Abstract
Oxidant stress has been implicated in the etiology and pathogenesis of atherothrombotic vascular disease. Elevated levels of reactive oxygen species, resulting from increased production and/or decreased antioxidant capacity, modulate the vessel wall phenotype to create an environment that facilitates the progression of atherosclerosis. Herein, we review a number of biochemical mechanisms by which oxidant stress mediates atherosclerotic lesion formation and progression.
Atherosclerosis remains a significant cause of morbidity and mortality in developed nations around the world. Recent estimates from the World Health Organization attribute 16.7 millions deaths (> 29% of all deaths globally) to atherosclerotic cardiovascular disease [1]. While there is consensus regarding the nature of commonplace cardiovascular disease risk factors, including hypertension, aberrant lipid profiles, tobacco use, diabetes mellitus, and family history, controversy still exists at a cellular and molecular level as to the precise initial perturbation(s) to the vessel wall that incite the cascade of cellular events resulting in atherosclerotic lesion formation and consequent vascular dysfunction. As such, a number of hypotheses have been advanced to explain the etiology and progression of atherosclerosis; common to these theories is a disruption of normal homeostatic mechanisms to engender an environment that favors oxidant stress.
Oxidant stress defines a state in which the level of reactive oxygen species (ROS) exists in excess of antioxidant defenses. This imbalance in the redox milieu results in a switch from ROS-stimulated ambient signaling processes to ROS-mediated pathophysiological consequences. In the vasculature, oxidant stress may result from either overproduction of ROS and/or a decrease in antioxidant capacity; when either predominates in the vessel wall, the net result is a ROS-mediated decrease in bioavailable nitric oxide and oxidative modification of lipids and proteins leading to impaired vasomotor reactivity, inflammation, and dysregulated cell proliferation [2]. As this pro-oxidant environment recapitulates that observed in atherothrombotic vascular disease, it is, therefore, not surprising that oxidant stress has been implicated in the pathogenesis and progression of atherosclerosis and that evidence of increased oxidant stress has been confirmed in atherosclerotic human coronary arteries and plaques [3,4].
Determinants of cellular ROS levels
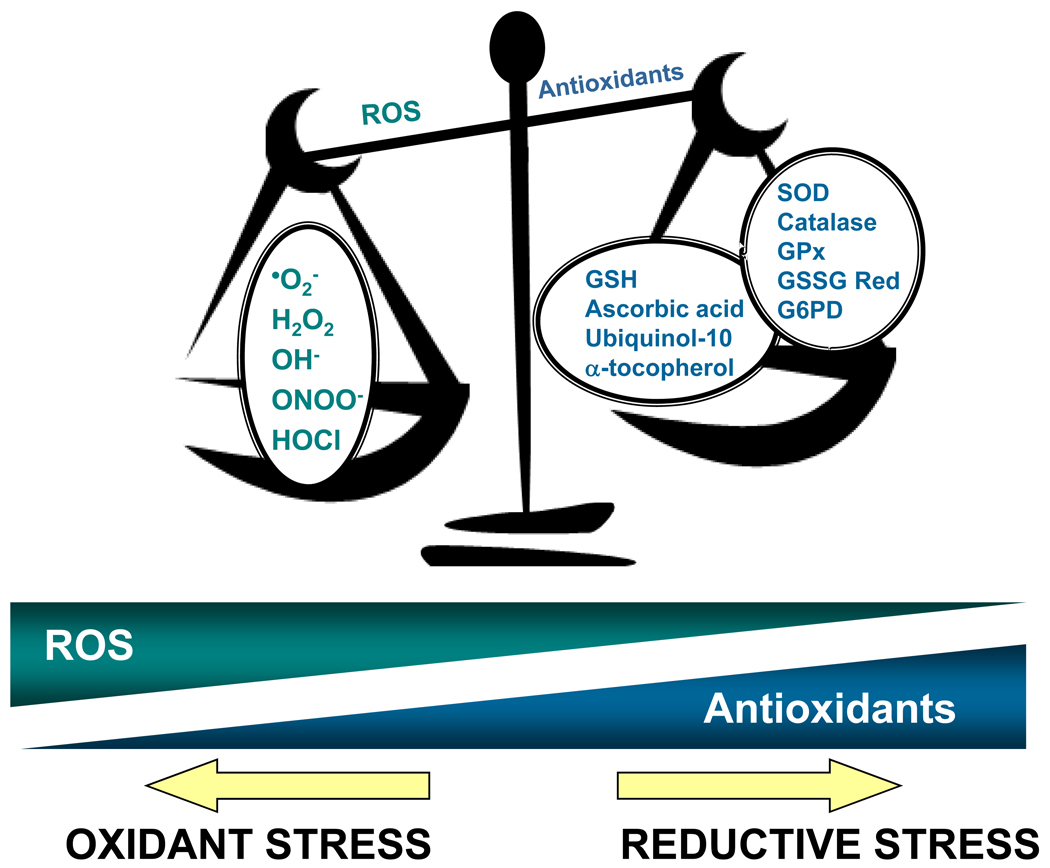
Oxidant stress may result from either an overproduction of ROS relative to antioxidant capacity, a decrease in antioxidant ability to limit ROS accumulation, or a combination of both mechanisms (Figure 1). Within the cell, there are numerous ROS generating and ROS metabolizing systems such that perturbation of any component will result in a state of oxidant stress. Under ambient conditions, basal levels of cellular respiration in an oxygen-rich environment yields abundant derivatives of redox-active partially reduced forms of molecular oxygen, which are collectively termed ROS [5]. Superoxide anion, a one-electron reduction product of oxygen, is the principal source from which most other ROS are derived. Superoxide anion is generated by metabolic and enzymatic sources, including mitochondrial respiration [6], NAD(P)H oxidases [7], xanthine oxidase [8], cyclooxygenases and lipoxygenases [9], and, when substrate or cofactors are deficient, is the byproduct of uncoupled nitric oxide synthase(s) [10–12]. It should be noted that superoxide anion, as well as its metabolic product hydrogen peroxide, serve as key molecules in signaling cascades that regulate homeostatic cellular functions. Transient elevations in ROS may beneficially amplify these processes when required; however, in the setting of a prolonged increased ROS accumulation in the absence of a physiological stimulus, oxidant stress ensues.
