same ecology with H. influenzae.
The average and the 95th percentile of the GC content within the KW20/Rd genome are 38% and 43.5%, respectively. We identified 50 (11%) novel features with GC content greater than 43.5%. The vast majority of them encode proteins involved in unknown functional roles (14, 28%), cell envelope (13, 26%), mobile elements (9, 18%), and DNA metabolism functions (6, 12 %, Table 2s). Within this group of high-GC features, we also found 12 pORFs predicted to encode putative virulence factors, including putative pili and pili ushers, outer membrane proteins, and adhesins.
Blast analysis revealed several genes with a close relationship to genes resident in N. meningitidis including an opaB (pII-homolog, ORF00736) precursor and two vapA orthologs (ORF00319, ORF00012). Opa proteins have been shown to be major virulence factors in both N. meningitidis and N. gonorrhoeae contributing to colonization and invasion [46–48]. Furthermore, opa genes encode highly variable surface exposed proteins that contribute to antigenic variation for evading the host immune response. Neisseria spp. vapA-homologs, lav and las, represent autotransporters found in H. influenzae and H. influenzae biogroup aegyptius, respectively [49]. The GC content of N. meningitidis serogroup B lav (vapA-homolog) is 40.0% closer to H. influenzae than Neisseria spp. (average of 52%). Therefore it has been hypothesized that this gene was a product of a recent transfer from Haemophilus to Neisseria [49–52] Protein database searches revealed that ORF00109 and ORF00300 shared significant homology with the “Ubiquitous Surface exposed Protein A” (UspA1) from Moraxella catarrhalis. Although phylogenetically distant, this gram-negative aerobe is also a respiratory tract pathogen sharing a common environment with H. influenzae. M. catarrhalis UspA1 and its homolog, UspA2, are high molecular weight outer membrane proteins. It has been demonstrated that these proteins mediate cytadherence and biofilm formation [53–56].
Finally, we investigated the distribution pattern of HK1212 VAGs among 16 publicly available H. influenzae genomes. Results of this analysis indicated that less than half of the HK1212 VAG set (39 ORFs) have orthologs in other H. influenzae genomes, a number varies between 15–35 ORFs/genome. The frequency of all these ORFs among the 16 genomes is also highly variable ranging 6–100%. Taken together, it is evident that the evolution of the strain HK1212 involved the acquisition of a comprehensive and large number of virulence factors via multiple horizontal gene transfer events. These events involved exchanges predominantly with its closest phylogenetic relatives and other pathogens residing in the same host environment.
H. influenzae Multi-Genome DNA Microarray Design
We performed bioinformatic analysis on publicly available sequences (NCBI release 152) from Hi genomes as well as those discovered from strain HK1212 in this study, to identify unique genes encoded in these genomes. The unique genomic features represented by 4,578 unique 70-mer oligonucleotides in this array define approximately 2617 orthologous (alleic) groups, which were then used as the basis for estimating the genomic content of the query strains using CGH data.
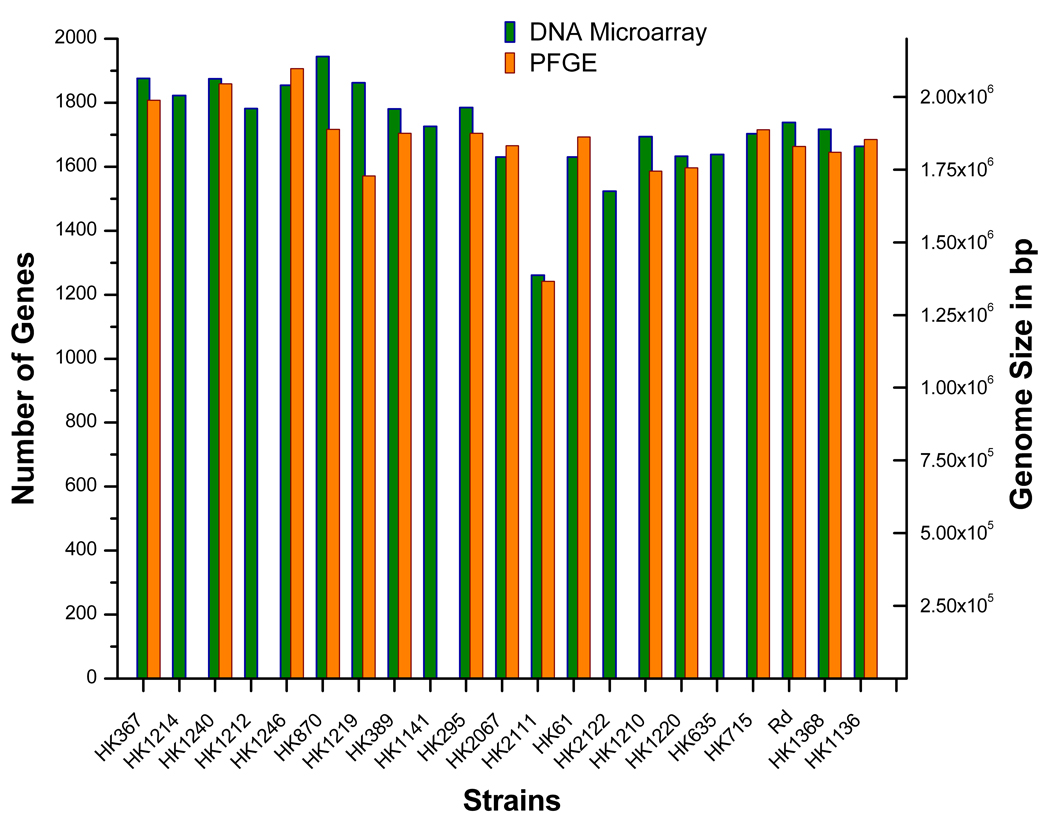
In order to assess the comprehensiveness of the DNA microarray with respect to gene representation of the H. influenzae species and its member clades, we compared gene number estimates based on hybridization data for each of the 21 query genomes to genome size estimates based on PFGE. The error rate for the KW20/Rd gene count varied between 0.5–6% (average 3.7%) across all hybridization experiments. In addition, we found a strong linear correlation between the total gene estimates resulting from the CGH data to the genome size approximations determined by PFGE (Figure 4). Taken collectively, our data indicate that the gene content represented on this microarray reflects a large proportion of the gene pool shared by Hi group members.
Figure 4.
Total gene content predictions derived from the CGH analysis using the species microarray and PFGE-based genome size estimates for the strains characterized in this study.
