Abstract
Pulmonary involvement is common in patients with non-Hodgkin's lymphoma (NHL). 90Y- and 131I-anti-CD20 antibodies (ibritumomab tiuxetan and tositumomab, respectively) have been approved for the treatment of refractory low-grade follicular NHL. In this work, we used Monte Carlo–based dosimetry to compare the potential of 90Y and 131I, based purely on their emission properties, in targeted therapy for NHL lung metastases of various nodule sizes and tumor burdens.
Methods
Lung metastases were simulated as spheres, with radii ranging from 0.2 to 5.0 cm, which were randomly distributed in a voxelized adult male lung phantom. Total tumor burden was varied from 0.2 to 1,641 g. Tumor uptake and retention kinetics of the 2 radionuclides were assumed equivalent; a uniform distribution of activity within tumors was assumed. Absorbed dose to tumors and lung parenchyma per unit activity in lung tumors was calculated by a Monte Carlo–based system using the MCNP4B package. Therapeutic efficacy was defined as the ratio of mean absorbed dose in the tumor to that in normal lung. Dosimetric analysis was also performed for a lung-surface distribution of tumor nodules mimicking pleural metastatic disease.
Results
The therapeutic efficacy of both 90Y and 131I declined with increasing tumor burden. In treating tumors with radii less than 2.0 cm, 131I targeting was more efficacious than 90Y targeting. 90Y yielded a broader distribution of tumor absorbed doses, with the minimum 54.1% lower than the average dose; for 131I, the minimum absorbed dose was 33.3% lower than the average. The absorbed dose to normal lungs was reduced when the tumors were distributed on the lung surface. For surface tumors, the reductions in normal-lung absorbed dose were greater for 90Y than for 131I, but 131I continued to provide a greater therapeutic ratio across different tumor burdens and sizes.
Conclusion
Monte Carlo–based dosimetry was performed to compare the therapeutic potential of 90Y and 131I targeting of lung metastases in NHL patients. 131I provided a therapeutic advantage over 90Y, especially in tumors with radii less than 2.0 cm and at lower tumor burdens. For both 90Y- and 131I-labeled antibodies, treatment is more efficacious when applied to metastatic NHL cases with lower tumor burdens. 131I has advantages over 90Y in treating smaller lung metastases.
Keywords: non-Hodgkin's lymphoma, pulmonary metastases, dosimetry, Monte Carlo, 90Y, 131I
Intrathoracic involvement is common in non-Hodgkin's lymphoma (NHL). Pulmonary involvement is found in about 24% of NHL patients (1,2). Chylothorax due to lymphatic obstruction and leakage leads to pleural effusion, which is usually associated with widely disseminated disease and a poor prognosis (1). Many NHL patients will relapse after treatment, and previously indolent lymphomas can transform into a more aggressive histologic type. Once transformation occurs, NHL becomes highly refractory to most conventional therapies (3,4). Radioimmunotherapy, using anti-CD20 antibodies, has been approved as a new treatment modality for NHL. Both 90Y-labeled antibodies (Zevalin [ibritumomab tiuxetan]; IDEC Pharmaceuticals) and 131I-labeled antibodies (Bexxar [tositumomab]; GlaxoSmithKline) have shown good therapeutic efficacy for patients with relapsed or refractory low-grade follicular NHL (4–9). Both radioimmunoconjugates have been investigated with autologous hematopoietic stem cell transplantation and in combination with chemotherapy (10–14). In this setting, the lung was the dose-limiting organ, with a maximum tolerated dose of 27 Gy for 131I-tositumomab (10,12,15). In trials with myeloablative 90Y-ibritumomab tiuxetan, the dose-limiting organ was less well established, with normal organs receiving a target dose of 10 Gy (14). With both agents, the high-dose, myeloablative approach showed promising efficacy, especially when combined with chemotherapy (10,12).
Although both 90Y-ibritumomab tiuxetan and 131I-tositumomab have produced a good overall response in the treatment of low-grade refractory or relapsed transformed NHL, they are different in their pharmacokinetics and in vivo stability. Most importantly, the conjugated radionuclide 90Y is a pure β-emitter with an average energy of 935 keV and a half-life of 64 h, whereas 131I emits both β-rays with an average energy of 183 keV and γ-rays with a half-life of 192.5 h (Table 1). The physical properties of the 2 radioisotopes are a critical parameter in the choice of the 2 anti-CD20 radioimmunoconjugates. The putative advantages of 90Y in tumor targeting are its higher energy and longer pathlength (0.25 cm), which make it suitable for treating NHL tumors with diameters larger than 1.0 cm or with necrotic cores that require a cross-fire effect in order to be sterilized, whereas 131I is more suitable for targeting micrometastases (16). The higher doses delivered by 90Y to normal organs, however, limits the radioactivity that may be administered for therapy.