Figure 1. Determinants of redox balance.
In the vessel wall, the redox milieu is tightly regulated and determined by the net balance between reactive oxygen species (ROS) generation and metabolism by antioxidant small molecules and enzymes. When there is an increase in ROS production and/or a decrease in antioxidant capacity, a state of oxidant stress ensues. In contrast, when ROS are depleted to the extent that ambient ROS-dependent signaling processes are affected and the net redox balance favors a reducing environment, a state of reductive stress exists. This paradigm highlights the importance of maintaining the balance between ROS accumulation and antioxidant function. •O2−, superoxide; H2O2, hydrogen peroxide; OH−, hydroxyl radical; ONOO−, peroxynitrite; HOCl, hypochlorous acid; GSH, reduced glutathione; SOD, superoxide dismutase(s); GPx, glutathione peroxidase(s); GSSG Red, glutathione reductase; G6PD, glucose-6-phosphate dehydrogenase.
To limit basal ROS accumulation and pathophysiological sequelae, the cellular redox state is balanced by a number of small molecule antioxidants, including reduced glutathione, ascorbic acid, α-tocopherol, and ubiquinol-10; and antioxidant enzymes that limit intra- and extra-cellular accrual of deleterious reactive oxygen metabolites. These antioxidant enzymes include the superoxide dismutases, which convert superoxide anion into hydrogen peroxide; catalase, which reduces hydrogen peroxide to water; the glutathione peroxidases, which reduce both hydrogen peroxide and lipid peroxides to water and lipid alcohols, respectively; glutathione reductase, which reduces glutathione disulfide to reduced glutathione to maintain intracellular levels of this small molecule antioxidant species; the glutathione-S- transferases, which glutathiolate oxidants as a detoxification mechanism; the thiol-disulfide oxidoreductases and peroxiredoxins, which preserve protein thiol redox state and limit oxidative modification of protein thiols; heme oxygenase, which degrades heme to biliverdin and carbon monoxide (CO) (with the release of free iron ions); and glucose-6-phosphate dehydrogenase, the principal intracellular source of NADPH that is utilized as both a reducing equivalent and cofactor for other antioxidant enzymes. As the activity of these antioxidant enzymes are a critical determinant of cellular redox balance, it is, therefore, not surprising that heritable polymorphisms that decrease expression or activity of these enzymes contribute significantly to increased oxidant stress and have been associated with atherothrombotic vascular disease (reviewed in: [12]).
Mechanism of ROS-mediated oxidation of lipids and proteins
Once formed, superoxide anions are free to react with other species containing unpaired electrons, such as nitric oxide, or with side chains of lipids and proteins in the local environment. In this manner, other reactive species with pathophysiological relevance for the pathogenesis of atherosclerosis, such as hydroxyl radicals, hydrogen and lipid peroxides, and peroxynitrites, are formed [13]. These reactive species, in turn, may engage in further reactions to generate more potent oxidants, as occurs when neutrophil myeloperoxidase catalyzes the reaction of hydrogen peroxide and chloride anion to yield hypochlorous acid [13].
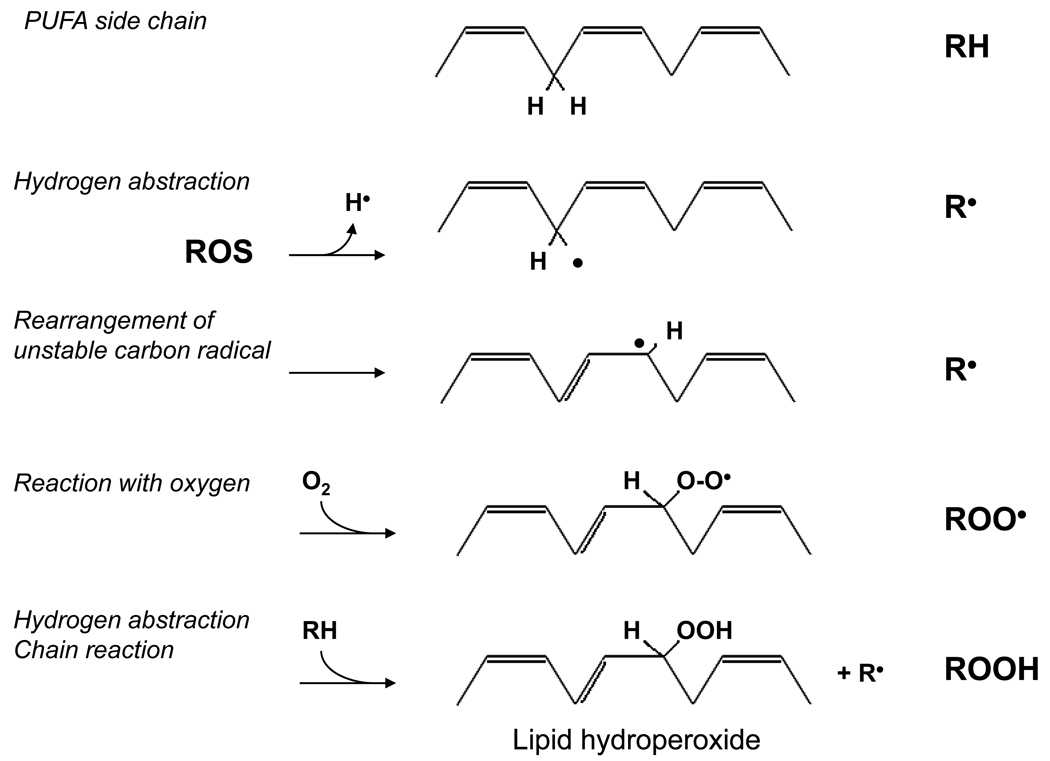
The reaction between superoxide anion and nitric oxide, which itself contains an unpaired electron, to form peroxynitrite occurs rapidly and is diffusion-limited. Superoxide anion or other free radicals with unpaired electrons may also react with other non-radical species in a process that involves donation or abstraction of a hydrogen radical (H•) from a C-H, O-H, or S-H bond present in the non-radical compound. These types of reactions tend to occur preferentially between free radicals and low-molecular-weight antioxidants, nucleic acids, sugar moieties, proteins, and lipids [14]. This, in fact, is the mechanism by which free radicals initiate lipid peroxidation and, thereby, atherosclerotic lesion formation. Reactive oxygen species react with hydrogen atoms attached to carbons of fatty acyl side chains rendering the remaining carbon radical available to react freely with molecular oxygen and generate a lipid peroxyl radical. The newly formed lipid peroxyl radical is highly reactive and itself initiates a chain reaction by reacting with nearby lipid molecules to yield additional lipid peroxyl radicals and lipid hydroperoxides (Figure 2) [14].