Phylogenomic Relationships and Clade-Specific Characteristics of Haemophilus Strains
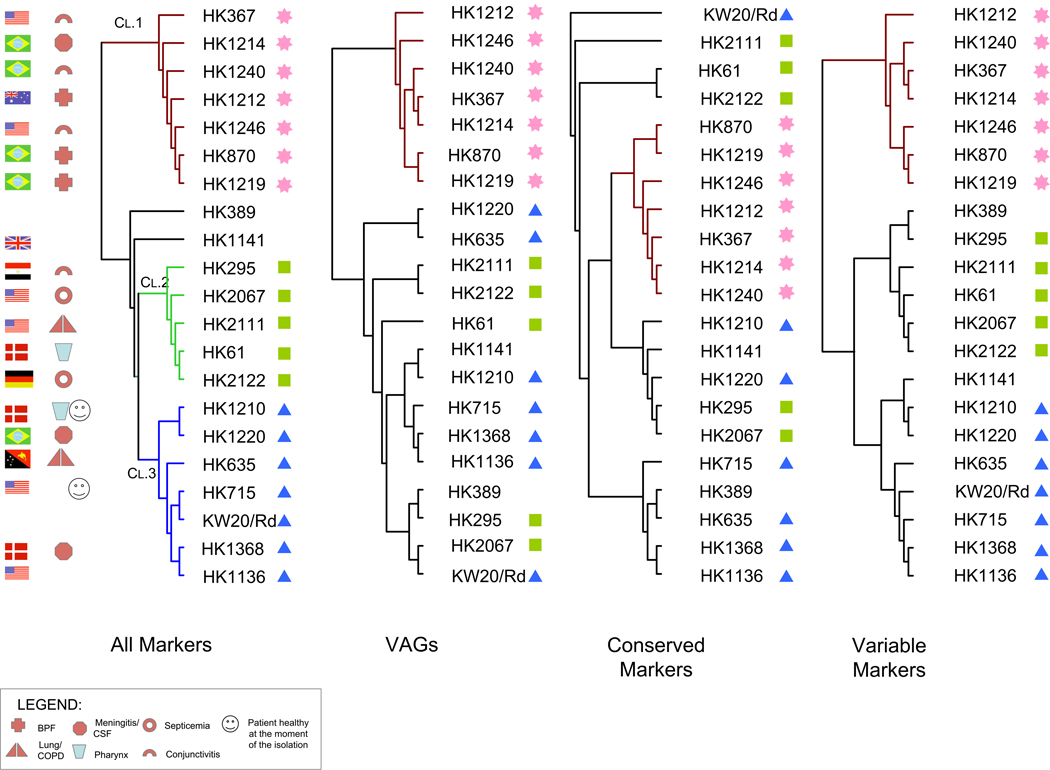
We further evaluated the phylogenomic relationships among Haemophilus group members with a focus on the Hi, HiBae and Hae, to identify genomic events associated with the BPF clone emergence. For this purpose, we have performed genome clustering using a variety of gene sets Figure 5. The first CGH dendrogram represents a summary of phylogenetic relationships based on global hybridization data wherein each gene is assigned the designation (“absent”, “divergent” or “present”). The second dendrogram is based on a set of 329 markers corresponding to putative virulence associated genes, such as cytadherence-related proteins and iron transporters. The third dendrogram is based on the ratio percentiles of a set of 2,097 conserved markers (present in ≥70% of the strains examined). Finally, the fourth dendrogram is based on 2,481 markers corresponding to the variable genomic loci (present in <70% of the strains examined).
Figure 5.
Phylogenomic relationships among Haemophilus strains based on the CGH patterns using different markers sets. A. CGH clustering based on the global gene hybridization patterns of all 4578 70-mers using the trinary designations “0”, “0.5” and “1” representing those coding sequences determined to be “absent”, “divergent” and “present” respectively. Three major lineages, i.e. Clades 1, 2 and 3 are color-coded in brown, green and blue respectively. B. Dendrogram is based on the trinary designations from the 329 markers representing putative virulence associated genes. C. Clustering approach was based on the ratio percentile values of the conserved markers. D. Dendrogram is based on the trinary designations from the markers representing variable genes.
We noted an overall congruency among all dendrograms (Figure 5). The dendrogram based on all genes indicated that the query strains form three major clades. The first one contained seven strains including all four Hae, two HiBae (Brazilian BPF) as well as the Australian BPF-like (HK1212). The second group consisted of five strains, two of which were from patients with septicemia, whereas the third group contained seven Hi, two originating from patients with meningitis. Regardless of the clustering approach (marker sets) used to investigate phylogenomic relationships, only Clade 1 (the “BPF/aegyptoids” clade) remained unchanged.
Genome clustering based on all genomic markers suggest that BPF clones are highly related to conjunctivitis isolates. As shown in Figure 5, conjunctivitis isolates are located closer to the node of the clade compared to the BPF clones. Phylogenomic relationships among BPF/aegyptoids revealed through all CGH clustering approaches are consistent with previous studies based on phenotypic characterizations with regard to Hae and HiBae isolates [7].
Cladistic Analysis Based on Genomic Flux
Based on the identified phylogenomic relationships, we conducted comparative analysis of the gene complements of isolates within each distinct clade. As illustrated in Figure 4, BPF/aegyptoids (Clade 1) appeared to have somewhat larger genomes than the other Hi strains. We estimated that genomes belonging to BPF/aegyptoids possessed an average of 182 additional genes compared to all other clades (1,859 vs. 1,677 genes). Among BPF-related strains HK1212 appeared to share 83% of its gene content with HK870 and HK1219, while these latter two share 90–93 % of their gene contents with each other.
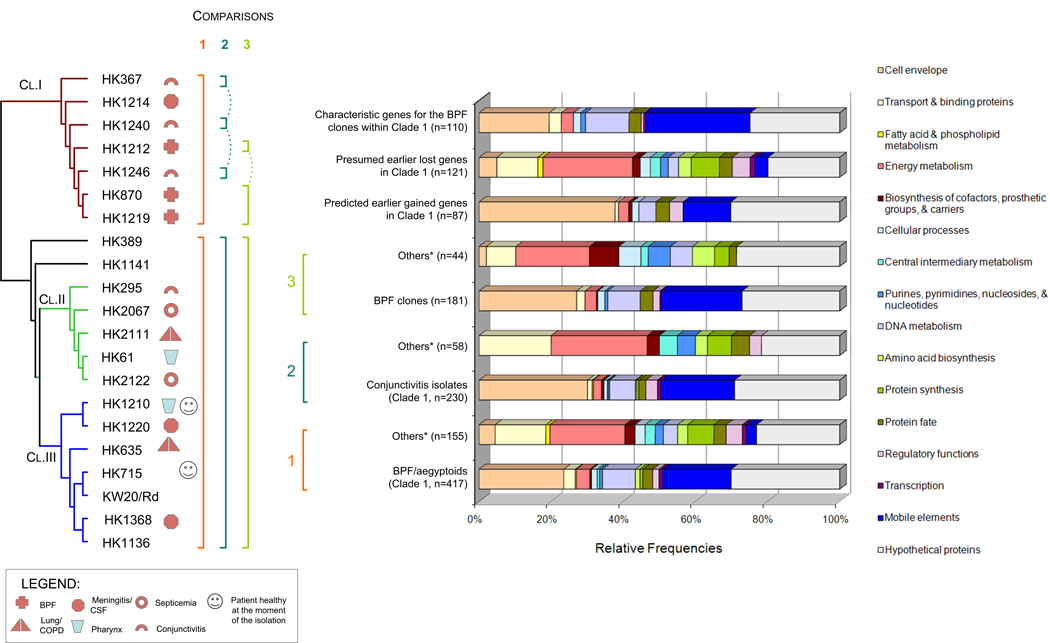
In order to identify genomic events associated with the BPF clone emergence, we conducted a detailed marker association analysis (MAA) based on the Fisher’s Exact Test. Initially we compared BPF/aegyptoids (Clade 1) with the remaining 14 query genomes. We identified 572 allelic groups that are characteristic of BPF/aegyptoids lineage (Table 3s). Among these clade-specific genomic events, 155 represent unique gene loss events, whereas 417 represent gene acquisition events (p<0.05), corresponding to a net gene gain of 262 genes (∼280 Kb). These genomic events resulted in significant functional modifications of strains within this lineage. Figure 6 summarizes MAA results as they relate to predicted cellular functional role categories. Although the number of genes characteristic for the BPF/aegyptoids clade is relatively large, they correspond to a limited set of functions related most predominantly to mobile elements, proteins targeted to the cell surface, DNA metabolism and protein fate. Conversely, functions such as transport, energy metabolism, transcription, protein synthesis and fate, as well as synthesis of co-factors and amino acids were less prevalent among the BPF/aegyptoids. We also investigated the differential metabolic profiling between BPF/aegyptoids and the other H. influenzae strains in more detail. While can identify apparently missing gene functions by CGH, we cannot rule out the possibility that the non-orthologous gene displacement events, including genes of unknown functions, have not occurred. We analyzed KEGG pathways containing ≥3 genes, and conservatively considered a pathway to be incomplete if ≥2 CDSs were absent in one clade compared to others. Results of this survey indicated that over 11 pathways may be incomplete or impaired due to gene absence among Clade 1 members. These fuctions impact the metabolism of: arginine and proline, glycine, serine and threonine, glyoxylate and dicarboxylate, pentose and glucuronate interconversions, fructose and mannose, amino sugars and nucleotide sugars, sucrose, glycerolipids, methane, one carbon pool