TABLE 1.
Physical Properties of 90Y and 131I
| Isotope | Half-life (h) | Radiation type | Energy (keV) | Mean range (cm) |
|---|---|---|---|---|
| 90Y | 64 | β | 935/2,280 | 0.25 |
| 131I | 192.5 | β | 183/807 | 0.04 |
| γ | 378/364 (81.7%) |
Data were obtained from Lund/LBNL Nuclear Data Search. β-Energy is presented as mean/maximum. γ-Energy is presented as mean/most abundant (abundance).
The presence of pulmonary metastases in NHL patients increases the dose absorbed by normal lungs, thus limiting the prescribed treatment activity. Accurate calculation of lung absorbed dose is difficult with the conventional whole-organ S value–based dosimetry or point-kernel approaches when disseminated tumor nodules are in the lungs (17). The low density of lung tissue invalidates the assumption that electron energy will be locally deposited, and disseminated disease leads to a heterogeneous tissue composition, coupled with a nonuniform activity distribution, thereby complicating the dose calculation. Monte Carlo–based dosimetry is better suited for such cases.
In this work, we used Monte Carlo–based dosimetry to compare the potential of 90Y and 131I, based purely on their emission properties, in labeled monoclonal antibody treatment of NHL lung metastases of various nodule sizes and tumor burdens. Assuming that the lungs are the dose-limiting organ in the myeloablative setting, the absorbed dose delivered to normal lung tissue served as the basis for comparing therapeutic efficacy.
MATERIALS AND METHODS
To investigate the relative efficacy of 90Y and 131I in the targeted therapy of lung metastases, we estimated the absorbed dose to simulated lung tumors and to normal lung parenchyma using Monte Carlo–based dosimetry. A voxelized representation of the Cristy–Eckerman adult male lung phantom was used as the normal-lung model into which tumor nodules were placed to simulate lung metastases (18). The comparison was based on the therapeutic ratio, defined as the absorbed dose to tumor divided by the absorbed dose to normal lung parenchyma.
Lung Phantom
Every voxel whose center lay within the surface of the lungs was included as part of the phantom. The lung phantom thus generated contained 52,700 voxels of 4 × 4 × 4 mm within a 72 × 64 × 48 voxel cube (Figs. 1A–1C). A 4-mm voxel size was chosen because it is a common voxel size in SPECT and PET. The mass of the voxelized lung phantom was 998 g, about 0.2% lower than that of the Cristy–Eckerman lung phantom. This phantom was then converted into the appropriate Monte Carlo code input format for dose calculation.
FIGURE 1.

(A–C) Voxelized Cristy-Eckerman lung phantom shown in transverse (A), coronal (B), and sagittal (C) projection views. (D) Coronal projection view of 72 randomly distributed tumors of 1.0-cm radius in voxelized lung phantom.
Monte Carlo Code
The MCNP4B Monte Carlo code (Oak Ridge National Laboratory) was used to perform the dosimetry calculations (19). The Monte Carlo methodology developed for lung dosimetry using this code, as well as its validation, has previously been described (17). The full photon and electron spectrums of 131I and 90Y were obtained from The Lund/LBNL Nuclear Data Search (version 2.0, 1999; http://nucleardata.nuclear.lu.se/nucleardata/toi/) (20). For all cases, a sufficient number of electron and photon transport histories were generated to produce statistically reliable energy tallies, with relative errors less than 0.10. Validation involved reproducing the 131I lung-to-lung S values found in OLINDA. The calculation was repeated for 90Y. The lung-to-lung self-absorbed doses for 131I and 90Y were about 6.0% lower and 13.4% lower, respectively, than those obtained from the OLINDA software package (21). The differences are likely explained by the fact that, in the MCNP4B package, electrons are assumed not to be deposited locally but to be transported explicitly.
Impact of Density on Self-Dose and Cross Dose
To examine the impact of density differences and β-particle emission energy on electron transport for 90Y relative to 131I, we calculated the energy deposition by electrons from 131I and 90Y in spheres with densities of 1.0 or 0.296 g/cm3. Spheres with radii of 50 μm to 5.0 cm were filled with water at a density of 1.0 g/cm3 or lung tissue at a density of 0.296 g/cm3. Activity was uniformly distributed within the spheres.