Figure 2. Reactive oxygen species and lipid peroxidation.
Reactive oxygen species (ROS) initiate lipid peroxidation by abstracting H• from a polyunsaturated fatty acid (PUFA) side chain of a lipid species (RH). The resulting lipid carbon radical (R•) is unstable and undergoes molecular rearrangement to form a conjugated diene. This radical reacts with molecular oxygen (O2) to form a highly reactive lipid peroxyl radical (ROO•). The lipid peroxyl radical may then abstract H• from another lipid species to form a lipid hydroperoxide (ROOH) and commence a chain reaction of lipid peroxidation.
In addition, non-radical ROS such as hydrogen peroxide promote oxidative events in the vessel wall. Hydrogen peroxide may oxidize protein thiol (R-SH) groups and interact with heme-containing proteins to release iron or form a ferryl heme moiety. Alternatively, hydrogen peroxide, itself a weak oxidant, may undergo decomposition via transition metals to form a hydroxyl radical (Fenton reaction), which is a potent and highly reactive oxidant species [5,14]. Interestingly, many non-radical ROS are involved in oxidation of proteins rather than lipids. These derivatives target cysteinyl and methioninyl residues of proteins as well as tyrosinyl, tryptophanyl and lysyl residues resulting in oxidative modifications that can alter protein function. The reaction between these non-radical ROS and amino acids is dependent upon the local environment. Vicinal thiol and methionine groups are exquisitely sensitive to oxidation, and a local milieu that favors transition of thiol groups to thiolate anions (i.e., lowers the apparent pKa of the - SH functionality) renders these residues highly susceptible to oxidation [5,14].
Oxidative modification of lipoproteins
The aforementioned reactions between free radical and non-radical ROS and lipids and proteins support the premise that oxidant stress plays a key role in the pathogenesis of atherosclerosis. A number of hypotheses have been derived to explain the etiology of atherosclerosis and have focused on disruption of different, but related, aspects of vessel wall homeostasis. For example, the response-to-injury hypothesis contends that endothelial injury and/or denudation is the inciting event in atherosclerosis [15], while the response-to-retention hypothesis suggests that neointima formation results from uptake of lipoproteins by the vascular wall [5]. In contrast, the oxidative modification hypothesis of atherosclerosis implicates oxidant stress as a mechanistic necessity to initiate atherosclerotic lesion formation [16]. It has, in fact, been demonstrated that low-density lipoproteins (LDL) in their native state are not atherogenic; however, once oxidized, these oxidized low-density lipoproteins (oxLDL) are taken up by macrophages causing them to undergo phenotype modulation to become foam cells and form the nidus for the developing atherosclerotic plaque [17].
Low-density lipoproteins
In the process of oxidation of LDL, ROS initially target polyunsaturated fatty acids containing bisallyllic hydrogen atoms. These oxidized polyunsaturated fatty acids subsequently degenerate to yield reactive aldehyde species that react with lysyl residues of apolipoprotein B-100 to alter the surface charge of LDL particles [18]. In the absence of oxidative modification of apolipoprotein B-100, LDL may be oxidized at other sites, and these minimally oxidized forms of oxLDL aid in the recruitment of inflammatory mediators by inducing endothelial and vascular smooth muscle cell synthesis of chemokines (monocyte chemotactic protein-1) [14]. Once this oxidative modification has occurred, LDL is taken up by macrophages via scavenger receptor pathways to yield cholesterol rich foam cells. Similarly, oxLDL may also be taken up by endothelial cells via the lectin-like oxidized LDL receptor-1 (LOX-1); intracellular accumulation of oxLDL results in decreased endothelial cell nitric oxide production, induction of lecukocyte adhesion molecules, loss of an antithrombotic surface, and the production of smooth muscle mitogenic factors [19]. These lipid-laden endothelial cells act in concert with atherogenic macrophage-derived foam cells to advance plaque formation [20]. The presence of oxLDL in atherosclerotic plaques has been confirmed: immunohistochemistry of human atherosclerotic lesions reveals considerable staining for apolipoprotein B-100, which is not detectable in normal arteries without evidence of atherosclerosis [14].
A small fraction of oxLDL exits the subendothelial space and reenters the circulation to comprise approximately 0.5% of the total pool of LDL in the bloodstream. The presence of circulating oxLDL has been strengthened by the observation that human plasma possesses immunoreactivity to epitopes generated from oxLDL, and a number of epitopes within apolipoprotein B-100 have been shown to generate an immune response [20] (vide infra). Increased plasma levels of oxLDL, which presumably mirror the vascular burden of oxLDL, correlate with acute coronary syndromes and the presence of coronary artery disease documented by angiography [21]. Elevated levels of plasma oxLDL also associate with serum lipoprotein(a), which is known to bind to oxidized phospholipids present in LDL, and is a recognized biomarker of atherosclerotic vascular disease [20,21].