by folate, and purines. Furthermore, BPF/aegyptoids had fewer proteins involved in transport functions than other strains —19 vs. 26. Over eight transport systems [57] appear to be incomplete among BPF/aegyptoids with respect to other genomes – four ABC transporters, three ion channels and one secondary metabolite transporter. Besides the differences in the number of proteins involved in transports functions, we noticed significant variation in the types of transporters that were characteristic for each group. For example, BFP/aegyptoids were devoid of the ABC transporter system responsible for xylose, cysteine, trace elements such as molybdenum, zinc and cobalt, while enriched for those involved in the uptake of galactosides, dipeptide/oligopeptide/nickel transport system, unspecified amino acids (COG0765) and thiamine. Although BPF/aegyptoids and other Hi strains appeared to have the same number of proteins involved in iron transport, there were still notable differences – yfeD (for chelated iron uptake, COG1108) type was characteristic for BPF/aegyptoids whereas afuC (for ferric uptake, COG3841) was characteristic for the other H. influenzae strains. The two component regulatory system for sensing low levels of nitrogen from the environment also appears absent in BPF/aegyptoids due to the absence of glnD. Finally, another gene of interest was tryptophan synthase beta subunit (trpB, HI1431) whose absence among all BPF/aegyptoids correlated 100% with the phenotypic assay for the tryptophanase activity [58].
Figure 6.
Summary of functions characteristic for the BPF/aegyptoids (Clade 1) group members based on the marker association analysis using Fisher’s Exact Test. Findings are summarized according to the putative cellular functional role categories of the coding DNA sequences. Numbers in brackets represent the number of characteristic genes for each group. The group of strains designate as “Others*” refer to Clade 2, Clade 3, HK369 and HK1141 genomes.
After identifying characteristic features for the Clade 1 members (BPF/aegyptoids), we further applied the MAA within this group to delineate the extent of genome relatedness between conjunctivitis isolates and the BPF clones. Accordingly, we compared the genomes of three conjunctivitis isolates (HK367, HK1240 and HK1246) with the remainder of the Hi strains excluding BPF-associated isolates (HK1212, HK870 and HK1219). Next, we compared genomes of the BPF-associated isolates with the remainder of the Hi strains (including or excluding three conjunctivitis isolates of Clade 1). The direct comparison between the two sub-groups of Clade 1 was not conducted due small number of genomes comprising each group. MAA results from these comparisons, summarized in the form of functional role categories, are shown in Figure 6. Relative to the other Hi strains, we identified 230 and 181 CDSs (allele groups) characteristic for the conjunctivitis strains and the BPF-related isolates respectively (p<0.05). These two sub-groups shared 87 allele groups in common relative to the remainder of Hi clades (Table 3s). It is also worth noting here that HK1212-derived oligonucleotides that were added onto the multi-genome arrays constitute a large fraction of marker sets that were found characteristic for BPF/aegyptoids (74%), non-invasive conjunctivitis strains of Clade 1 (76%) and especially BPF case-related strains (89%).
In addition to identifying the shared genomic features that appeared early in the BPF/aegyptoids (Clade 1) evolution, we were able to identify those genes that are characteristic for either non-invasive conjunctivitis isolates or BPF-associated isolates. We found 110 CDSs that are characteristic for the BPF-related isolates. This gene set highlights the acquisition events associated with the emergence of the BPF clones. The majority (81 %) of the gained BPF-specific novel functions were those related to cell envelope (18%), DNA metabolism (11%), mobile elements (27%) and genes with yet unknown cellular roles (24%). Among the 143 genes that are characteristic of Clade 1 non-invasive conjunctivitis strains we note the same trends in over-representation of functional role categories as the BPF clones. Eighty-five percent of CDSs specific for the conjunctivitis strains encoded proteins predicted to be localized in the cell envelope (25%), mobile elements (24%), DNA metabolism (8%), or proteins of unknown functions (28%).
We applied additional attention to the CDSs encoding putative VAGs to gain insights to the virulence of the BPF clones. The earlier acquisition events appear to have included sequence variants of genes encoding proteins involved in cytadherence, e.g. hifB, hifC, Hsf, uspA1, hmwA, hep_hag adhesins, pilins and pilin ushers (chaperones (Table 3s). Several of these genes had been previously found in HK1212 (Table 2). Interestingly, later events that may have taken place specifically in the genomes of BPF-related isolates involved the acquisition of additional genes functioning in cytadherence and invasion including bpf001, bpf002, hifD, a variant of hifB, heme/hemopexin-binding protein, and several pili and pili ushers unrelated to the previous group. Among the 181 genes found characteristic for the BPF-related strains only 38 (21%) are thought to contribute to the virulence. The CGH analysis also shows that the VAG set shared among BPF-related genomes does not have a uniform distribution among clades. For example, strains HK870, HK1219 and Clade 1 members as group share 84%, 81% and 47% of their VAG sets with HK1212 respectively. By contrast, the Clade 2 and Clade 3 group members share <1% of the HK1212 VAGs on the array.