When tumors are present in the lung tissue, explicitly transported electrons are able to reach nearby tumors or normal lung tissues, delivering to tumors or normal tissues “cross-fire” doses with a pure β-emitter such as 90Y. To demonstrate this effect, we simulated a simplified scenario in which 2 spheres—a source sphere and a target sphere—of the same radius were filled with water and placed side by side in direct contact with each other. They were surrounded by either water or lung tissue. Activity was uniformly distributed in the source sphere, and the fraction of electron energy deposited in the neighboring target sphere was calculated when both sphere radii were varied simultaneously.
Therapeutic Efficacy of Tumors in Lungs
The therapeutic efficacy of 90Y- and 131I-labeled antibody in targeting different sizes of tumors at various tumor burdens was evaluated. The densities of tumor and normal lung tissue are 1.04 and 0.2958 g/cm3, respectively, in all subsequent calculations. Therapeutic efficacy was defined as the absorbed dose to tumors per unit of tumor-cumulated activity (mGy/MBq-s) divided by the absorbed dose to normal lungs per unit of tumor-cumulated activity (mGy/MBq-s). A series of phantoms were generated. Each phantom contained a uniformly distributed set of randomly placed computer-generated tumors of the same radius. Tumor radii ranged from 0.2 to 5.0 cm. None of the tumors overlapped. An example projection image of a lung phantom containing 72 tumors with a radius of 1 cm is shown in Figure 1D. A tumor with a radius of 1.0 cm on average consists of 81 voxels of 4 × 4 × 4 mm each. A tumor burden range of about 0.2–1,641 g, for each tumor size, was achieved by changing the number of tumors. Activity was assumed to be uniformly distributed in the tumors only, and no activity was assigned to normal lungs. Differences in pharmacokinetics and nonuniform antibody penetration in tumor nodules were not evaluated, nor were the possible differences in the fate of the radionuclide after radioantibody internalization. Nontumor voxels were designated as normal lung tissues. The specific absorbed dose to both tumors and lungs was then calculated after MCNP4B electron and photon transport for every tumor size and tumor burden. Therapeutic efficacies for both 90Y and 131I were calculated and compared.
A nonuniform absorbed dose distribution in tumors and lungs could compromise the dose response in tumors. The spatial distribution of absorbed dose was obtained for 1-cm tumor nodules randomly distributed throughout the lungs and a tumor burden of 5.4 g. The spatial distribution of absorbed dose by 131I and 90Y in both tumor and normal lung was represented using dose-volume histograms. The effects of a nonuniform dose distribution in the tumors were not investigated.
Surface-Distributed Tumors
The spatial distribution of tumor nodules within the lungs may considerably affect the absorbed dose to lungs and also to tumor. To examine this effect, we performed a dosimetric analysis with the centers of the tumors placed on the lung surfaces. The same radius and tumor burden distribution were used except that all the tumors were now randomly and uniformly distributed on the lung surfaces. For both 90Y and 131I, the absorbed dose to normal lungs was compared with that obtained when tumors were uniformly distributed throughout the lungs.
RESULTS
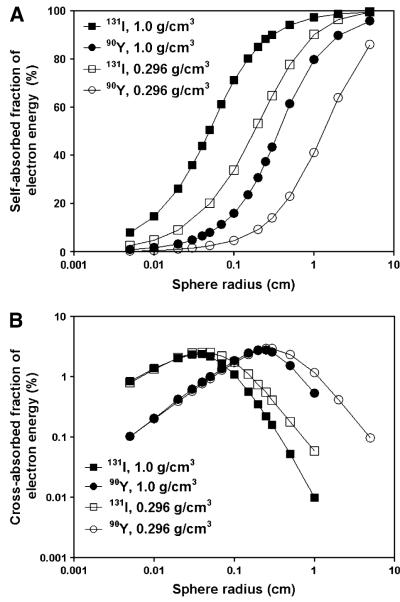
The impact of normal lung density on energy deposition by electrons is shown in Figure 2. Spheres of lung density absorbed a smaller fraction of electron energy both for 131I and for 90Y (Fig. 2A). The fraction of energy absorbed in spheres of radius 1.0 cm decreased from 97.3% to 90.2% for 131I and from 79.8% to 41.2% for 90Y. When tumors were surrounded by normal lung tissue, both 131I and 90Y electrons were able to deliver more cross-fire doses to the target sphere (Fig. 2B). Electrons from 90Y, because of their higher mean energy, have a longer mean range in both water and lung tissue than do electrons from 131I. For spheres with a 1.0-cm radius, the fraction of electron energy absorbed in the target sphere was 1.2% for 90Y, compared with 0.1% for 131I. The fraction of electron energy absorbed in the target sphere increased with increasing target sphere size but decreased with increasing source sphere radius. The maximum absorbed energy fraction (2.54% for 131I and 2.94% for 90Y) in the target sphere was reached at 0.04 cm for 131I and 0.25 cm for 90Y when the radii of both source and target sphere were the same.