Remnant lipoproteins
In addition to oxLDL cholesterol, remnant lipoproteins, referred to as remnant-like lipoprotein particle-cholesterol (RLP-C), may exist in oxidized forms and contribute to atherogenesis. RLP-C levels are elevated in patients with endothelial dysfunction and coronary artery disease, including those patients with normal total cholesterol or triglyceride levels [20]. In the circulation, RLP-C undergoes oxidative modification owing to its high phosphatidylcholine hydroperoxide content, and has been shown to be extensively oxidized compared to very-low-density lipoproteins. RLP-C also contains a high concentration of the atherogenic and inflammatory mediator lysophosphatidylcholine in chylomicron remnants. RLP-C may also increase LOX-1 expression in the vascular endothelium, activate NADPH oxidase to increase further local superoxide production, upregulate adhesion molecule and MCP-1 expression, degrade cell surface membranes, as well as initiate apoptosis of endothelial cells [20].
High-density lipoproteins
High-density lipoprotein (HDL) cholesterol, recognized for its anti-atherogenic properties, is also subject to oxidative modification resulting in “dysfunctional HDL” that paradoxically contributes to atherosclerotic plaque formation. The HDL particle is comprised of free cholesterol, phospholipid, and apolipoproteins, of which apolipoprotein A-I is the most abundant. HDL particles also contain a number of enzymes including the antioxidant enzymes paraoxonase-1, lecithin:cholesterol acyltransferase (LCAT), and cholesteryl ester transfer protein [22,23]. Together, these enzyme systems modulate the redox milieu and ROS levels within both LDL and HDL cholesterol particles as well as the vessel wall.
Normally, HDL promotes reverse cholesterol transport by enhancing apolipoprotein A-1 efflux of cholesterol from macrophages via interaction with the ATP-binding cassette (ABC)A1 and LCAT-mediated esterification of cholesterol, limits phospholipid oxidation in LDL cholesterol through apolipoprotein A1 and antioxidant enzymes, and decreases expression of inflammatory cytokines and vascular cell adhesion molecules. In this manner, oxidative modification of LDL cholesterol is interrupted and uptake by macrophages is limited [22–24].
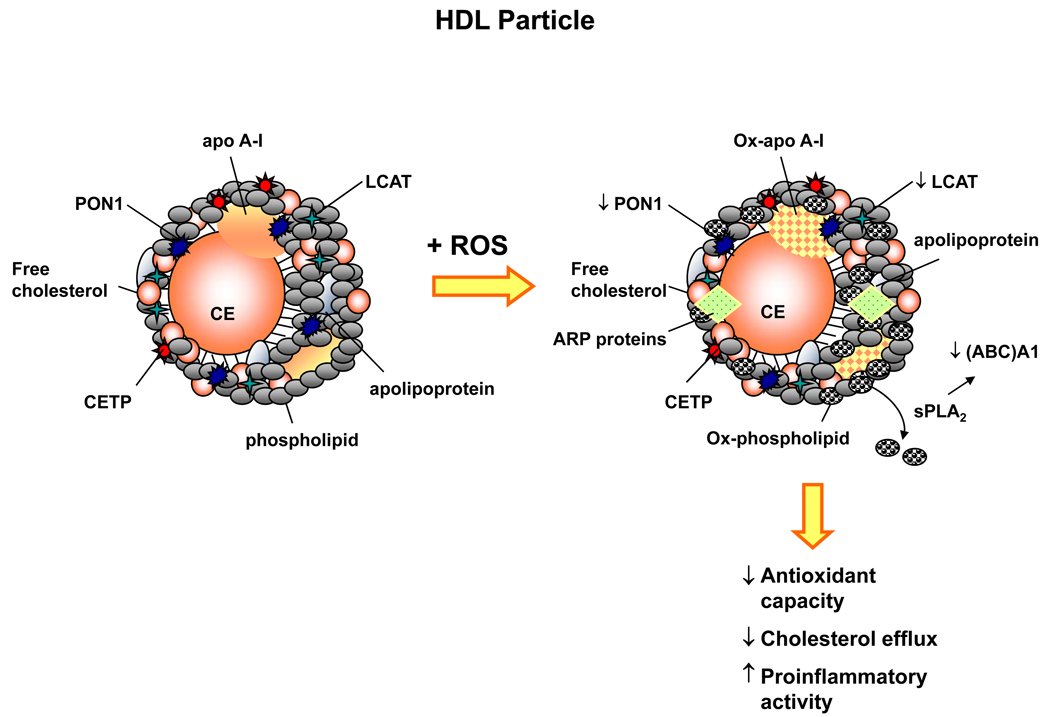
In contrast, in the setting of inflammation and increased oxidant stress, as may occur in a pro-atherosclerotic environment, HDL cholesterol itself achieves proinflammatory properties and promotes the oxidation of LDL cholesterol (Figure 3) [22]. HDL cholesterol is also subject to glycation when exposed to high concentrations of glucose; this occurs due to a decrease in paraoxonase activity resulting in decreased HDL antioxidant capacity [25]. Furthermore, HDL cholesterol undergoes structural modification such that apolipoprotein A-I, apolipoprotein A-II, paraoxonase, and LCAT levels are decreased, and the particle becomes enriched in serum amyloid A and apolipoprotein J signaling a transition to an inflammatory HDL phenotype [26]. Myeloperoxidase has also been shown to modify HDL cholesterol oxidatively by promoting nitrotyrosination and chlorotyrosination within apolipoprotein A-I, influencing particle function adversely. Oxidized HDL cholesterol upregulates the expression of other gene products linked to atherosclerosis including cyclooxygenase, plasminogen activator inhibitor-1 antigen, and proteases [23].
Figure 3. Reactive oxygen species modulate HDL cholesterol function.