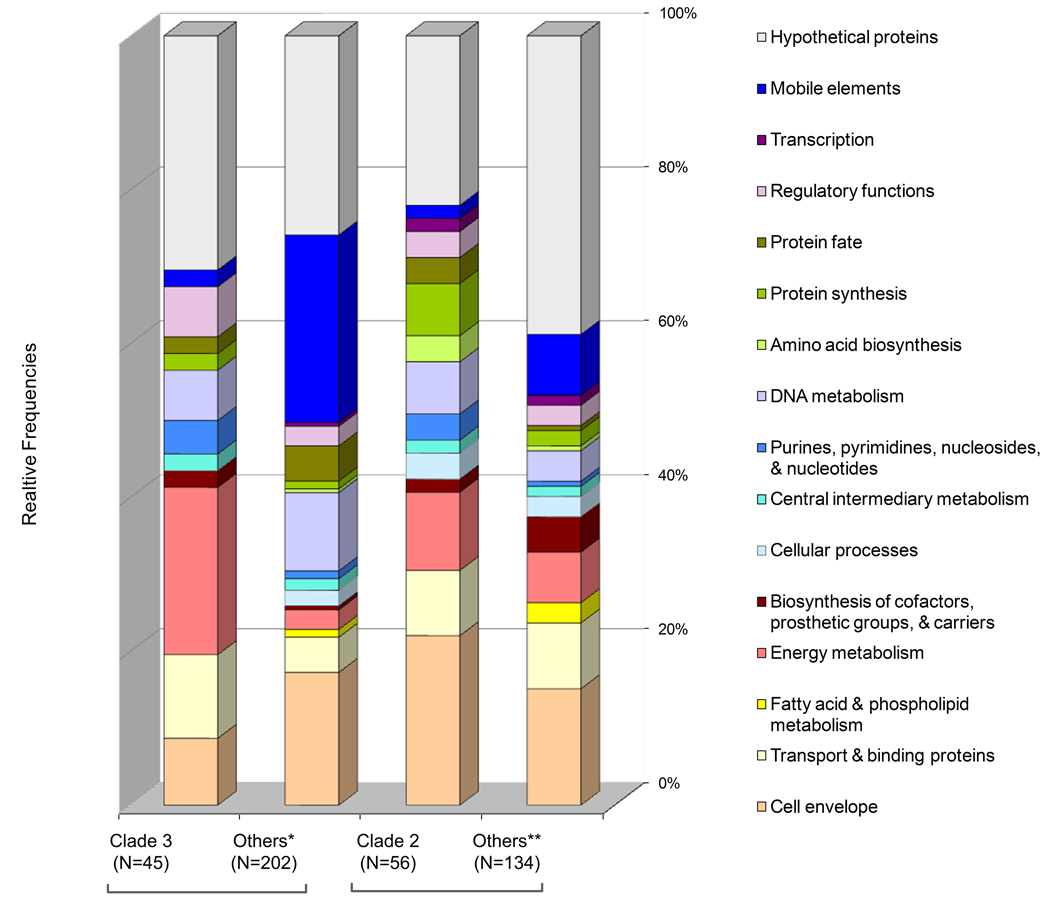
Finally, Figure 7 summarizes the MAA results for Clades 2 and 3 (Figure 5), each containing two isolates associated with septicemia and two with meningitis, respectively. In contrast to the results regarding Clade 1 gene profiling, the number of absent CDSs exceeded the number of those found characteristic in Clades 2 and 3 by 2- and 4-fold respectively (Table 3s). It therefore appears that H. influenzae genome evolution is proceeding in such a way that some lineages are expanding their gene complements, while other clades may be moving toward more streamlined genomes. Our marker profile analysis indicated that each clade had its own particular sequence variants for genes involved in lipo-polysaccharide biosynthesis or outer membrane proteins (allelic groups 576, 745, and 1035). In addition, we noticed that members of the Clade 2 also seem to have characteristic sequence variants for the urease α-subunit and periplasmic-binding protein yfeA, which is involved in iron acquisition.
Figure 7.
Summary of functions characteristic for strains of Clades 2 and 3 (Figure 3) based on the marker association analysis using Fisher’s Exact Test. Findings are summarized according to the putative cellular functional role categories of the coding DNA sequences. Numbers in brackets represent the number of characteristic genes for each group. The group of strains designated as “Others*” refer to Clade 1, Clade 2, HK369 and HK1141 genome, whereas “Others**” refer to Clade 1, Clade 3, HK369 and HK1141 genomes.
DISCUSSION
This study using a H. influenzae species microarray enabled the elucidation of phylogenomic relationships among of members of this taxonomic group, in particular, clonal variants associated with BPF. In addition, by investigating the extent of H. influenzae species diversity, we identified numerous genomic features associated with the emergence of the BPF variants. However, the large number of genes of unknown function (∼40%) discovered in the HK1212 accessory genome underscores that many gaps remain in our understanding of the genotype-phenotype relationship of these isolates. The genomic factors that contribute to emergence and the pathobiology of this highly virulent organism appear to be nominally related to a relatively large group of cell envelope proteins involved in cytadherence and invasion. Our findings support the previous hypothesis that BPF isolates have an unusual membrane composition, especially with regard to pili [44,59,60]. The features encoding for pili-related functions made up a significant portion of the putative virulence genes identified in the BPF-like isolate, HK1212. Mobile elements, almost exclusively composed of phage proteins, were also highly represented among acquired genes. Likewise, this group of features (mobile elements) represented ∼20% of the genetic content characteristic for the BPF/aegyptoids genomes. This pattern is very similar to the one observed in the genome of enterohemorrhagic E. coli O157:H7 [61], and this finding underscores the significance of mobile elements as vehicles of horizontal gene transfer and as such, they contribute extensively to the generation of genetic diversity [62–64].
In conjunction with the acquisition of a limited set of acquired functional roles, it is apparent that genomic flux among BPF/aegyptoids genomes is characterized by significant alterations in selected role categories. Several genes involved in housekeeping cellular functions such as energy metabolism, transcription, translation, synthesis of co-factors, amino acids and proteins, as well as transport, appear to have been lost. Our findings suggest that, in addition to deficiencies in a few two-component regulatory systems and transport systems, several pathways involved in the metabolism of certain carbohydrates (xylose, sucrose, fructose, mannose and nucleotide sugars), vitamins, nucleotides and amino acid metabolism (arginine and proline, glycine, serine and threonine) are impaired in BPF/aegyptoids. Consequently, adaptation to the host environment may be driving the loss of genes that are no longer needed in the host environment. Without exception, the gene loss profile among Clade 1 members, and especially BPF-related strains, suggests that genome evolution is driving the bacterial cell toward deeper dependency on the host for energy and metabolites (e.g. loss of genes involved in energy metabolism and transport). This process has been accompanied by a sequential acquisition of genes associated with particular functional roles that have enabled the cell to invade host tissues with remarkable efficiency. Almost all genes VAGs found in HK1212 and characteristic for the BPF-related stains as a group appear to be involved in cytadherence and invasion.
Extensive research on H. influenzae pathogenesis has resulted in the identification and characterization of many virulence factors. However, the screening of clinical isolates has indicated that their distribution is not universal [65]. With the exception of the cap genes, we identified an unusually large set of previously described H. influenzae virulence factors in the genome of HK1212. Based on our literature review [15,16,19,65,66], CGH data, as well as comparative sequence analysis, this is quite different from known patterns of virulence gene content discovered within the genomes of various members of H. influenzae. Although the genome content revealed by our study is a snapshot of HK1212 evolution, it is clear that a gene complement supporting high invasiveness in a capsule independent manner has emerged. As a consequence, we postulate that during this developmental process, cap genes may have been counter-selected and lost. It is possible that the presence of the capsule may interfere or be incompatible with the function of other surface-localized virulence factors. This speculation is consistent with the demonstration that cap pneumococci adhere more efficiently to epithelial cells and that such strains are more associated with conjunctivitis [67].
CGH-based phylogenomic analysis revealed three major groups, and for the majority of these strains, phylogenomic relationships were essentially congruent among all clustering approaches independent of the gene set used. The strongest relationships were observed among the seven members of Clade 1, including all four Hae, two HiBae (Brazilian BPF) as well as the Australian BPF-like (HK1212). Regardless of the clustering approach, the members of this group remained the same, indicating that “BPF/aegyptoids” form a coherent clade. The second clade included two strains obtained from patients with septicemia, whereas the third clade contained two isolates associated with meningitis. Despite major clade separation, it was noticed that virulent strains clustered together with those isolated from healthy individuals or patients with milder forms of disease.