FIGURE 2.
(A) Absorbed fraction of electron energy deposited in spheres with radii ranging from 50 μm to 5.0 cm. Spheres were filled with water (density of 1.0 g/cm3) or lung tissue (density of 0.296 g/cm3) and uniformly distributed with 90Y or 131I. (B) Absorbed fraction of electron energy deposited in neighboring target sphere by 90Y or 131I. Both source and target sphere were filled with water. They were surrounded by either water or lung tissue. Solid symbols indicate water-filled spheres; open symbols indicate lung-tissue-filled spheres.
The therapeutic efficacies of both 131I and 90Y declined as tumor burden increased. This reduction occurred regardless of tumor nodule size (Figs. 3A and 3B). For example, for tumors 1.0 cm in radius, the therapeutic efficacy ratio dropped from 720.3 to 2.8 for 90Y and from 1,607.9 to 6.1 for 131I when the tumor burden was increased from 5.4 to 1,552.7 g. This sharp deterioration can be attributed mainly to the increase in lung dose when activity concentration remains constant for larger tumor burdens. Therapeutic efficacy, however, was better for larger-diameter tumors at a constant tumor burden. This improvement was more evident when using 90Y than when using 131I. At a tumor burden that was approximately 12% of the total lung mass, the therapeutic efficacy for 2.0-cm tumors improved relative to that for 0.2-cm tumors by a factor of 6.1 with 90Y but only 2.2-fold with 131I. This difference in efficacy as a function of tumor size for the 2 isotopes is caused by the fact that 90Y is a pure β-emitter and, thus, that tumor absorbed energy is largely determined by tumor size. 131I also emits photons, the energy deposition of which, however, is less sensitive to tumor size.
FIGURE 3.
(A and B) Therapeutic efficacy of 131I (A) and 90Y (B) in treating tumor nodules in lungs. Tumors with radii ranging from 0.2 to 5.0 cm were randomly and uniformly distributed in lungs. Total tumor burden was varied from 0.2 to 1,641 g by varying the number of tumors. (C) Therapeutic efficacy ratio of 131I to 90Y for various tumor sizes and tumor burdens.
As shown in Figure 3C, the therapeutic efficacy of 131I was greater than that of 90Y across all tumor nodule sizes and tumor burdens considered. The therapeutic ratio calculated for 131I was 6.0 times higher than that for 90Y when tumor radius was 0.2 cm and tumor burden was 0.2 g; the ratio was 3.0 when total tumor burden was 859.6 g. This decline can be attributed in part to an increased tumor dose from 90Y at a higher tumor burden because of the cross-fire effects. Although electron energy is usually assumed to be deposited locally, for 90Y electrons of an average 935 keV, electron energy could still reach neighboring tumors in low-density lung tissue. For larger tumors, the therapeutic advantage of 131I over 90Y decreased. When tumor radius reached 2.0 cm, both 131I and 90Y yielded therapeutic efficacy ratios of 1.4. For 5.0-cm tumors, the therapeutic efficacy for 131I was about 30% smaller than that for 90Y. The indicated tumor burden range does not seem to affect the therapeutic efficacy ratio between 131I and 90Y for tumors with radii larger than 1.0 cm. As shown in Figure 2B, this is most likely because the cross-fire dose to adjacent tumor nodules decreased with increasing tumor size.
The dose–volume histograms of absorbed dose within tumors and normal lungs are shown in Figure 4. With higher-energy electrons, 90Y delivers a greater average absorbed dose to tumors per disintegration than does 131I. The average absorbed doses to 1.0-cm randomly positioned tumors were 2.72 × 10−2 and 6.86 × 10−3 mGy/MBq-s for 90Y and 131I, respectively. The dose–volume histograms, however, revealed a wider dose distribution for 90Y relative to that for 131I: The minimum absorbed doses for 90Y and 131I were 54.1% less and 33.3% less, respectively, than the respective mean values. Such a distribution will lead to tumor underdosing by 90Y when the average tumor absorbed dose from 131I matches that of 90Y. Although absorbed dose in every voxel of 90Y is larger than that of 131I, this is counterbalanced by toxicity constraints. Therefore, the administered activity of 90Y is usually smaller than that of 131I. The dose–volume histogram for normal-lung volume is shown in Figure 4B. The average absorbed doses to the lungs were 3.06 × 10−5 and 3.45 × 10−6 mGy/MBq-s for 90Y and 131I, respectively. The maximum single-voxel doses reached 9.43 × 10−3 and 1.94 × 10−4 mGy/MBq-s, respectively. 90Y and 131I also have distinctive dose distributions in normal lungs. Because 90Y emits only electrons, the normal-lung volume it can irradiate is limited, with about 7% of the lung receiving a radiation dose greater than 1.28 × 10−9 mGy/MBq-s when a tumor of radius 1.0 cm is the single source. For 131I, however, every single voxel (100%) of normal-lung volume had significant energy deposited in it, mainly from the 131I photons. As a result, lung voxels that do receive energy from 90Y have much higher doses than the corresponding mean lung dose, whereas the dose distribution from 131I is more uniform than that from 90Y and the absorbed dose to a given normal-lung voxel is closer to the mean lung dose. This is demonstrated by the fact that for 90Y, the maximum single-voxel dose was 3.1 times higher than the mean dose, and for 131I, the maximum single-voxel dose was 55.4% higher than the mean dose.