High-density lipoprotein (HDL) cholesterol is comprised of free cholesterol and cholesteryl esters (CE), phospholipids, apolipoproteins, and enzymes involved in reverse cholesterol transport, including lecithin:cholesterol acyltransferase (LCAT) and cholesteryl ester transfer protein (CETP). HDL cholesterol also contains the antioxidant enzyme paraoxonase-1 (PON1) that regulates the local redox environment. HDL cholesterol possesses anti-atherogenic and anti-inflammatory properties; however, when exposed to excess reactive oxygen species (ROS), HDL cholesterol may undergo structural and functional oxidative modifications. Increased ROS decreases PON1 and LCAT activity to alter the redox milieu, oxidizes apolipoprotein A-I (Ox-apo A-1) to render it ineffective, and oxidizes phospholipids (Ox-phospholipid). Elevated levels of the secretory protein phospholipase A2 (sPLA2) promote the release of oxidized phospholipids from HDL and impair cholesterol efflux by decreasing the expression of the ATP-binding cassette (ABC)A1. In addition, ROS increases circulating acute phase reactants, which may incorporate into the HDL particle in the place of atheroprotective proteins. Together, these oxidative modifications of HDL result in a HDL particle that possesses decreased antioxidant capacity, diminished cholesterol efflux, and enhanced proinflammatory activity. apo A-I, apolipoprotein A-I; ARP proteins, acute phase reactant proteins.
Clinical studies support the concept of dysfunctional HDL cholesterol and associate it with atherosclerosis. Patients with established coronary heart disease in the setting of very high levels of HDL cholesterol (≥84 mg/dl) demonstrate impaired HDL functional responses: HDL isolated from these patients exhibits a proinflammatory monocyte chemotactic assay response and increases LDL oxidation as compared to HDL isolated from healthy control subjects [27]. Similar responses have been observed in patients with stable coronary artery disease as well as poorly controlled diabetes, in whom HDL was found to have decreased antioxidant enzyme activity and increased content of lipid hydroperoxides [27,28].
Oxidant stress and immunomodulation of atherosclerosis
Atherosclerosis is recognized as an inflammatory disease process, and immunohistochemical analysis has identified polyclonal memory T lymphocytes in human atherosclerotic plaques, suggesting that atherosclerosis is influenced by innate and adaptive immune responses. These CD4+ T cells were found to be reactive against epitopes present in oxidatively modified LDL, thereby implicating oxidant stress as a component of the cellular immune response associated with atherosclerosis [29,30].
There is abundant evidence to support the concept that oxLDL plays a significant role in the observed immune response in atherosclerosis. In apoE knockout mice with aortic plaques, CD4+ and CD8+ T cells have been found in the lesions, and the mice have elevated circulating levels of antibodies against modified LDL. Furthermore, scavenger receptors have been shown to modulate the uptake of oxLDL for presentation to antigen-specific T cells, and T cells specific for epitopes present on oxLDL have been identified in human atherosclerotic lesions [29,30]. In advanced human atheromas, T cells comprise approximately 10–20% of the total cell population and are often localized to sites prone to rupture. These T cells are of the T helper-1 subset and secrete proinflammatory cytokines, including interferon-γ, interleukin-2, and tumor necrosis factor-α, that activate macrophages to increase further ROS production [31].
In addition to oxLDL, a second group of candidate autoantigens responsive to oxidant stress is the heat shock protein (Hsp) family. Mycobacterial Hsp65 and chlamydial HSP60, which resemble human Hsp60, have been localized to atherosclerotic lesions in experimental animal models, and immunization with Hsp65 or Hsp60 augments atherosclerotic plaque formation in mice and rabbits [30,32]. Interestingly, macrophage antioxidant enzymes regulate the chronicity of Chlamydia pneumoniae infection. Macrophages with decreased antioxidant enzyme capacity and increased indices of oxidant stress demonstrate increased levels and duration of Hsp60 protein expression. This finding suggests that oxidant stress-mediated Hsp60 expression in macrophages resident in the atherosclerotic plaque may contribute to the inflammatory immune response [33].
Reactive oxygen species may also serve as a mechanistic link between the innate and adaptive immune response to atherosclerosis. ROS have been shown to modulate T cell expression of CD40 ligand suggesting that oxidant stress may importantly mediate B cell formation of antibodies to putative target epitopes and antigens in atherosclerosis [34]. In fact, it has been shown that activated T cells express CD40 ligand that binds to its receptor CD40 on B cells to generate circulating antibodies to oxLDL. These anti-oxLDL antibodies have been demonstrated in patients with advanced atherosclerosis [30,32]. Furthermore, oxidant stress has been shown to perpetuate the immune response by rendering T cells hyporesponsive to stimuli that signal for diminished function to limit the immune response. As such, the T cells remain refractory to deactivation and continue the inflammatory cascade [35]. These observations suggest that immunomodulation of atherosclerosis as a therapeutic intervention may benefit from a concomitant reduction in the burden of oxidant stress.
Hemodynamic forces, oxidant stress, and atherosclerosis
In addition to the myriad of biochemical mediators of atherosclerosis that increase vascular oxidant stress, disturbance of homeostatic pulsatile flow and mechanical forces similarly increases ROS accumulation and, thereby, contributes to atherosclerotic plaque formation. In vivo, blood vessels are exposed continually to mechanical forces including shear stress and stretch as a result of pulsatile blood flow and blood pressure; however, it has been recognized that the protective effects of these hemodynamic forces are often lost at arterial branch points where flow becomes turbulent and increases the predilection for atherosclerotic plaque formation in these regions [36].