All clustering approaches provided complementary information for elucidating overall genome content relatedness. The phylogenomic relationships inferred by clustering based on gene presence, in general, do not reproduce the phylogeny of a species in terms of vertical evolution, but instead represent the overall relatedness of genomes to one another, and provide a means to evaluate genome evolution within the species. The MLST clustering approach indicated initially that the two Brazilian BPF clones (HK870 and HK1219) are ancestrally distant from the Australian isolate (HK1212); these observations were further supported by the clustering based on conserved loci (Figure 1). At the same time, we found that the BPF clones as well as their conjunctivitis close relatives share similar genomic compositions.
Hae and Hibae are recognized as a separate phylogenetic group within the species H. influenzae [1,7]. This group (containing both Hae and Hibae) is characterized by a distinct cell morphology, microcolony formation on conjunctival epithelial cells, a strong hemagglutinating activity, the inability to metabolize xylose, and a decaying hap gene [7]. Both Hae and Hibae cause acute eye infections but the latter has invasive potential similar to serotype b strains. It is remarkable that the phylogenetically distinct, non-encapsulated strains HK870, HK1219 and HK1212 each share a unique and invasive pathogenic potential that is consistent with their patterns of gene acquisition and gene loss. Based on the hap pseudogene analysis, it has been hypothesized that the BPF-associated H. influenzae biogroup aegyptius and H. aegyptius evolved recently as a distinct group from H. influenzae [1,7]. The patterns of present and missing genes further suggest that the BPF-associated H. influenzae biogroup aegyptius (HK870 and HK1219) share a recent common ancestor with H. aegyptius (HK1246) and HK1212.
Based on the CGH results, this evolutionary hypothesis suggests that gene acquisition events resulting in the recent clone emergence, have taken place independently. Therefore, characterization of gene presence/absence patterns has fundamental epidemiological significance. This type of analytical approach will help improve diagnostics and open the avenues for developing predictive cladistic models to improve our understanding of genome evolution associated with clone emergence. Future efforts will seek to broaden our coverage of genomes to include additional sequenced strains with the goal of identifying genomic markers that can be used for diagnostic purposes and finally assist both drug- and vaccine design [24,68].
Supplementary Material
Figure 1s. Gene Discovery (GD). An HK1212 random genomic library was generated. Cloned genomic fragments are common (uncolored boxes) or unique (colored boxes). Plasmid DNAs are printed onto glass slide microarrays and subsequently probed with Cy-labeled (either Cy3 or Cy5) HK1212 and KW20/Rd in a competitive hybridization. Those plasmids that do not hybridize with KW20/Rd were considered to contain unique sequences and therefore were sequenced. Other plasmids that hybridized weakly were considered to contain divergent sequences were also sequenced.
Figure 2s. Microarray data analysis approach.
A. The Histogram Mode Centering (HMC) algorithm was used to normalize the signal intensity data to fit the log-ratio frequencies (gray dots) of the main peak to a Gaussian distribution function. We estimated the best fit with minimal sum of squared residuals between raw histogram data and a theoretical Gaussian curve (green line). Upon finding the best fit, the mode of the Gaussian function is calculated. The arrow represents the value shift (data transformation) based on the difference between the expected theoretical log-ratio (zero) and the mode of the fit normal distribution model. The standard deviation value of the normal distribution model was then used to calculate the percentiles (see below) corresponding to each log-ratio based on the z-score table.
B. Criteria used to assign gene presence in the reference or query genomes based on the log-ratio percentile values from the HMC.
Figure 3s Comparative cluster analysis of H. influenza strains. A. CGH clustering based on the global gene hybridization patterns (of 1720 70-mers, single-genome-array based on KW20/Rd genome). B. MLST-based dendrogram [7]. C. Dendrogram represents MLEE clustering (using Unweighted Pair Group Method with Arithmetic Mean analysis (UPGMA)) [7].
Acknowledgments
L. Papazisi and S. Ratnayake contributed equally to this work with writing and data analysis. B. Remortel and G. Bock contributed equally to this work with regard to the CGH data generation. We thank Dr. Marcus Jones and Dr. Timothy Minogue for their critical review of the manuscript, and Dr. Chun-Hua Wan for his assistance regarding microarray data formatting and submission to the GEO database. This work was supported by the NIAID contract No. N01-AI-15447 to the Pathogen Functional Genomics Resource Center (PFGRC) at the JCVI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Sequence Accession Numbers.
The sequence data from this study have been submitted to the NCBI under accession number ABFC00000000.
Microarray data deposition.
The sequence data from this study have been submitted to the NCBI Gene Expression omnibus (GEO) under accession number GSE8300.
REFERENCES
- 1.Kilian M. Haemophilus. In: Murray PR, Baron EJ, Jorgensen JH, Landry ML, Pfaller MA, editors. Manual of Clinial Microbiology. 9th ed. Washinton D.C.: American Society of Microbiology; 2007. pp. 636–648. [Google Scholar]
- 2.Meats E, Feil EJ, Stringer S, Cody AJ, Goldstein R, Kroll JS, Popovic T, Spratt BG. Characterization of encapsulated and noncapsulated Haemophilus influenzae and determination of phylogenetic relationships by multilocus sequence typing. J Clin Microbiol. 2003;41:1623–1636. doi: 10.1128/JCM.41.4.1623-1636.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peltola H. Worldwide Haemophilus influenzae type b disease at the beginning of the 21st century: global analysis of the disease burden 25 years after the use of the polysaccharide vaccine and a decade after the advent of conjugates. Clin Microbiol Rev. 2000;13:302–317. doi: 10.1128/cmr.13.2.302-317.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kroll JS, Loynds B, Brophy LN, Moxon ER. The bex locus in encapsulated Haemophilus influenzae: a chromosomal region involved in capsule polysaccharide export. Mol Microbiol. 1990;4:1853–1862. doi: 10.1111/j.1365-2958.1990.tb02034.x. [DOI] [PubMed] [Google Scholar]