FIGURE 4.
Dose-volume histograms calculated voxel by voxel with Monte Carlo-based dosimetry for tumors (A) and normal lung tissues (B) using either 131I or 90Y as sources. Mean specific absorbed doses to tumors and normal lungs are also shown for 131I (dotted line) and 90Y (dashed line).
The effect of surface distribution versus random distribution of tumor nodules was examined in terms of the absorbed dose ratio to normal lungs. Calculations were performed for both 131I and 90Y (Figs. 5A and B). The results showed that larger tumors tended to result in a relatively lower lung dose when they were located on the surface. For instance, with a tumor burden of about 60 g (about 6% of lung mass) in the lungs, the 131I absorbed dose ratio from surface tumors to randomly positioned tumors decreased from 0.77 for tumors with radii of 0.2 cm to 0.54 for tumors with radii of 2.0 cm. The reduction in normal-lung absorbed dose for lung-surface tumors with larger radii occurred because the tumor tissue was farther from the lung surface, resulting in less dose delivered to the lung tissues. The decrease in normal-lung absorbed dose leads to an improved therapeutic efficacy for both 90Y and 131I. However, this improvement is more evident for 90Y than for 131I, because photons from 131I make the lung dose less sensitive to variations in source positions. As shown in Figure 5C, the therapeutic efficacy of 131I is still higher than that of 90Y: For tumors with a radius of 0.2 cm, the maximum therapeutic efficacy ratio for 131I to 90Y decreased from 6.0 when the tumors were uniformly distributed in the lungs (Fig. 3C) to 5.3 when the tumors were on the lung surface.
FIGURE 5.
(A and B) Ratios of specific absorbed doses by 131I (A) and 90Y (B) to normal lungs when tumors were uniformly distributed in lungs or on lung surface. (C) Therapeutic efficacy ratio of 131I to 90Y. Tumor radii are from 0.2 to 2.0 cm.
DISCUSSION
Both 90Y-ibritumomab tiuxetan and 131I-tositumomab have been approved for treating relapsed or refractory low-grade follicular NHL and have shown similar overall objective responses. The choice of radioimmunoconjugates is of great interest for NHL patients at various stages of disease and with different tumor burdens and tumor nodule sizes. Pulmonary involvement in NHL is common clinically. In this work, we used Monte Carlo–based dosimetry to compare the efficacies of 90Y-ibritumomab tiuxetan and 131I-tositumomab, based purely on the emission properties of 90Y and 131I, in treating lung metastases in NHL patients. The absorbed dose to normal lung parenchyma served as the basis for comparison.
The 131I-based radioimmunoconjugate showed substantially better therapeutic efficacy than the 90Y-labeled radioantibody when targeting smaller tumors (radius < 2.0 cm) at a low total tumor burden. For larger tumors (radius > 2.0 cm), 90Y-labeled radioantibody showed increasingly better therapeutic efficacy. These findings were obtained with the assumption that both radiolabeled antibodies had identical targeting and pharmacokinetic properties. This approach made it possible to evaluate the impact of differences in their emission properties, all other things being equal. The pharmacokinetics could, however, greatly affect the limiting toxic doses and, thereby, the clinically achievable therapeutic efficacy. 131I-Tositumomab has a more varied clearance among patients and therefore required pretreatment patient-specific dosimetry analysis (3,22). In a myeloablative setting, the lungs have been reported to be the dose-limiting organ for these patients, especially if there is pulmonary involvement. In such cases, the amount administered for therapy will be driven by the absorbed dose expected in the lungs. Assuming a maximum tolerated dose to the lungs, the ratio of activity retention between tumor and lung becomes the critical parameter in determining therapeutic efficacy. For whole-antibody–based radioimmunotherapy, the ratio of tumor to normal tissue is usually between 1.0 and 2.0, depending on the type of cancer being targeted (23). A past study has also reported a tumor-to-lung absorbed dose ratio of 1.5 using myeloablative doses of 131I-tositumomab and stem cell transfer (15). In this study, we assumed, under the ideal situation, that activity was distributed only within the tumors. Including activity within the normal lungs would certainly lower the therapeutic efficacy for both 90Y and 131I. Given the high-energy β-emissions of 90Y, however, it may be expected that the reduction would be greater for 90Y than for 131I.