Experimental models have shown that static vascular endothelial cells exposed to laminar flow demonstrate a transient increase in ROS production via activation of NAD(P)H oxidase suggesting, in this setting, that ROS play a role in ambient signaling processes [37,38]. In contrast, prolonged exposure to turbulent flow activated both NAD(P)H oxidase and xanthine oxidase and increased superoxide and hydrogen peroxide production [37,39,40]. Interestingly, when human coronary arteries were subjected to an acute rise in shear stress, increased levels of hydrogen peroxide resulted from enhanced mitochondrial ROS production [41]. The balance between ROS accumulation and ROS metabolism is modulated further by flow-mediated changes in gene expression of antioxidant enzymes. In fact, in vascular endothelial cells laminar, but not turbulent, flow is associated with increased gene expression of a number of antioxidant enzymes including manganese superoxide dismutase, glutathione peroxidase, heme oxygenase-1, as well as enzymes that maintain reduced glutathione stores such as glutathione S-transferase and gamma-glutamylcysteine synthase [36].
As atherosclerotic plaques tend to form at sites of turbulent flow, it is, therefore, not surprising that flow-mediated ROS production contributes to the development of atherosclerotic plaques [42]. ROS produced by turbulent flow decreases levels of bioavailable nitric oxide rendering endothelial cells susceptible to apoptosis, increasing cell permeability, upregulating surface expression of inflammatory cell adhesion molecules, and promoting vascular smooth muscle cell proliferation [36]. Turbulent flow also increases levels of chemokines and growth factors necessary to initiate leukocyte recruitment, adhesion, and extravasation to the subendothelial matrix [43]. Other hemodynamic forces, such as mechanical strain also contribute to atherosclerotic plaque formation. Increased mechanical strain that is associated with high blood pressure correlates well with atheroma burden identified by intima-media thickness in human carotid arteries [44]. Mechanical strain has also been linked to elevated levels of circulating proinflammatory soluble intracellular adhesion molecule-1 and vascular cell adhesion molecule-1 in patients with hypertension [45]. In vitro, endothelial cell expression of these adhesion molecules was prevented by coincubation with the antioxidants N-acetylcysteine or catalase, thereby implicating ROS as a mechanistic link between inflammation and atherosclerotic plaque formation [46].
Conclusion
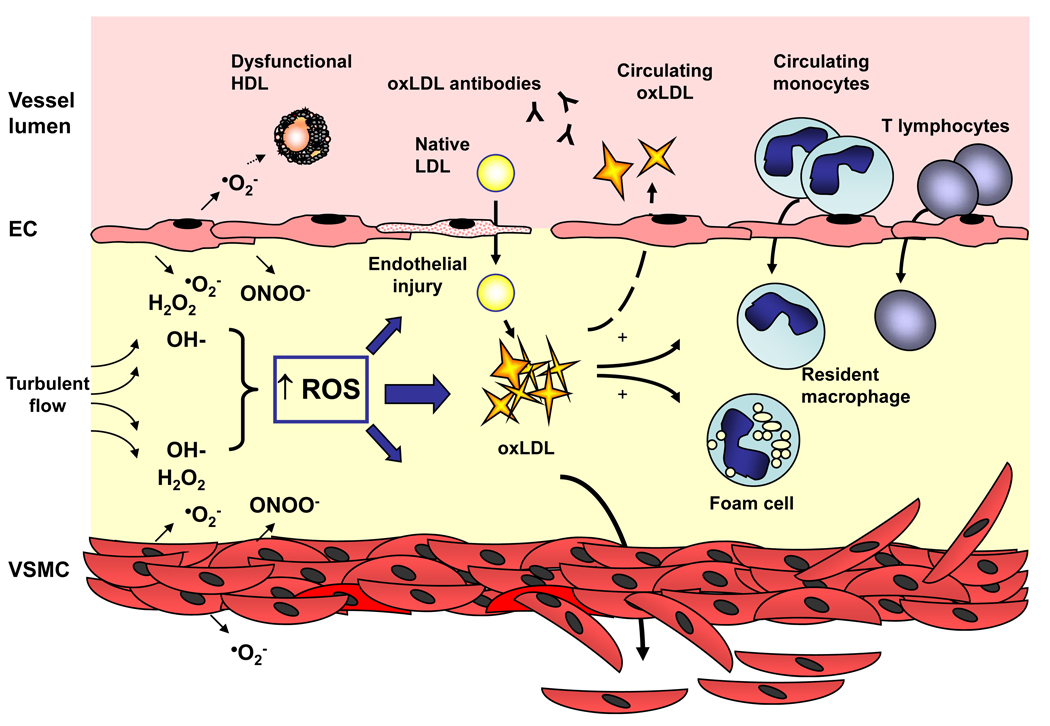
In the vessel wall, an increase in ROS accumulation, resulting from either increased production and/or decreased antioxidant capacity, signals for a vascular phenotype switch to an environment that is permissive for oxidative modification of lipids and proteins that otherwise maintain vascular homeostasis (Figure 4). In this perturbed redox milieu, the ensuing oxidation of low-density lipoproteins results in uptake by macrophages that transition to foam cells and take up residence in the vessel wall to provide the nidus for atherosclerotic lesion formation. Increased levels of ROS augment this response by disabling the antiatherogenic properties of high-density lipoproteins and by adversely modulating the immune response. Furthermore, disruption of homeostatic laminar flow subjects the arterial wall to turbulent flow, typically at sites of arterial branching, to increase local ROS production and promote plaque formation. Taken together, these observations demonstrate that elevated levels of ROS resulting in vascular oxidant stress play an integral mechanistic role in the pathogenesis of atherothrombotic vascular disease.
Figure 4. Reactive oxygen species and the pathogenesis of atherosclerosis.
In the vessel wall, an increase in reactive oxygen species (ROS) accumulation may result as a consequence of increased production and/or decreased antioxidant capacity. This imbalance in the ambient redox state promotes endothelial cell injury and facilitates oxidation of native low-density-lipoproteins (LDL). These oxidized low-density lipoproteins (oxLDL) are chemotactic for both circulating monocytes and T lymphocytes, are taken up by resident macrophages to form foam cells, and stimulate vascular smooth muscle cell proliferation and migration to serve as the nidus for atherosclerotic lesion formation. Moreover, excess ROS augments these effects by rendering high-density lipoprotein (HDL) dysfunctional, reacting with bioavailable nitric oxide to generate peroxynitrite (ONOO−) and promote endothelial dysfunction, and stimulating vascular smooth muscle cell growth directly. Increased levels of ROS may also function as a link between the innate and adaptive immune response to atherosclerosis as circulating antibodies to oxLDL have been detected and in the setting of increased ROS levels, T lymphocytes have been shown to be hyporesponsive to stimuli that limit the immune response. EC, endothelial cell; VSMC, vascular smooth muscle cell; •O2−, superoxide; H2O2, hydrogen peroxide; OH−, hydroxyl radical.