- 5.Harrison LH, Simonsen V, Waldman EA. Emergence and disappearance of a virulent clone of Haemophilus influenzae biogroup aegyptius, cause of Brazilian purpuric fever. Clin Microbiol Rev. 2008;21:594–605. doi: 10.1128/CMR.00020-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Musser JM, Selander RK. Brazilian purpuric fever: evolutionary genetic relationships of the case clone of Haemophilus influenzae biogroup aegyptius to encapsulated strains of Haemophilus influenzae. J Infect Dis. 1990;161:130–133. doi: 10.1093/infdis/161.1.130. [DOI] [PubMed] [Google Scholar]
- 7.Kilian M, Poulsen K, Lomholt H. Evolution of the paralogous hap and iga genes in Haemophilus influenzae: evidence for a conserved hap pseudogene associated with microcolony formation in the recently diverged Haemophilus aegyptius and H. influenzae biogroup aegyptius. Mol Microbiol. 2002;46:1367–1380. doi: 10.1046/j.1365-2958.2002.03254.x. [DOI] [PubMed] [Google Scholar]
- 8.Smoot LM, Franke DD, McGillivary G, Actis LA. Genomic analysis of the F3031 Brazilian purpuric fever clone of Haemophilus influenzae biogroup aegyptius by PCR-based subtractive hybridization. Infect Immun. 2002;70:2694–2699. doi: 10.1128/IAI.70.5.2694-2699.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brenner DJ, Mayer LW, Carlone GM, Harrison LH, Bibb WF, Brandileone MC, Sottnek FO, Irino K, Reeves MW, Swenson JM, et al. Biochemical, genetic, and epidemiologic characterization of Haemophilus influenzae biogroup aegyptius (Haemophilus aegyptius) strains associated with Brazilian purpuric fever. J Clin Microbiol. 1988;26:1524–1534. doi: 10.1128/jcm.26.8.1524-1534.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McIntyre P, Wheaton G, Erlich J, Hansman D. Brasilian purpuric fever in central Australia. Lancet. 1987;2:112. doi: 10.1016/s0140-6736(87)92788-7. [DOI] [PubMed] [Google Scholar]
- 11.Dobson SR, Kroll JS, Moxon ER. Insertion sequence IS1016 and absence of Haemophilus capsulation genes in the Brazilian purpuric fever clone of Haemophilus influenzae biogroup aegyptius. Infect Immun. 1992;60:618–622. doi: 10.1128/iai.60.2.618-622.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li MS, Farrant JL, Langford PR, Kroll JS. Identification and characterization of genomic loci unique to the Brazilian purpuric fever clonal group of H. influenzae biogroup aegyptius: functionality explored using meningococcal homology. Mol Microbiol. 2003;47:1101–1111. doi: 10.1046/j.1365-2958.2003.03359.x. [DOI] [PubMed] [Google Scholar]
- 13.Kroll JS, Farrant JL, Tyler S, Coulthart MB, Langford PR. Characterisation and genetic organisation of a 24-MDa plasmid from the Brazilian Purpuric Fever clone of Haemophilus influenzae biogroup aegyptius. Plasmid. 2002;48:38–48. doi: 10.1016/s0147-619x(02)00020-3. [DOI] [PubMed] [Google Scholar]
- 14.Fleischmann RD, Adams MD, White O, Clayton RA, Kirkness EF, Kerlavage AR, Bult CJ, Tomb JF, Dougherty BA, Merrick JM, et al. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science. 1995;269:496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- 15.Erwin AL, Nelson KL, Mhlanga-Mutangadura T, Bonthuis PJ, Geelhood JL, Morlin G, Unrath WC, Campos J, Crook DW, Farley MM, Henderson FW, Jacobs RF, Muhlemann K, Satola SW, van Alphen L, Golomb M, Smith AL. Characterization of genetic and phenotypic diversity of invasive nontypeable Haemophilus influenzae. Infect Immun. 2005;73:5853–5863. doi: 10.1128/IAI.73.9.5853-5863.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrison A, Dyer DW, Gillaspy A, Ray WC, Mungur R, Carson MB, Zhong H, Gipson J, Gipson M, Johnson LS, Lewis L, Bakaletz LO, Munson RS., Jr Genomic sequence of an otitis media isolate of nontypeable Haemophilus influenzae: comparative study with H. influenzae serotype d, strain KW20. J Bacteriol. 2005;187:4627–4636. doi: 10.1128/JB.187.13.4627-4636.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hogg JS, Hu FZ, Janto B, Boissy R, Hayes J, Keefe R, Post JC, D G. Ehrlich, Characterization and modeling of the Haemophilus influenzae core and supragenomes based on the complete genomic sequences of Rd and 12 clinical nontypeable strains. Genome Biol. 2007;8:R103. doi: 10.1186/gb-2007-8-6-r103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernaays MM, Lesse AJ, Sethi S, Cai X, Murphy TF. Differential genome contents of nontypeable Haemophilus influenzae strains from adults with chronic obstructive pulmonary disease. Infect Immun. 2006;74:3366–3374. doi: 10.1128/IAI.01904-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen K, Antalis P, Gladitz J, Sayeed S, Ahmed A, Yu S, Hayes J, Johnson S, Dice B, Dopico R, Keefe R, Janto B, Chong W, Goodwin J, Wadowsky RM, Erdos G, Post JC, Ehrlich GD, Hu FZ. Identification, distribution, and expression of novel genes in 10 clinical isolates of nontypeable Haemophilus influenzae. Infect Immun. 2005;73:3479–3491. doi: 10.1128/IAI.73.6.3479-3491.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 21.Moore DD. Overview of recombinant DNA libraries. In: Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, al e, editors. Current protocols in molecular biology. New York: John Wiley & Sons; 1993. pp. 5.1.1–5.1.3. [Google Scholar]
- 22.Peterson SN, Sung CK, Cline R, Desai BV, Snesrud EC, Luo P, Walling J, Li H, Mintz M, Tsegaye G, Burr PC, Do Y, Ahn S, Gilbert J, Fleischmann RD, Morrison DA. Identification of competence pheromone responsive genes in Streptococcus pneumoniae by use of DNA microarrays. Mol Microbiol. 2004;51:1051–1070. doi: 10.1046/j.1365-2958.2003.03907.x. [DOI] [PubMed] [Google Scholar]
- 23.Sutton G, White O, Adams MD, Kerlavage AR. A New Tool for Assembling Large Shotgun Sequencing Projects. Genome Science & Technology. 1995;1:9–19. [Google Scholar]
- 24.Tettelin H, Masignani V, Cieslewicz MJ, Donati C, Medini D, Ward NL, Angiuoli SV, Crabtree J, Jones AL, Durkin AS, Deboy RT, Davidsen TM, Mora M, Scarselli M, Margarit y Ros I, Peterson JD, Hauser CR, Sundaram JP, Nelson WC, Madupu R, Brinkac LM, Dodson RJ, Rosovitz MJ, Sullivan SA, Daugherty SC, Haft DH, Selengut J, Gwinn ML, Zhou L, Zafar N, Khouri H, Radune D, Dimitrov G, Watkins K, O’Connor KJ, Smith S, Utterback TR, White O, Rubens CE, Grandi G, Madoff LC, Kasper DL, Telford JL, Wessels MR, Rappuoli R, Fraser CM. Genome analysis of multiple pathogenic isolates of Streptococcus agalactiae: implications for the microbial "pan-genome". Proc Natl Acad Sci U S A. 2005;102:13950–13955. doi: 10.1073/pnas.0506758102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delcher AL, Harmon D, Kasif S, White O, Salzberg SL. Improved microbial gene identification with GLIMMER. Nucleic Acids Res. 1999;27:4636–4641. doi: 10.1093/nar/27.23.4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riley M. Functions of the gene products of Escherichia coli. Microbiol Rev. 1993;57:862–952. doi: 10.1128/mr.57.4.862-952.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 28.Huang X, Madan A. CAP3: A DNA sequence assembly program. Genome Res. 1999;9:868–877. doi: 10.1101/gr.9.9.