Abstract
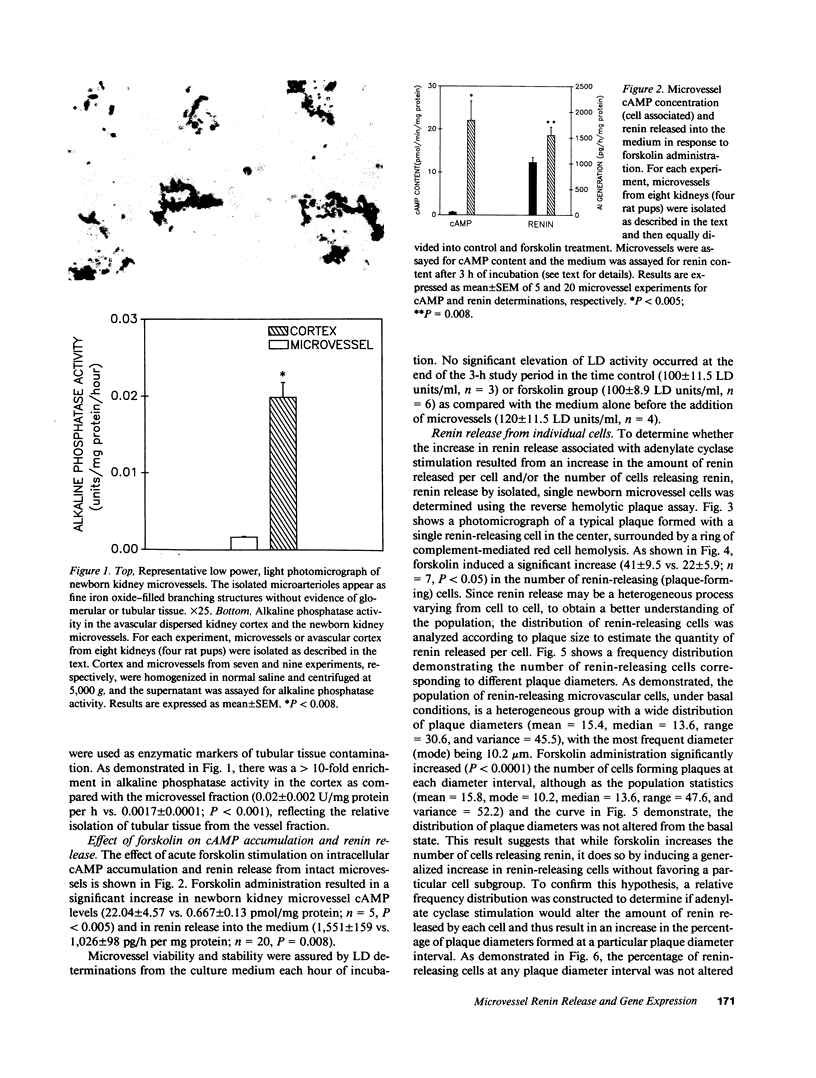
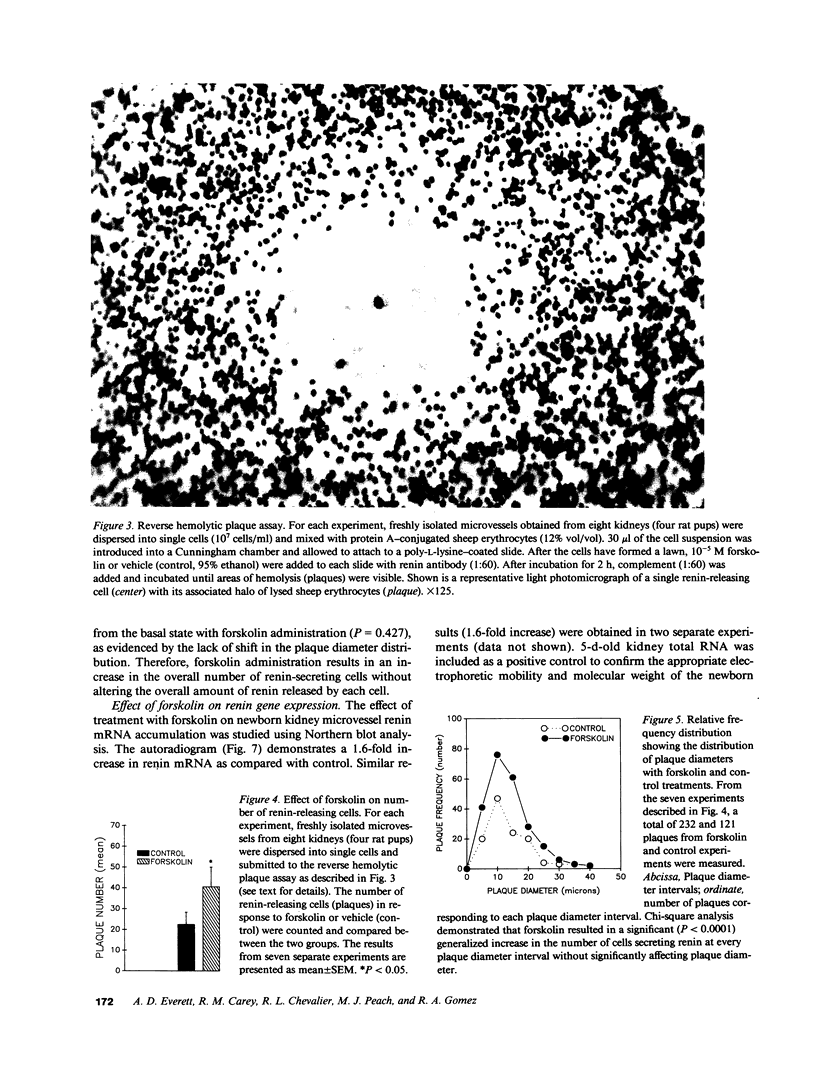
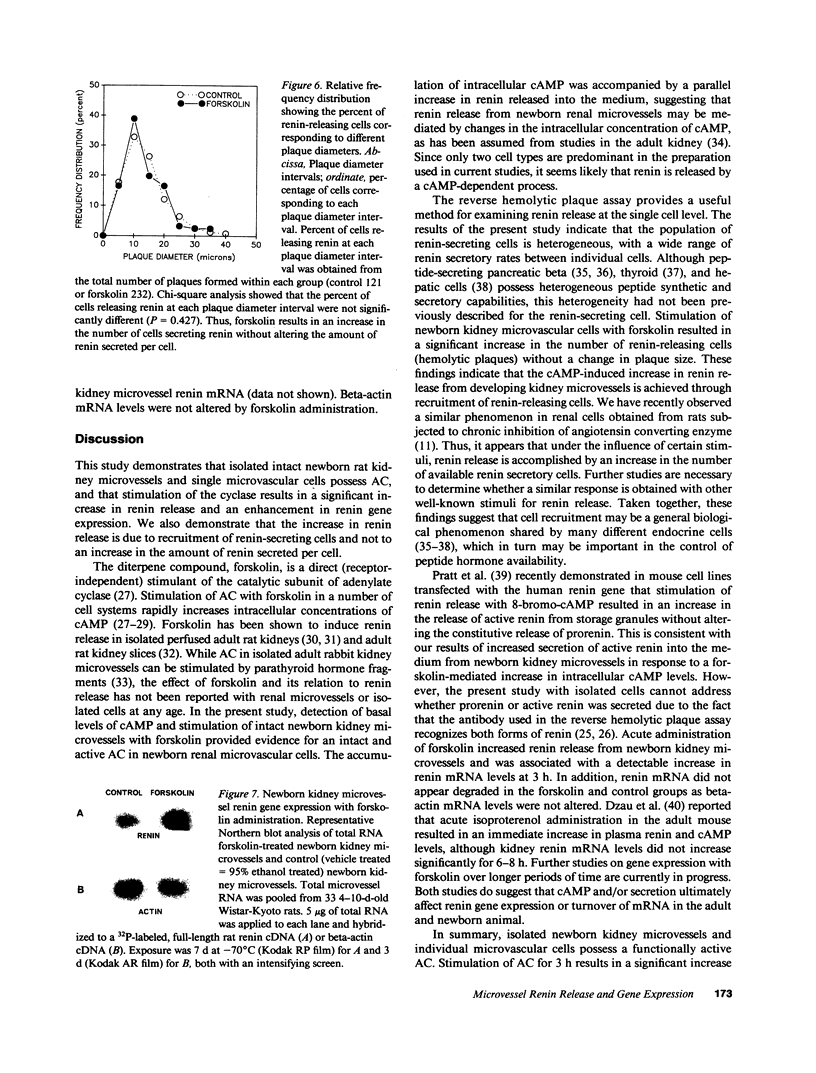
To investigate whether newborn kidney microvessels and isolated single microvascular cells have the capacity to release renin and/or alter the expression of the renin gene in response to adenylate cyclase stimulation, newborn kidney microvessels were isolated and purified (95%) using an iron perfusion/enzymatic digestion technique. Incubation of microvessels with either vehicle (control; C) or 10(-5) M forskolin (F) in media resulted in an increase in microvessel cAMP (0.67 +/- 0.13 vs. 22 +/- 4.6 pmol/min per mg protein) (P less than 0.005) and renin released into the culture media (1,026 +/- 98 vs. 1,552 +/- 159 pg angiotensin I/h per mg protein) (P = 0.008) (C vs. F). Renin mRNA levels in the newborn kidney microvessels increased 1.6-fold with forskolin treatment. Renin release by isolated, single microvascular cells (with or without forskolin) was assessed using the reverse hemolytic plaque assay. Forskolin administration resulted in an increase in the number of renin-secreting cells without changes in the amount of renin secreted by individual cells. In conclusion, newborn kidney microvessels and isolated renin-releasing microvascular cells possess a functionally active adenylate cyclase whose short-term stimulation results in accumulation of cAMP, a significant increase in renin release, and an enhancement of renin gene expression. The increase in renin release is due to recruitment of microvascular cells secreting renin. Recruitment of hormone-secreting cells in response to stimuli may prove to be a mechanism of general biological importance shared by many endocrine cell types.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burnham C. E., Hawelu-Johnson C. L., Frank B. M., Lynch K. R. Molecular cloning of rat renin cDNA and its gene. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5605–5609. doi: 10.1073/pnas.84.16.5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celio M. R., Groscurth P., Inagami T. Ontogeny of renin immunoreactive cells in the human kidney. Anat Embryol (Berl) 1985;173(2):149–155. doi: 10.1007/BF00316297. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill P. C. Second messengers in renin secretion. Am J Physiol. 1985 Aug;249(2 Pt 2):F175–F184. doi: 10.1152/ajprenal.1985.249.2.F175. [DOI] [PubMed] [Google Scholar]
- Davis J. O., Freeman R. H. Mechanisms regulating renin release. Physiol Rev. 1976 Jan;56(1):1–56. doi: 10.1152/physrev.1976.56.1.1. [DOI] [PubMed] [Google Scholar]
- Deutsch P. J., Hoeffler J. P., Jameson J. L., Lin J. C., Habener J. F. Structural determinants for transcriptional activation by cAMP-responsive DNA elements. J Biol Chem. 1988 Dec 5;263(34):18466–18472. [PubMed] [Google Scholar]
- Egerer G., Taugner R., Tiedemann K. Renin immunohistochemistry in the mesonephros and metanephros of the pig embryo. Histochemistry. 1984;81(4):385–390. doi: 10.1007/BF00514334. [DOI] [PubMed] [Google Scholar]
- Fray J. C., Park C. S. Forskolin and calcium: interactions in the control of renin secretion and perfusate flow in the isolated rat kidney. J Physiol. 1986 Jun;375:361–375. doi: 10.1113/jphysiol.1986.sp016121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukamizu A., Nishi K., Cho T., Saitoh M., Nakayama K., Ohkubo H., Nakanishi S., Murakami K. Structure of the rat renin gene. J Mol Biol. 1988 May 20;201(2):443–450. doi: 10.1016/0022-2836(88)90151-9. [DOI] [PubMed] [Google Scholar]
- Gomez R. A., Lynch K. R., Chevalier R. L., Wilfong N., Everett A., Carey R. M., Peach M. J. Renin and angiotensinogen gene expression in maturing rat kidney. Am J Physiol. 1988 Apr;254(4 Pt 2):F582–F587. doi: 10.1152/ajprenal.1988.254.4.F582. [DOI] [PubMed] [Google Scholar]