REFERENCES
- 1.Association AH. International Cardiovascular Disease Statistics. 2007 [Google Scholar]
- 2.Yung LM, et al. Reactive oxygen species in vascular wall. Cardiovasc Hematol Disord Drug Targets. 2006;6(1):1–19. doi: 10.2174/187152906776092659. [DOI] [PubMed] [Google Scholar]
- 3.Sorescu D, et al. Superoxide production and expression of nox family proteins in human atherosclerosis. Circulation. 2002;105(12):1429–1435. doi: 10.1161/01.cir.0000012917.74432.66. [DOI] [PubMed] [Google Scholar]
- 4.Azumi H, et al. Superoxide generation in directional coronary atherectomy specimens of patients with angina pectoris: important role of NAD(P)H oxidase. Arterioscler Thromb Vasc Biol. 2002;22(11):1838–1844. doi: 10.1161/01.atv.0000037101.40667.62. [DOI] [PubMed] [Google Scholar]
- 5.Stocker R, Keaney JF., Jr Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004;84(4):1381–1478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- 6.Turrens JF, Boveris A. Generation of superoxide anion by the NADH dehydrogenase of bovine heart mitochondria. Biochem J. 1980;191(2):421–427. doi: 10.1042/bj1910421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Griendling KK, et al. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res. 2000;86(5):494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 8.Jarasch ED, et al. Localization of xanthine oxidase in mammary-gland epithelium and capillary endothelium. Cell. 1981;25(1):67–82. doi: 10.1016/0092-8674(81)90232-4. [DOI] [PubMed] [Google Scholar]
- 9.Romano M, Claria J. Cyclooxygenase-2 and 5-lipoxygenase converging functions on cell proliferation and tumor angiogenesis: implications for cancer therapy. Faseb J. 2003;17(14):1986–1995. doi: 10.1096/fj.03-0053rev. [DOI] [PubMed] [Google Scholar]
- 10.Vasquez-Vivar J, et al. Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc Natl Acad Sci U S A. 1998;95(16):9220–9225. doi: 10.1073/pnas.95.16.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xia Y, et al. Superoxide generation from endothelial nitric-oxide synthase. A Ca2+/calmodulin-dependent and tetrahydrobiopterin regulatory process. J Biol Chem. 1998;273(40):25804–25808. doi: 10.1074/jbc.273.40.25804. [DOI] [PubMed] [Google Scholar]
- 12.Leopold JA, Loscalzo J. Oxidative enzymopathies and vascular disease. Arterioscler Thromb Vasc Biol. 2005;25(7):1332–1340. doi: 10.1161/01.ATV.0000163846.51473.09. [DOI] [PubMed] [Google Scholar]
- 13.Madamanchi NR, et al. Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol. 2005;25(1):29–38. doi: 10.1161/01.ATV.0000150649.39934.13. [DOI] [PubMed] [Google Scholar]
- 14.Stocker R, Keaney JF., Jr New insights on oxidative stress in the artery wall. J Thromb Haemost. 2005;3(8):1825–1834. doi: 10.1111/j.1538-7836.2005.01370.x. [DOI] [PubMed] [Google Scholar]
- 15.Ross R, Glomset JA. Atherosclerosis and the arterial smooth muscle cell: Proliferation of smooth muscle is a key event in the genesis of the lesions of atherosclerosis. Science. 1973;180(93):1332–1339. doi: 10.1126/science.180.4093.1332. [DOI] [PubMed] [Google Scholar]
- 16.Steinberg D, et al. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989;320(14):915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- 17.Goldstein JL, et al. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc Natl Acad Sci U S A. 1979;76(1):333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hazell LJ, Stocker R. Oxidation of low-density lipoprotein with hypochlorite causes transformation of the lipoprotein into a high-uptake form for macrophages. Biochem J. 1993;290(Pt 1):165–172. doi: 10.1042/bj2900165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vohra RS, et al. Atherosclerosis and the Lectin-like OXidized low-density lipoprotein scavenger receptor. Trends Cardiovasc Med. 2006;16(2):60–64. doi: 10.1016/j.tcm.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Nakajima K, et al. The oxidative modification hypothesis of atherosclerosis: the comparison of atherogenic effects on oxidized LDL and remnant lipoproteins in plasma. Clin Chim Acta. 2006;367(1–2):36–47. doi: 10.1016/j.cca.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 21.Tsimikas S, et al. Oxidized phospholipids, Lp(a) lipoprotein, and coronary artery disease. N Engl J Med. 2005;353(1):46–57. doi: 10.1056/NEJMoa043175. [DOI] [PubMed] [Google Scholar]
- 22.Ansell BJ, et al. The paradox of dysfunctional high-density lipoprotein. Curr Opin Lipidol. 2007;18(4):427–434. doi: 10.1097/MOL.0b013e3282364a17. [DOI] [PubMed] [Google Scholar]
- 23.Ansell BJ, et al. High-density lipoprotein function recent advances. J Am Coll Cardiol. 2005;46(10):1792–1798. doi: 10.1016/j.jacc.2005.06.080. [DOI] [PubMed] [Google Scholar]
- 24.Brewer HB., Jr High-density lipoproteins: a new potential therapeutic target for the prevention of cardiovascular disease. Arterioscler Thromb Vasc Biol. 2004;24(3):387–391. doi: 10.1161/01.ATV.0000121505.88326.d2. [DOI] [PubMed] [Google Scholar]