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 30.Bozdech Z, Zhu J, Joachimiak MP, Cohen FE, Pulliam B, DeRisi JL. Expression profiling of the schizont and trophozoite stages of Plasmodium falciparum with a long-oligonucleotide microarray. Genome Biol. 2003;4:R9. doi: 10.1186/gb-2003-4-2-r9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGillivary G, Tomaras AP, Rhodes ER, Actis LA. Cloning and sequencing of a genomic island found in the Brazilian purpuric fever clone of Haemophilus influenzae biogroup aegyptius. Infect Immun. 2005;73:1927–1938. doi: 10.1128/IAI.73.4.1927-1938.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saeed AI, Sharov V, White J, Li J, Liang W, Bhagabati N, Braisted J, Klapa M, Currier T, Thiagarajan M, Sturn A, Snuffin M, Rezantsev A, Popov D, Ryltsov A, Kostukovich E, Borisovsky I, Liu Z, Vinsavich A, Trush V, Quackenbush J. TM4: a free, open-source system for microarray data management and analysis. Biotechniques. 2003;34:374–378. doi: 10.2144/03342mt01. [DOI] [PubMed] [Google Scholar]
- 33.Kim CC, Joyce EA, Chan K, Falkow S. Improved analytical methods for microarray-based genome-composition analysis. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-11-research0065. RESEARCH0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boyd EF, Porwollik S, Blackmer F, McClelland M. Differences in gene content among Salmonella enterica serovar typhi isolates. J Clin Microbiol. 2003;41:3823–3828. doi: 10.1128/JCM.41.8.3823-3828.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Porwollik S, Wong RM, McClelland M. Evolutionary genomics of Salmonella: gene acquisitions revealed by microarray analysis. Proc Natl Acad Sci U S A. 2002;99:8956–8961. doi: 10.1073/pnas.122153699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Champion OL, Gaunt MW, Gundogdu O, Elmi A, Witney AA, Hinds J, Dorrell N, Wren BW. Comparative phylogenomics of the food-borne pathogen Campylobacter jejuni reveals genetic markers predictive of infection source. Proc Natl Acad Sci U S A. 2005;102:16043–16048. doi: 10.1073/pnas.0503252102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leavis HL, Willems RJ, van Wamel WJ, Schuren FH, Caspers MP, Bonten MJ. Insertion sequence-driven diversification creates a globally dispersed emerging multiresistant subspecies of E. faecium. PLoS Pathog. 2007;3:e7. doi: 10.1371/journal.ppat.0030007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- 39.Stabler RA, Gerding DN, Songer JG, Drudy D, Brazier JS, Trinh HT, Witney AA, Hinds J, Wren BW. Comparative Phylogenomics of Clostridium difficile Reveals Clade Specificity and Microevolution of Hypervirulent Strains. J. Bacteriol. 2006;188:7297–7305. doi: 10.1128/JB.00664-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zar JH. Biostatistical analysis. 4th ed. Upper Saddle River NJ, USA: Prentice Hall; 1999. [Google Scholar]
- 41.Sheskin DJ. Handbood of Parametric and Nonparametric Statistical Procedures. Chapman & Hall/CRC; 2004. [Google Scholar]
- 42.Lomholt H, Kilian M. Distinct antigenic and genetic properties of the immunoglobulin A1 protease produced by Haemophilus influenzae biogroup aegyptius associated with Brazilian purpuric fever in Brazil. Infect Immun. 1995;63:4389–4394. doi: 10.1128/iai.63.11.4389-4394.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.St Geme WJ, 3rd, de la Morena ML, Falkow S. A Haemophilus influenzae IgA protease-like protein promotes intimate interaction with human epithelial cells. Mol Microbiol. 1994;14:217–233. doi: 10.1111/j.1365-2958.1994.tb01283.x. [DOI] [PubMed] [Google Scholar]
- 44.Gilsdorf JR, Marrs CF, Foxman B. Haemophilus influenzae: genetic variability and natural selection to identify virulence factors. Infect Immun. 2004;72:2457–2461. doi: 10.1128/IAI.72.5.2457-2461.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jin H, Ren Z, Whitby PW, Morton DJ, Stull TL. Characterization of hgpA, a gene encoding a haemoglobin/haemoglobin-haptoglobin-binding protein of Haemophilus influenzae. Microbiology. 1999;145(Pt 4):905–914. doi: 10.1099/13500872-145-4-905. [DOI] [PubMed] [Google Scholar]
- 46.Toleman M, Aho E, Virji M. Expression of pathogen-like Opa adhesins in commensal Neisseria: genetic and functional analysis. Cell Microbiol. 2001;3:33–44. doi: 10.1046/j.1462-5822.2001.00089.x. [DOI] [PubMed] [Google Scholar]
- 47.I. M, de Jonge Hamstra HJ, van Alphen L, Dankert J, van der Ley P. Mapping the binding domains on meningococcal Opa proteins for CEACAM1 and CEA receptors. Mol Microbiol. 2003;50:1005–1015. doi: 10.1046/j.1365-2958.2003.03749.x. [DOI] [PubMed] [Google Scholar]
- 48.Naids FL, Belisle B, Lee N, Rest RF. Interactions of Neisseria gonorrhoeae with human neutrophils: studies with purified PII (Opa) outer membrane proteins and synthetic Opa peptides. Infect Immun. 1991;59:4628–4635. doi: 10.1128/iai.59.12.4628-4635.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Davis J, Smith AL, Hughes WR, Golomb M. Evolution of an autotransporter: domain shuffling and lateral transfer from pathogenic Haemophilus to Neisseria. J Bacteriol. 2001;183:4626–4635. doi: 10.1128/JB.183.15.4626-4635.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ochsner UA, Vasil AI, Johnson Z, Vasil ML. Pseudomonas aeruginosa fur overlaps with a gene encoding a novel outer membrane lipoprotein, OmlA. J Bacteriol. 1999;181:1099–1109. doi: 10.1128/jb.181.4.1099-1109.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grizot S, Buchanan SK. Structure of the OmpA-like domain of RmpM from Neisseria meningitidis. Molecular Microbiology. 2004;51:1027–1037. doi: 10.1111/j.1365-2958.2003.03903.x. [DOI] [PubMed] [Google Scholar]
- 52.May BJ, Zhang Q, Li LL, Paustian ML, Whittam TS, Kapur V. Complete genomic sequence of Pasteurella multocida, Pm70. Proc Natl Acad Sci U S A. 2001;98:3460–3465. doi: 10.1073/pnas.051634598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hill DJ, Virji M. A novel cell-binding mechanism of Moraxella catarrhalis ubiquitous surface protein UspA: specific targeting of the N-domain of carcinoembryonic antigen-related cell adhesion molecules by UspA1. Mol Microbiol. 2003;48:117–129. doi: 10.1046/j.1365-2958.2003.03433.x. [DOI] [PubMed] [Google Scholar]