- Gomez R. A., Lynch K. R., Sturgill B. C., Elwood J. P., Chevalier R. L., Carey R. M., Peach M. J. Distribution of renin mRNA and its protein in the developing kidney. Am J Physiol. 1989 Nov;257(5 Pt 2):F850–F858. doi: 10.1152/ajprenal.1989.257.5.F850. [DOI] [PubMed] [Google Scholar]
- Harper J. F., Brooker G. Femtomole sensitive radioimmunoassay for cyclic AMP and cyclic GMP after 2'0 acetylation by acetic anhydride in aqueous solution. J Cyclic Nucleotide Res. 1975;1(4):207–218. [PubMed] [Google Scholar]
- Helwig J. J., Mandel P., Bollack C. Distribution of some phosphohydrolases in glomerular and tubular fractions isolated from rabbit kidney. Arch Int Physiol Biochim. 1974 Dec;82(5):907–916. doi: 10.3109/13813457409072338. [DOI] [PubMed] [Google Scholar]
- Helwig J. J., Yang M. C., Bollack C., Judes C., Pang P. K. Response of isolated renal microvessel and tubule adenylate cyclases to PTH fragments. Kidney Int Suppl. 1988 Sep;25:S45–S48. [PubMed] [Google Scholar]
- Ice K. S., Geary K. M., Gomez R. A., Johns D. W., Peach M. J., Carey R. M. Cell and molecular studies of renin secretion. Clin Exp Hypertens A. 1988;10(6):1169–1187. doi: 10.1080/07300077.1988.11878809. [DOI] [PubMed] [Google Scholar]
- Johns D. W., Carey R. M., Gomez R. A., Lynch K., Inagami T., Saye J., Geary K., Farnsworth D. E., Peach M. J. Isolation of renin-rich rat kidney cells. Hypertension. 1987 Nov;10(5):488–496. doi: 10.1161/01.hyp.10.5.488. [DOI] [PubMed] [Google Scholar]
- Keeton T. K., Campbell W. B. The pharmacologic alteration of renin release. Pharmacol Rev. 1980 Jun;32(2):81–227. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lacasse J., Ballak M., Mercure C., Gutkowska J., Chapeau C., Foote S., Ménard J., Corvol P., Cantin M., Genest J. Immunocytochemical localization of renin in juxtaglomerular cells. J Histochem Cytochem. 1985 Apr;33(4):323–332. doi: 10.1177/33.4.3884706. [DOI] [PubMed] [Google Scholar]