- 25.Hedrick CC, et al. Glycation impairs high-density lipoprotein function. Diabetologia. 2000;43(3):312–320. doi: 10.1007/s001250050049. [DOI] [PubMed] [Google Scholar]
- 26.Rohrer L, et al. High density lipoproteins in the intersection of diabetes mellitus, inflammation and cardiovascular disease. Curr Opin Lipidol. 2004;15(3):269–278. doi: 10.1097/00041433-200406000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Ansell BJ, et al. Inflammatory/antiinflammatory properties of high-density lipoprotein distinguish patients from control subjects better than high-density lipoprotein cholesterol levels and are favorably affected by simvastatin treatment. Circulation. 2003;108(22):2751–2756. doi: 10.1161/01.CIR.0000103624.14436.4B. [DOI] [PubMed] [Google Scholar]
- 28.Gowri MS, et al. Decreased protection by HDL from poorly controlled type 2 diabetic subjects against LDL oxidation may Be due to the abnormal composition of HDL. Arterioscler Thromb Vasc Biol. 1999;19(9):2226–2233. doi: 10.1161/01.atv.19.9.2226. [DOI] [PubMed] [Google Scholar]
- 29.Hansson GK. Vaccination against atherosclerosis: science or fiction? Circulation. 2002;106(13):1599–1601. doi: 10.1161/01.cir.0000035275.64667.a3. [DOI] [PubMed] [Google Scholar]
- 30.Hansson GK, et al. Innate and adaptive immunity in the pathogenesis of atherosclerosis. Circ Res. 2002;91(4):281–291. doi: 10.1161/01.res.0000029784.15893.10. [DOI] [PubMed] [Google Scholar]
- 31.Nilsson J, et al. Immunomodulation of atherosclerosis: implications for vaccine development. Arterioscler Thromb Vasc Biol. 2005;25(1):18–28. doi: 10.1161/01.ATV.0000149142.42590.a2. [DOI] [PubMed] [Google Scholar]
- 32.Nicoletti A, et al. Immunomodulation of atherosclerosis: myth and reality. J Intern Med. 2000;247(3):397–405. doi: 10.1046/j.1365-2796.2000.00660.x. [DOI] [PubMed] [Google Scholar]
- 33.Azenabor AA, et al. Macrophage antioxidant enzymes regulate Chlamydia pneumoniae chronicity: evidence of the effect of redox balance on host-pathogen relationship. Immunobiology. 2006;211(5):325–339. doi: 10.1016/j.imbio.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 34.Salmen S, et al. CD40/CD40L expression in leukocytes from chronic granulomatous disease patients. Apmis. 2007;115(8):939–947. doi: 10.1111/j.1600-0463.2007.apm_613.x. [DOI] [PubMed] [Google Scholar]
- 35.Griffiths HR. ROS as signalling molecules in T cells--evidence for abnormal redox signalling in the autoimmune disease, rheumatoid arthritis. Redox Rep. 2005;10(6):273–280. doi: 10.1179/135100005X83680. [DOI] [PubMed] [Google Scholar]
- 36.Lehoux S. Redox signalling in vascular responses to shear and stretch. Cardiovasc Res. 2006;71(2):269–279. doi: 10.1016/j.cardiores.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 37.De Keulenaer GW, et al. Oscillatory and steady laminar shear stress differentially affect human endothelial redox state: role of a superoxide-producing NADH oxidase. Circ Res. 1998;82(10):1094–1101. doi: 10.1161/01.res.82.10.1094. [DOI] [PubMed] [Google Scholar]
- 38.Hsieh HJ, et al. Increase of reactive oxygen species (ROS) in endothelial cells by shear flow and involvement of ROS in shear-induced c-fos expression. J Cell Physiol. 1998;175(2):156–162. doi: 10.1002/(SICI)1097-4652(199805)175:2<156::AID-JCP5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 39.Hwang J, et al. Pulsatile versus oscillatory shear stress regulates NADPH oxidase subunit expression: implication for native LDL oxidation. Circ Res. 2003;93(12):1225–1232. doi: 10.1161/01.RES.0000104087.29395.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McNally JS, et al. Role of xanthine oxidoreductase and NAD(P)H oxidase in endothelial superoxide production in response to oscillatory shear stress. Am J Physiol Heart Circ Physiol. 2003;285(6):H2290–H2297. doi: 10.1152/ajpheart.00515.2003. [DOI] [PubMed] [Google Scholar]
- 41.Liu Y, et al. Mitochondrial sources of H2O2 generation play a key role in flow-mediated dilation in human coronary resistance arteries. Circ Res. 2003;93(6):573–580. doi: 10.1161/01.RES.0000091261.19387.AE. [DOI] [PubMed] [Google Scholar]
- 42.Malek AM, et al. Hemodynamic shear stress and its role in atherosclerosis. Jama. 1999;282(21):2035–2042. doi: 10.1001/jama.282.21.2035. [DOI] [PubMed] [Google Scholar]
- 43.Walpola PL, et al. Expression of ICAM-1 and VCAM-1 and monocyte adherence in arteries exposed to altered shear stress. Arterioscler Thromb Vasc Biol. 1995;15(1):2–10. doi: 10.1161/01.atv.15.1.2. [DOI] [PubMed] [Google Scholar]
- 44.O'Leary DH, et al. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340(1):14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 45.Buemi M, et al. Cold pressor test raises serum concentrations of ICAM-1, VCAM-1, and E-selectin in normotensive and hypertensive patients. Hypertension. 1997;30(4):845–847. doi: 10.1161/01.hyp.30.4.845. [DOI] [PubMed] [Google Scholar]
- 46.Cheng JJ, et al. Cyclic strain-induced reactive oxygen species involved in ICAM-1 gene induction in endothelial cells. Hypertension. 1998;31(1):125–130. doi: 10.1161/01.hyp.31.1.125. [DOI] [PubMed] [Google Scholar]