The model-derived results provided here, however, neglect several potentially important factors that might affect response and toxicity. A particularly important one is dose rate (24). With a half-life of 64 h, a greater dose-rate effect may be expected from 90Y than from 131I, which has a 192.5-h half-life. The repair rate of normal lung parenchymal cells versus NHL tumor cells and the growth rate of the tumor will affect the degree to which dose rate is important Thus, the ability of normal lung tissue to recover from radiation damage, and the growth of specific NHL tumors that can be controlled by dose rate, need to be determined (24).
Although 90Y has been predicted to be less effective in smaller tumors (radius < 2.0 cm), this prediction was based on the assumption that activity is uniformly distributed in the whole tumor volume. This prediction is not, however, realistic, because tumors with a diameter larger than 2.0 cm may demonstrate nonuniform uptake of the radiolabeled antibody. Under such circumstances, a 90Y-labeled radioantibody may have a better therapeutic efficacy because of the cross-fire effects from its more energetic electrons. Correspondingly, the dose distribution on the dose–volume histogram may be better for 90Y than for 131I. Clinically, NHL tumors with diameters larger than 1.0 cm may show better responses with 90Y-ibritumomab tiuxetan (16).
Radioiodine-labeled antibody is internalized and catabolized by tumor cells to release iodotyrosine, which accumulates rapidly in the thyroid and stomach. Such antibody deiodination will not only increase thyroid dose but also directly affect retention of radioiodine at the tumor site. Tumor doses may therefore be smaller for 131I-labeled antibody than for radionuclides using stable metal chelates that achieve higher tumor retention. Normal-lung dose, which is part of therapeutic efficacy, will also decrease because of dehalogenation. Accurate dose estimation requires awareness of the dehalogenation kinetics from both tumors and normal lungs. Improved iodine labeling with residualizing peptide helps to enhance radioiodine retention in tumors and the therapeutic efficacy of 131I-labeled radioantibodies (25).
Several animal studies have compared the therapeutic efficacies of 90Y- and 131I-labeled antibodies. In most subcutaneous xenograft models (including a lung carcinoma model), 90Y appears to have superior therapeutic efficacy over 131I (26–30), whereas in peritoneal (31) and lung metastasis (32) models, 131I has resulted in better tumor responses. These findings are consistent with the results of this study and with the conclusion that 131I is more suitable than 90Y for treatment of small lung metastases at a low tumor burden.
CONCLUSION
Monte Carlo–based dosimetry was performed to compare the therapeutic potentials of 90Y- and 131I-labeled antibodies in targeting lung metastases in NHL patients. Therapeutic efficacy was better for 131I-labeled antibody than for 90Y-labeled antibody for smaller tumors with radii less than 2.0 cm and at lower tumor burdens. The comparison considered only the emission properties of the 2 radionuclides. The impact of differences in pharmacokinetics, dose-rate effects, nonuniform activity distribution, and radioimmunoconjugate stability was discussed.
ACKNOWLEDGMENTS
This work was supported, in part, by grant R01CA116477 from the National Institutes of Health, grant DE-FG02-05ER63967 from the Department of Energy, and grant BC044176 from the Department of Defense (a Multidisciplinary Postdoctoral Fellowship to one of the authors).