- 54.Lafontaine ER, Cope LD, Aebi C, Latimer JL, McCracken GH, Jr, Hansen EJ. The UspA1 protein and a second type of UspA2 protein mediate adherence of Moraxella catarrhalis to human epithelial cells in vitro. J Bacteriol. 2000;182:1364–1373. doi: 10.1128/jb.182.5.1364-1373.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pearson MM, Laurence CA, Guinn SE, Hansen EJ. Biofilm formation by Moraxella catarrhalis in vitro: roles of the UspA1 adhesin and the Hag hemagglutinin. Infect Immun. 2006;74:1588–1596. doi: 10.1128/IAI.74.3.1588-1596.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoiczyk E, Roggenkamp A, Reichenbecher M, Lupas A, Heesemann J. Structure and sequence analysis of Yersinia YadA and Moraxella UspAs reveal a novel class of adhesins. EMBO J. 2000;19:5989–5999. doi: 10.1093/emboj/19.22.5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ren Q, Chen K, Paulsen IT. TransportDB: a comprehensive database resource for cytoplasmic membrane transport systems and outer membrane channels. Nucleic Acids Res. 2007;35:D274–D279. doi: 10.1093/nar/gkl925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martin K, Morlin G, Smith A, Nordyke A, Eisenstark A, Golomb M. The tryptophanase gene cluster of Haemophilus influenzae type b: evidence for horizontal gene transfer. J Bacteriol. 1998;180:107–118. doi: 10.1128/jb.180.1.107-118.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Read TD, Satola SW, Farley MM. Nucleotide sequence analysis of hypervariable junctions of Haemophilus influenzae pilus gene clusters. Infect Immun. 2000;68:6896–6902. doi: 10.1128/iai.68.12.6896-6902.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Read TD, Dowdell M, Satola SW, Farley MM. Duplication of pilus gene complexes of Haemophilus influenzae biogroup aegyptius. J Bacteriol. 1996;178:6564–6570. doi: 10.1128/jb.178.22.6564-6570.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hayashi T, Makino K, Ohnishi M, Kurokawa K, Ishii K, Yokoyama K, Han CG, Ohtsubo E, Nakayama K, Murata T, Tanaka M, Tobe T, Iida T, Takami H, Honda T, Sasakawa C, Ogasawara N, Yasunaga T, Kuhara S, Shiba T, Hattori M, Shinagawa H. Complete genome sequence of enterohemorrhagic Escherichia coli O157:H7 and genomic comparison with a laboratory strain K-12. DNA Res. 2001;8:11–22. doi: 10.1093/dnares/8.1.11. [DOI] [PubMed] [Google Scholar]
- 62.Frost LS, Leplae R, Summers AO, Toussaint A. Mobile genetic elements: the agents of open source evolution. Nat Rev Microbiol. 2005;3:722–732. doi: 10.1038/nrmicro1235. [DOI] [PubMed] [Google Scholar]
- 63.Dobrindt U, Hochhut B, Hentschel U, Hacker J. Genomic islands in pathogenic and environmental microorganisms. Nat Rev Microbiol. 2004;2:414–424. doi: 10.1038/nrmicro884. [DOI] [PubMed] [Google Scholar]
- 64.Woese CR. On the evolution of cells. Proc Natl Acad Sci U S A. 2002;99:8742–8747. doi: 10.1073/pnas.132266999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.J N. Haemophilus influenzae. Medical Molecular Microbiolgy: Academic Press; 2001. High; pp. 1967–1988. [Google Scholar]
- 66.Munson RS, Jr, Harrison A, Gillaspy A, Ray WC, Carson M, Armbruster D, Gipson J, Gipson M, Johnson L, Lewis L, Dyer DW, Bakaletz LO. Partial analysis of the genomes of two nontypeable Haemophilus influenzae otitis media isolates. Infect Immun. 2004;72:3002–3010. doi: 10.1128/IAI.72.5.3002-3010.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martin M, Turco JH, Zegans ME, Facklam RR, Sodha S, Elliott JA, Pryor JH, Beall B, Erdman DD, Baumgartner YY, Sanchez PA, Schwartzman JD, Montero J, Schuchat A, Whitney CG. An outbreak of conjunctivitis due to atypical Streptococcus pneumoniae. N Engl J Med. 2003;348:1112–1121. doi: 10.1056/NEJMoa022521. [DOI] [PubMed] [Google Scholar]
- 68.Read TD, Peterson SN, Tourasse N, Baillie LW, Paulsen IT, Nelson KE, Tettelin H, Fouts DE, Eisen JA, Gill SR, Holtzapple EK, Okstad OA, Helgason E, Rilstone J, Wu M, Kolonay JF, Beanan MJ, Dodson RJ, Brinkac LM, Gwinn M, DeBoy RT, Madpu R, Daugherty SC, Durkin AS, Haft DH, Nelson WC, Peterson JD, Pop M, Khouri HM, Radune D, Benton JL, Mahamoud Y, Jiang L, Hance IR, Weidman JF, Berry KJ, Plaut RD, Wolf AM, Watkins KL, Nierman WC, Hazen A, Cline R, Redmond C, Thwaite JE, White O, Salzberg SL, Thomason B, Friedlander AM, Koehler TM, Hanna PC, Kolsto AB, Fraser CM. The genome sequence of Bacillus anthracis Ames and comparison to closely related bacteria. Nature. 2003;423:81–86. doi: 10.1038/nature01586. [DOI] [PubMed] [Google Scholar]
- 69.Kunze W, Muller L, Kilian M, Schuhmann MU, Baumann L, Handrick W. Recurrent posttraumatic meningitis due to nontypable Haemophilus influenzae: case report and review of the literature. Infection. 2008;36:74–77. doi: 10.1007/s15010-007-6048-5. [DOI] [PubMed] [Google Scholar]
- 70.Urwin G, Krohn JA, Deaver-Robinson K, Wenger JD, Farley MM. Invasive disease due to Haemophilus influenzae serotype f: clinical and epidemiologic characteristics in the H. influenzae serotype b vaccine era. The Haemophilus influenzae Study Group. Clin Infect Dis. 1996;22:1069–1076. doi: 10.1093/clinids/22.6.1069. [DOI] [PubMed] [Google Scholar]
- 71.Kilian M. A taxonomic study of the genus Haemophilus, with the proposal of a new species. J Gen Microbiol. 1976;93:9–62. doi: 10.1099/00221287-93-1-9. [DOI] [PubMed] [Google Scholar]
- 72.Murphy TF, Brauer AL, Sethi S, Kilian M, Cai X, Lesse AJ. Haemophilus haemolyticus: a human respiratory tract commensal to be distinguished from Haemophilus influenzae. J Infect Dis. 2007;195:81–89. doi: 10.1086/509824. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 1s. Gene Discovery (GD). An HK1212 random genomic library was generated. Cloned genomic fragments are common (uncolored boxes) or unique (colored boxes). Plasmid DNAs are printed onto glass slide microarrays and subsequently probed with Cy-labeled (either Cy3 or Cy5) HK1212 and KW20/Rd in a competitive hybridization. Those plasmids that do not hybridize with KW20/Rd were considered to contain unique sequences and therefore were sequenced. Other plasmids that hybridized weakly were considered to contain divergent sequences were also sequenced.
Figure 2s. Microarray data analysis approach.
A. The Histogram Mode Centering (HMC) algorithm was used to normalize the signal intensity data to fit the log-ratio frequencies (gray dots) of the main peak to a Gaussian distribution function. We estimated the best fit with minimal sum of squared residuals between raw histogram data and a theoretical Gaussian curve (green line). Upon finding the best fit, the mode of the Gaussian function is calculated. The arrow represents the value shift (data transformation) based on the difference between the expected theoretical log-ratio (zero) and the mode of the fit normal distribution model. The standard deviation value of the normal distribution model was then used to calculate the percentiles (see below) corresponding to each log-ratio based on the z-score table.
B. Criteria used to assign gene presence in the reference or query genomes based on the log-ratio percentile values from the HMC.
Figure 3s Comparative cluster analysis of H. influenza strains. A. CGH clustering based on the global gene hybridization patterns (of 1720 70-mers, single-genome-array based on KW20/Rd genome). B. MLST-based dendrogram [7]. C. Dendrogram represents MLEE clustering (using Unweighted Pair Group Method with Arithmetic Mean analysis (UPGMA)) [7].