- Legrand A. B., Narayanan T. K., Ryan U. S., Aronstam R. S., Catravas J. D. Modulation of adenylate cyclase activity in cultured bovine pulmonary arterial endothelial cells. Effects of adenosine and derivatives. Biochem Pharmacol. 1989 Feb 1;38(3):423–430. doi: 10.1016/0006-2952(89)90381-x. [DOI] [PubMed] [Google Scholar]
- Leong D. A., Lau S. K., Sinha Y. N., Kaiser D. L., Thorner M. O. Enumeration of lactotropes and somatotropes among male and female pituitary cells in culture: evidence in favor of a mammosomatotrope subpopulation in the rat. Endocrinology. 1985 Apr;116(4):1371–1378. doi: 10.1210/endo-116-4-1371. [DOI] [PubMed] [Google Scholar]
- Lin C. T., Palmer W., Wu J. Y., Chan L. Estrogen induction of very low density apolipoprotein II synthesis, a major avian liver yolk protein, involves the recruitment of hepatocytes. Endocrinology. 1986 Feb;118(2):538–544. doi: 10.1210/endo-118-2-538. [DOI] [PubMed] [Google Scholar]
- Minuth M., Hackenthal E., Poulsen K., Rix E., Taugner R. Renin immunocytochemistry of the differentiating juxtaglomerular apparatus. Anat Embryol (Berl) 1981;162(2):173–181. doi: 10.1007/BF00306489. [DOI] [PubMed] [Google Scholar]
- Nakamura K. T., Page W. V., Sato T., Klinkefus J. M., Robillard J. E. Ontogeny of isoproterenol-stimulated renin secretion from sheep renal cortical slices. Am J Physiol. 1989 Jun;256(6 Pt 2):R1258–R1263. doi: 10.1152/ajpregu.1989.256.6.R1258. [DOI] [PubMed] [Google Scholar]
- Naruse K., Takii Y., Inagami T. Immunohistochemical localization of renin in luteinizing hormone-producing cells of rat pituitary. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7579–7583. doi: 10.1073/pnas.78.12.7579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C. S., Sigmon D. H., Han D. S., Honeyman T. W., Fray J. C. Control of renin secretion by Ca2+ and cyclic AMP through two parallel mechanisms. Am J Physiol. 1986 Sep;251(3 Pt 2):R531–R536. doi: 10.1152/ajpregu.1986.251.3.R531. [DOI] [PubMed] [Google Scholar]