REFERENCES
- 1.Berkman N, Breuer R, Kramer MR, Polliack A. Pulmonary involvement in lymphoma. Leuk Lymphoma. 1996;20:229–237. doi: 10.3109/10428199609051612. [DOI] [PubMed] [Google Scholar]
- 2.Maturen KE, Blane CE, Strouse PJ, Fitzgerald JT. Pulmonary involvement in pediatric lymphoma. Pediatr Radiol. 2004;34:120–124. doi: 10.1007/s00247-003-1080-9. [DOI] [PubMed] [Google Scholar]
- 3.Juweid ME. Radioimmunotherapy of B-cell non-Hodgkin's lymphoma: from clinical trials to clinical practice. J Nucl Med. 2002;43:1507–1529. [PubMed] [Google Scholar]
- 4.Fisher RI, Kaminski MS, Wahl RL, et al. Tositumomab and iodine-131 tositumomab produces durable complete remissions in a subset of heavily pretreated patients with low-grade and transformed non-Hodgkin's lymphomas. J Clin Oncol. 2005;23:7565–7573. doi: 10.1200/JCO.2004.00.9217. [DOI] [PubMed] [Google Scholar]
- 5.Witzig TE, Flinn IW, Gordon LI, et al. Treatment with ibritumomab tiuxetan radioimmunotherapy in patients with rituximab-refractory follicular non-Hodgkin's lymphoma. J Clin Oncol. 2002;20:3262–3269. doi: 10.1200/JCO.2002.11.017. [DOI] [PubMed] [Google Scholar]
- 6.Witzig TE, Gordon LI, Cabanillas F, et al. Randomized controlled trial of yttrium-90-labeled ibritumomab tiuxetan radioimmunotherapy versus rituximab immunotherapy for patients with relapsed or refractory low-grade, follicular, or transformed B-cell non-Hodgkin's lymphoma. J Clin Oncol. 2002;20:2453–2463. doi: 10.1200/JCO.2002.11.076. [DOI] [PubMed] [Google Scholar]
- 7.Vose JM, Wahl RL, Saleh M, et al. Multicenter phase II study of iodine-131 tositumomab for chemotherapy-relapsed/refractory low-grade and transformed low-grade B-cell non-Hodgkin's lymphomas. J Clin Oncol. 2000;18:1316–1323. doi: 10.1200/JCO.2000.18.6.1316. [DOI] [PubMed] [Google Scholar]
- 8.Kaminski MS, Tuck M, Estes J, et al. 131I-tositumomab therapy as initial treatment for follicular lymphoma. N Engl J Med. 2005;352:441–449. doi: 10.1056/NEJMoa041511. [DOI] [PubMed] [Google Scholar]
- 9.Kaminski MS, Estes J, Zasadny KR, et al. Radioimmunotherapy with iodine (131)I tositumomab for relapsed or refractory B-cell non-Hodgkin lymphoma: updated results and long-term follow-up of the University of Michigan experience. Blood. 2000;96:1259–1266. [PubMed] [Google Scholar]
- 10.Press OW, Eary JF, Gooley T, et al. A phase I/II trial of iodine-131-tositumomab (anti-CD20), etoposide, cyclophosphamide, and autologous stem cell transplantation for relapsed B-cell lymphomas. Blood. 2000;96:2934–2942. [PubMed] [Google Scholar]
- 11.Press OW, Eary JF, Appelbaum FR, et al. Radiolabeled-antibody therapy of B-cell lymphoma with autologous bone marrow support. N Engl J Med. 1993;329:1219–1224. doi: 10.1056/NEJM199310213291702. [DOI] [PubMed] [Google Scholar]
- 12.Gopal AK, Gooley TA, Maloney DG, et al. High-dose radioimmunotherapy versus conventional high-dose therapy and autologous hematopoietic stem cell transplantation for relapsed follicular non-Hodgkin lymphoma: a multivariable cohort analysis. Blood. 2003;102:2351–2357. doi: 10.1182/blood-2003-02-0622. [DOI] [PubMed] [Google Scholar]
- 13.Winter JN, Inwards D, Erwin W, et al. Zevalin dose escalation followed by high-dose BEAM and autologous peripheral blood progenitor cell (PBPC) transplant in non-Hodgkin's lymphoma: early outcome results. Blood. 2002;100(suppl 1):411a. abstract. [Google Scholar]
- 14.Nademanee A, Molina A, Forman SJ. A phase I/II trial of high-dose radioimmunotherapy (RIT) with Zevalin in combination with high-dose etoposide (VP-16) and cyclophosphamide (CY) followed by autologous stem cell transplant (ASCT) in patients with poor-risk or relapsed B-cell non-Hodgkin's lymphoma (NHL) Blood. 2002;100(suppl 1):182a. abstract. [Google Scholar]
- 15.Liu SY, Eary JF, Petersdorf SH, et al. Follow-up of relapsed B-cell lymphoma patients treated with iodine-131-labeled anti-CD20 antibody and autologous stem-cell rescue. J Clin Oncol. 1998;16:3270–3278. doi: 10.1200/JCO.1998.16.10.3270. [DOI] [PubMed] [Google Scholar]