- Pratt R. E., Flynn J. A., Hobart P. M., Paul M., Dzau V. J. Different secretory pathways of renin from mouse cells transfected with the human renin gene. J Biol Chem. 1988 Mar 5;263(7):3137–3141. [PubMed] [Google Scholar]
- Salomon D., Meda P. Heterogeneity and contact-dependent regulation of hormone secretion by individual B cells. Exp Cell Res. 1986 Feb;162(2):507–520. doi: 10.1016/0014-4827(86)90354-x. [DOI] [PubMed] [Google Scholar]
- Schuit F. C., In't Veld P. A., Pipeleers D. G. Glucose stimulates proinsulin biosynthesis by a dose-dependent recruitment of pancreatic beta cells. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3865–3869. doi: 10.1073/pnas.85.11.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwertschlag U., Hackenthal E. Forskolin stimulates renin release from the isolated perfused rat kidney. Eur J Pharmacol. 1982 Oct 15;84(1-2):111–113. doi: 10.1016/0014-2999(82)90165-0. [DOI] [PubMed] [Google Scholar]
- Seamon K. B., Padgett W., Daly J. W. Forskolin: unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3363–3367. doi: 10.1073/pnas.78.6.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson S. M., Jiang J. X., Chang Y. S., De Souza N. J., Pezzuto J. M. A rapid and sensitive bioassay involving cultured rat glioma cells to screen for substances capable of elevating intracellular cyclic AMP concentration. J Nat Prod. 1988 Sep-Oct;51(5):929–936. doi: 10.1021/np50059a019. [DOI] [PubMed] [Google Scholar]
- Takii Y., Figueiredo A. F., Inagami T. Application of immunochemical methods to the identification and characterization of rat kidney inactive renin. Hypertension. 1985 Mar-Apr;7(2):236–243. doi: 10.1161/01.hyp.7.2.236. [DOI] [PubMed] [Google Scholar]
- WROBLEWSKI F., LADUE J. S. Lactic dehydrogenase activity in blood. Proc Soc Exp Biol Med. 1955 Oct;90(1):210–213. doi: 10.3181/00379727-90-21985. [DOI] [PubMed] [Google Scholar]