- 16.Silverman DH, Delpassand ES, Torabi F, Goy A, McLaughlin P, Murray JL. Radiolabeled antibody therapy in non-Hodgkins lymphoma: radiation protection, isotope comparisons and quality of life issues. Cancer Treat Rev. 2004;30:165–172. doi: 10.1016/j.ctrv.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 17.Song H, He B, Prideaux A, et al. Lung dosimetry for radioiodine treatment planning in the case of diffuse lung metastases. J Nucl Med. 2006;47:1985–1994. [PMC free article] [PubMed] [Google Scholar]
- 18.Cristy M, Eckerman KF. Specific Absorbed Fractions of Energy at Various Ages for Internal Photon Sources. Oak Ridge National Laboratory; Oak Ridge, TN: 1987. pp. 25–26. ORNL/TM-8381. [Google Scholar]
- 19.Yoriyaz H, dos Santos A, Stabin MG, Cabezas R. Absorbed fractions in a voxel-based phantom calculated with the MCNP-4B code. Med Phys. 2000;27:1555–1562. doi: 10.1118/1.599021. [DOI] [PubMed] [Google Scholar]
- 20.Audi G, Wapstra AH. Masses: Q-values and nucleon separation energies—the 1995 update to the atomic mass evaluation. Nucl Phys A. 1995;595:409–480. [Google Scholar]
- 21.Stabin MG, Sparks RB, Crowe E. OLINDA/EXM: the second-generation personal computer software for internal dose assessment in nuclear medicine. J Nucl Med. 2005;46:1023–1027. [PubMed] [Google Scholar]
- 22.Wahl RL. Tositumomab and 131I therapy in non-Hodgkin's lymphoma. J Nucl Med. 2005;46(suppl 1):128S–140S. [PubMed] [Google Scholar]
- 23.Norrgren K, Strand SE, Nilsson R, Lindgren L, Sjogren HO. A general, extracorporeal immunoadsorption method to increase the tumor-to-normal tissue ratio in radioimmunoimaging and radioimmunotherapy. J Nucl Med. 1993;34:448–454. [PubMed] [Google Scholar]
- 24.Dale RG. Dose-rate effects in targeted radiotherapy. Phys Med Biol. 1996;41:1871–1884. doi: 10.1088/0031-9155/41/10/001. [DOI] [PubMed] [Google Scholar]
- 25.Stein R, Govindan SV, Mattes MJ, et al. Improved iodine radiolabels for monoclonal antibody therapy. Cancer Res. 2003;63:111–118. [PubMed] [Google Scholar]
- 26.Stein R, Chen S, Haim S, Goldenberg DM. Advantage of yttrium-90-labeled over iodine-131-labeled monoclonal antibodies in the treatment of a human lung carcinoma xenograft. Cancer. 1997;80(suppl 12):2636–2641. doi: 10.1002/(sici)1097-0142(19971215)80:12+<2636::aid-cncr39>3.3.co;2-n. [DOI] [PubMed] [Google Scholar]
- 27.Cardillo TM, Ying Z, Gold DV. Therapeutic advantage of (90)yttrium- versus (131)iodine-labeled PAM4 antibody in experimental pancreatic cancer. Clin Cancer Res. 2001;7:3186–3192. [PubMed] [Google Scholar]
- 28.Vallabhajosula S, Smith-Jones PM, Navarro V, Goldsmith SJ, Bander NH. Radioimmunotherapy of prostate cancer in human xenografts using monoclonal antibodies specific to prostate specific membrane antigen (PSMA): studies in nude mice. Prostate. 2004;58:145–155. doi: 10.1002/pros.10281. [DOI] [PubMed] [Google Scholar]
- 29.Brouwers AH, van Eerd JE, Frielink C, et al. Optimization of radio-immunotherapy of renal cell carcinoma: labeling of monoclonal antibody cG250 with 131I, 90Y, 177Lu, or 186Re. J Nucl Med. 2004;45:327–337. [PubMed] [Google Scholar]
- 30.Stein R, Govindan SV, Chen S, et al. Radioimmunotherapy of a human lung cancer xenograft with monoclonal antibody RS7: evaluation of 177Lu and comparison of its efficacy with that of 90Y and residualizing 131I. J Nucl Med. 2001;42:967–974. [PubMed] [Google Scholar]
- 31.Koppe MJ, Bleichrodt RP, Soede AC, et al. Biodistribution and therapeutic efficacy of 125/131I-, 186Re-, 88/90Y-, or 177Lu-labeled monoclonal antibody MN-14 to carcinoembryonic antigen in mice with small peritoneal metastases of colorectal origin. J Nucl Med. 2004;45:1224–1232. [PubMed] [Google Scholar]
- 32.Sharkey RM, Blumenthal RD, Behr TM, et al. Selection of radioimmunoconju-gates for the therapy of well-established or micrometastatic colon carcinoma. Int J Cancer. 1997;72:477–485. doi: 10.1002/(sici)1097-0215(19970729)72:3<477::aid-ijc16>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]